方案详情
文
红醋栗(一种浆果)蛋白富集组分作为油水乳状液乳化剂的潜力Potential of redcurrant protein-enriched fractions as emulsifier in oil–water-emulsions
方案详情

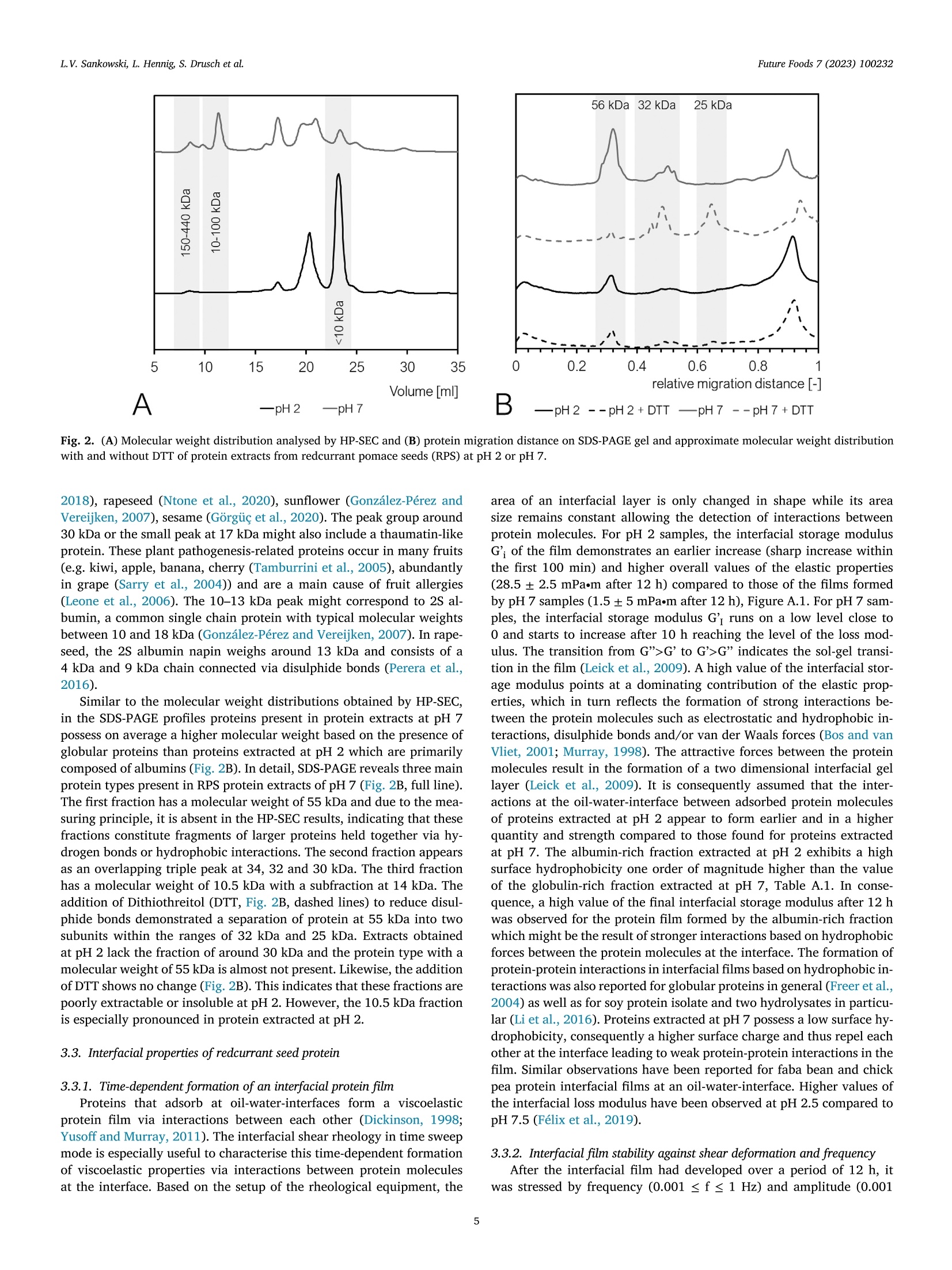
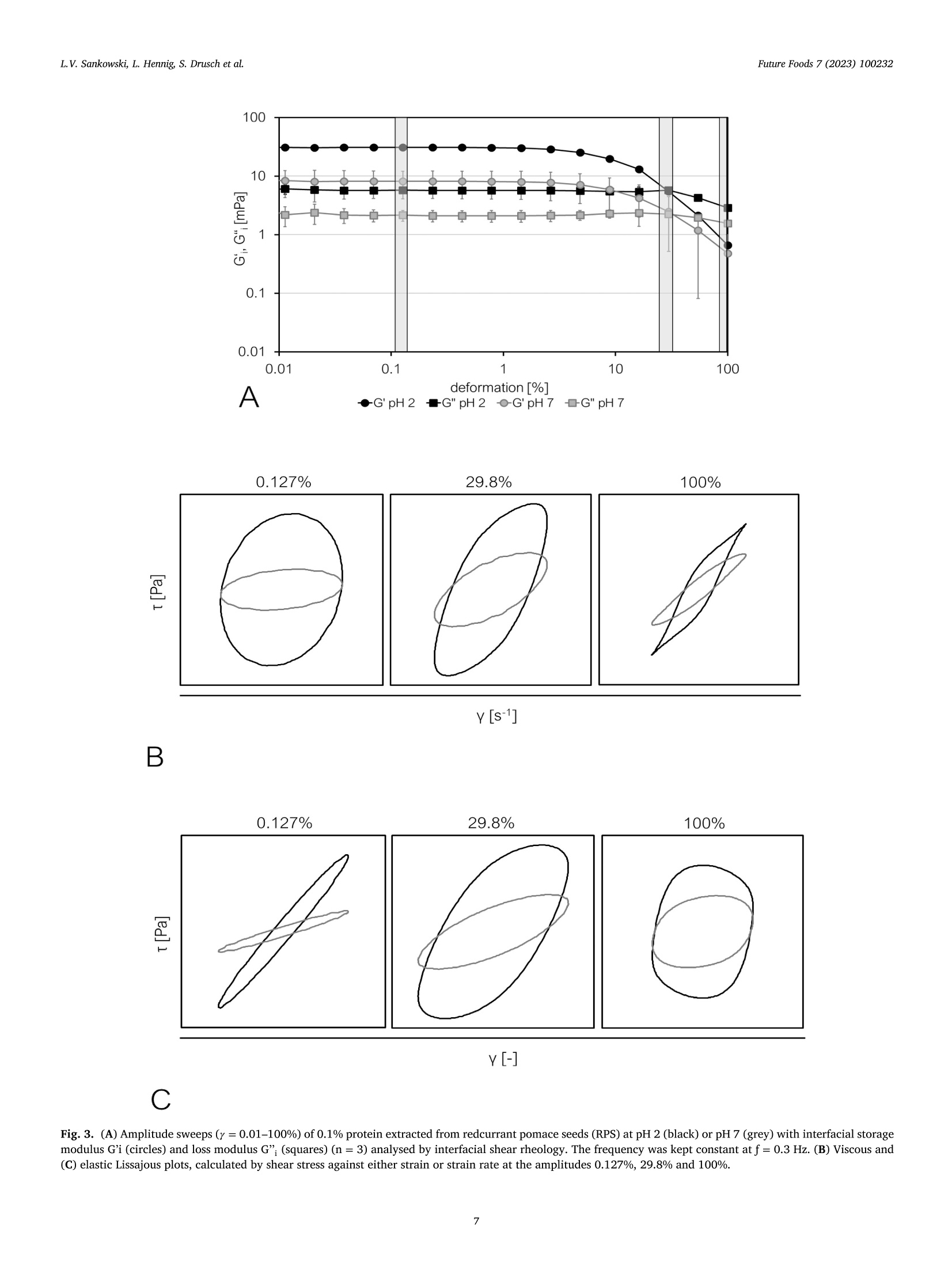
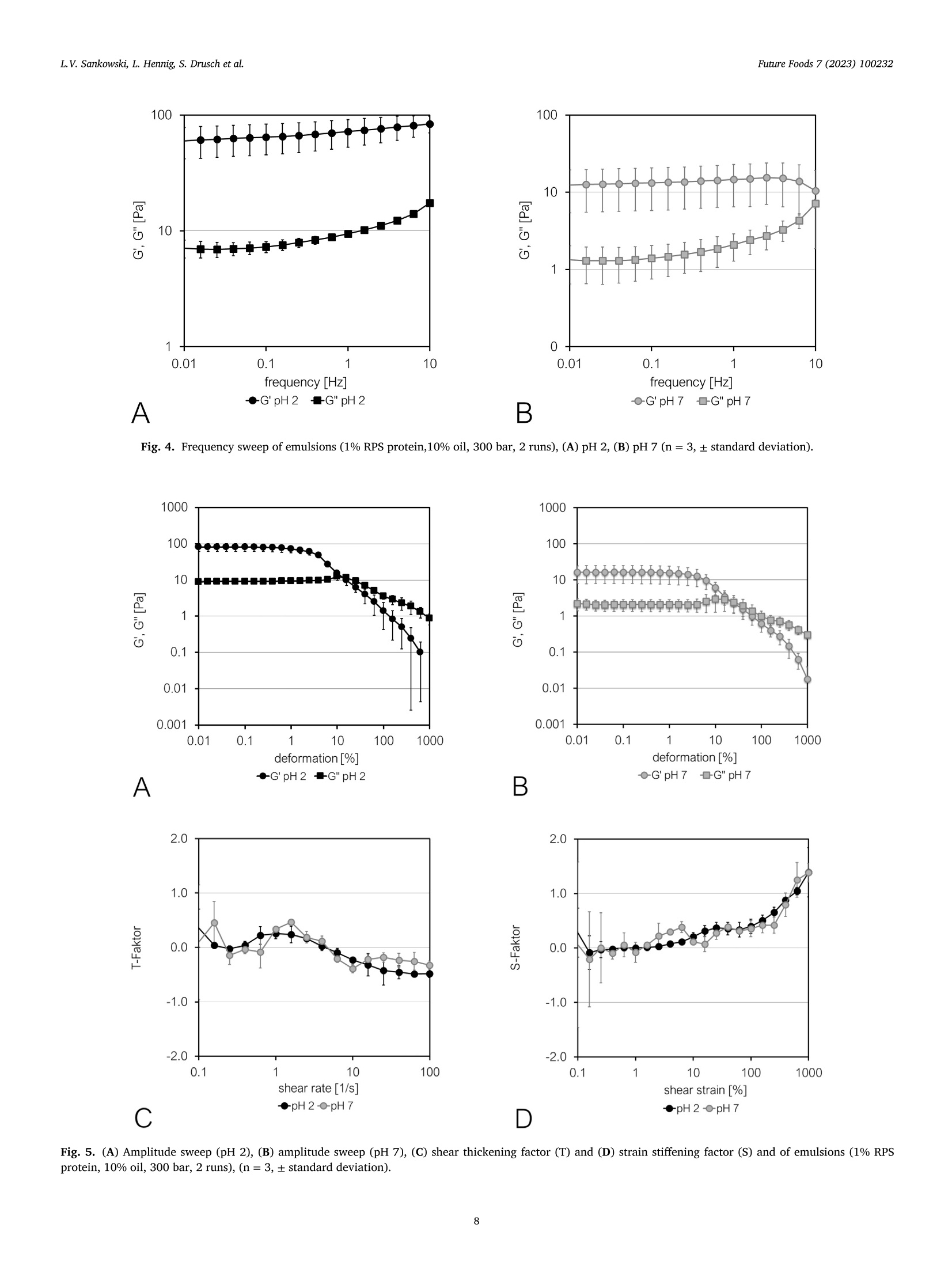
红醋栗(一种浆果)蛋白富集组分作为油水乳状液乳化剂的潜力Potential of redcurrant protein-enriched fractions as emulsifier in oil–water-emulsionsFuture Foods 7 (2023) 100232L.V. Sankowski, L. Hennig, S. Drusch et al. L.V. Sankowski, L. Hennig, S. Drusch et al.Future Foods 7 (2023) 100232 Contents lists available at ScienceDirect Future Foods journal homepage: www.elsevier.com/locate/fufo 红醋栗(一种浆果)蛋白富集组分作为油水乳状液乳化剂的潜力 Potential of redcurrant protein-enriched fractions as emulsiier in oil–water-emulsions L.V. Sankowski a , a , L. Hennig b , S. Drusch a , M. Brückner-Gühmann a 柏林理工大学食品技术和食品材料科学系 a Technische Universität Berlin, Department of Food Technology and Food Material Science, Königin-Luise-Str. 22, 14195 Berlin, Germany b Technische Universität Berlin, Department of Food Biotechnology and Process Engineering, Königin-Luise-Str. 22, 14195 Berlin, Germany ARTICLE INFO ABSTRACT 柏林理工大学食品生物技术与工艺工程系 浆果籽渣 Keywords: Berry protein, due to its functional properties, was extracted from redcurrant pomace seeds (RPS) in aqueous me- Redcurrant pomace dia to utilise side-streams and increase the sustainability of food production. Emulsions containing 1% redcurrant Plant protein seed protein and 10% rapeseed oil were prepared by high pressure homogenisation. The emulsions’ structure, By-product droplet surface charge, stability, rheological behaviour, and colour were characterised by microscopy, dynamic Emulsiication Utilisation of side-streams laser light scattering, storage tests and bulk rheology including large amplitude oscillatory shear rheology, re- Plant-based emulsiier spectively. Protein extracts of RPS proved to stabilise oil–water-interfaces by formation of a protein ilm having non-linear low characteristics. While at low to medium shear, the emulsions showed pseudoplastic behaviour, limited molecular mobility at the interface induced softening at high shear. The shear stability of the interfa- cial protein layer decreased with increasing pH, i.e. with increasing molecular charge. Emulsions were prone to locculation and subsequent creaming, either because of low surface charge at low pH or due to bridging by proteins and polymerised polyphenol complexes under neutral conditions. Although redcurrants only occupy a small market share in the berry sector, they are of high importance for specialised local producers and results presented here might evince possibilities for the utilisation of other pomace types. 1. Introduction The adoption of the UN Sustainable Development Goals (SDG) 2030deined the need for a sustainable development in our society. The con-tribution of the food industry to the SDGs has been a subject of particu-lar interest because the food production is directly associated with many goals including SDG 2 (no hunger). As a result, food industry should aim at an eicient valorisation of existing resources to reduce food waste and losses. One example for a currently under-exploited side-stream is berry pomace, including redcurrant pomace. From a botanical point of view, redcurrant berries comprise of a skin (exocarp), pulp (meso-carp), and seed (endosperm), each fulilling a distinct biological function ( Lopez and Barclay, 2017 ). During the juice production, after pressing, the remaining wet press cake may immediately be dried to prevent mi-crobial growth and milled for further application ( Reißner et al., 2019 ). Thus, the pomace constitutes a heterogeneous material containing skin, pulp, and seeds. Although it contains valuable nutrients, among others phenolic compounds, oil, ibre and protein ( Reißner et al., 2019; Sójka and Król, 2009; Venskutonis, 2020 ), direct application of pomace pow-der as an ingredient in food products is challenging. The high amount of insoluble dietary ibre and the comparatively high particle size (d 90~ 250 um) ( Reißner et al., 2019 ) cause undesirable sensory and func- tional attributes. Therefore, the reclassiication of the pomace into seed and pericarp fractions and subsequent characterisation of valuable com-ponents is inevitable. So far, studies on the valorisation of berry pomace have mainly focused on the extraction of polyphenols, antioxidants and lipids, as indicated by Dienaite et al. (2020) and Struck et al. (2016). The extraction, characterisation and utilisation of this protein as a food component could meet the increasing demand for animal-free, sustain-ably sourced protein for human nutrition ( Takefuji, 2021 ). Information about redcurrant protein is scarce and mainly covers the primary protein structure ( Pieszka et al., 2015 ). The protein types found in redcurrant pomace are assumed to resemble other fruit and seed proteins, especially the dominating presence of albumins and glob-ulins, as the genetic information about their composition is well pre-served across plant species ( Chéreau et al., 2016; Gazzola et al., 2014; González-Pérez and Vereijken, 2007 ). Assuming structural similarities to other plant protein species, extractability of redcurrant protein using aqueous media is expected to show good results and high protein yields between neutral and favourably alkaline pH which was reviewed by Sari et al. (2015) for various plant materials. The major drawback of ex-traction under alkaline conditions is the induction of chemical reactions ( Contreras et al., 2019; Karefyllakis et al., 2017 ), namely oxidation of phenols that might covalently interact with proteins resulting in protein https://doi.org/10.1016/j.fufo.2023.100232 2666-8335/© 2023 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY license ( http://creativecommons.org/licenses/by/4.0/) aggregation ( Prigent et al., 2003; Keppler et al., 2020; Sari et al., 2015 ) or alteration of the protein structure ( Li et al., 2020 ). These reactions are considered to negatively afect the proteins’ properties due to forma-tion of green-brown complexes and of-lavour substances ( Kroll et al.,2003; Laguna et al., 2018; Liang & Were, 2018a , 2018b ; Ozdal et al.,2013; Sabir et al., 1974; Wildermuth et al., 2016 ) reducing consumer ac-ceptance. In contrast, extraction under acidic conditions could have the potential to reduce covalent phenol-protein interactions ( Chéreau et al.,2016 ). The functionality of a protein determines its applicability in foods. In general, protein extracts from botanical species show good interfa-cial properties ( Pham et al., 2017; Shao et al., 2014 ), suggesting similar behaviour for proteins extracted from redcurrant pomace. While most literature focuses on the emulsiication properties of globular proteins ( Yang & Sagis, 2021 ), the albumin fraction in plant protein was also reported to have comparable or even better emulsiication or foaming properties ( Ghumman et al., 2016 ). Thus, in the present study extraction pH is controlled to generate two fractions: one being rich in globulin and the other being rich in albumins. The aim of this study was to extract pro-tein from redcurrant pomace and to analyse the interfacial properties as well as the behaviour of these protein extracts in emulsions. Protein was extracted from redcurrant seeds under acidic and neutral conditions. It was expected that the pH-value during extraction would inluence the molecular weight distribution of the fractions. It was hypothesised that albumins being smaller in size and having a higher surface hydrophobic-ity would develop increased protein-protein-interactions in the interfa-cial ilm which would alter the viscoelastic characteristics of the protein ilms and their network forming ability, which in turn would inluence both the emulsiication properties and the emulsion stability. 2. Materials and methods 2.1. Materials 浆果渣 Berry pomace was collected during the harvesting period of 2020. Ribes rubrum, redcurrant, were processed by Austria Juice GmbH, All-hartsberg, Austria. The pomace contained stems, seeds and skins and was stored at − 20 °C in bags in batches of 10 kg. After thawing for 24 h at 8 °C, redcurrant pomace was dried in a compact tray dryer (H01, Har-ter GmbH, Stiefenhofen, Germany) at 60 °C to a inal moisture content of 10%. The pomace was sieved (mesh size: 2.5, 2.0, 1.6 and 1.4 μ m) and sorted manually into seed and pericarp fractions. The seed fraction was milled in a centrifugal mill (ZM1, Retsch, Haan, Germany) using a 500 μ m ring sieve. All pomace materials were stored at room temper-ature (RT). The nitrogen contents of the pomace and its fractions were determined by the Dumas method with a DUMATHERM® (C. Gerhardt GmbH & Co. KG, Königswinter, Germany) using a conversion factor of 6.25 for the calculation of the protein content. The fat content was de-termined by extraction in a Soxhlet apparatus with petrol ether. 2.2. Extraction of redcurrant seed protein Protein extracts were prepared for the application in emulsions. In brief, 200 g of milled RPS were weighed into a 2 l beaker together with 1800 g distilled water, premixed, and the pH (pH 2 or pH 7) was ad-justed by addition of 10% NaOH or HCl, respectively. The suspension was stirred for 30 min with a paddle stirrer at approximately 150 min − 1revolutions (EUROSTAR digital, IKA-Werke, Staufen, Germany) at RT. Afterwards, the pH was readjusted, and the suspension was left to settle for 15 min at RT. The cream layer was partially removed with a pipette and the supernatant was poured through a metal sieve (mesh size ap-proximately 0.5 mm). The extract obtained from the supernatant (sol-uble fraction) was subsequently freeze-dried (Beta 1-8 LSCplus, Christ Gefriertrocknungsanlagen, Osterode am Harz, Germany). The use of the soluble fraction served to exclude possible sedimentation processes. 2.3. Emulsiication procedure Oil-water-emulsions were prepared using 10% rapeseed oil and 1%protein of extract. In brief, an aliquot of protein extract containing 3 g protein was illed up to 270 g with distilled water and stirred for 15 min at RT with 500 min − 1 (Multistirrer digital 15, VELP Scientiica, Usmate Velate, Italy) to solubilise the protein. The pH was adjusted with 1 M NaOH or HCl, respectively, to values of 2 or 7. Oil (25 g rapeseed oil) and aqueous phase (225 g) were premixed with a disperser (T25 basic ULTRA-TURRAX, IKA-Werke, Staufen, Germany) for 45 s at 9500 rpm. Subsequently, the premix was homogenised in a high-pressure homoge-nizer (PandaPLUS, GEA mechanical equipment, Parma, Italy) with two stages in two runs (irst run at 300/50 bar, second run at 200/50 bar). 2.4. Characterisation of the protein extracts 2.4.1. Determination of the protein and fat content The nitrogen contents of the protein extracts were determined by the Dumas method with a DUMATHERM® (C. Gerhardt GmbH & Co. KG, Königswinter, Germany) using a conversion factor of 6.25 for the calculation of the protein content which was used for the determi-nation of the protein content in pomace by Gazzola et al. (2014), Rezig et al. (2013) and Shao et al. (2014). The protein extraction yield (%) was expressed as the percentage of the protein content in the freeze-dried extracts in relation to the amount originally present in the RPS. The fat content was determined by extraction in a Soxhlet apparatus with petrol ether. The amount of extracted fat was determined gravi-metrically following the standard ISO 659:2009 (Deutsches Institut für Normung, 2009). 2.4.2. Determination of the molecular weight distributions The molecular weight distributions of protein extracts were deter-mined by high-performance liquid size exclusion chromatography (HP-SEC) and SDS-PAGE. HP-SEC was carried out according to Brückner-Gühmann et al. (2018) using the same equipment (HPLC ÄKTAbasicTM 10 system consisting of a separation unit: pump P-900, UV moni-tor UV-900, UV-low cell (10 mm), injection valve INV-907, mixer M-925, low restrictor FR-904; and software for system control and result analysis: UNICORN TM version 5.01 (Amersham Biosciences, GE Healthcare, Chicago (IL), USA)). A 0.1 M sodium phosphate bufer at pH 7 containing 0.1 M NaCl was degassed in an ultrasound bath (Sonorex super RK 103 H, BANDELIN electronic, Berlin, Germany) and the Superdex TM 200 Increase 10/300 GL chromatography column (GE healthcare GmbH, Chicago (IL), USA) was lushed with the same bufer as mobile phase for 30 min. Protein extracts were diluted to 0.1% protein with eluent bufer and iltered with a syringe ilter (pore size 0.45 μ m, Rotilabo syringe ilter, Carl Roth, Karlsruhe, Germany), whereupon the extract was inserted and the chromatography run with a low rate of 0.5 ml/min. Retention times were measured by UV extinction at 280 nm. Replicate absorbances were normalised and mean values plotted against elution time. To estimate the molecular weights of the peaks in the chromatograms of the samples a gel iltration calibration kit with low and high molecular weight standards (GE healthcare GmbH, Chicago (IL), USA) were purchased from Sigma-Aldrich Co. LLC (Taufkirchen, Germany): feritin (440 kDa), aldolase (158 kDa), conalbumin (75 kDa), ovalbumin (44 kDa), carbonic an-hydrase (29 kDa), ribonuclease (13.7 kDa), aprotinin (6.5 kDa), and somatostatin (1.6 kDa). SDS-PAGE was carried out based on the method introduced by Laemmli (1970). Extracts were diluted to a protein concentration of 0.2% with distilled water. The protein solutions were further diluted to 0.1% protein content with Laemmli sample bufer (Bio-Rad, Hercules (CA), USA) and heated to 95°C for 5 min. For reducing SDS-PAGE, the samples were prepared with a sample bufer containing 5% Dithiothre-itol (DTT) (Bio-Rad, Hercules (CA), USA), instead. A precast Tris-Glycine gel (Criterion TGX Gel 12%, 12 + 2/ 26 well type, Bio-Rad, Hercules (CA), USA) was loaded with 10 μ l of the protein solutions and 5 μ l of molecular weight marker, respectively (PageRuler Prestained Pro-tein Ladder 10 – 180 kDa, Thermo Fisher Scientiic, Waltham (MA), USA). The electrophoresis was run in an electrophoresis chamber (Cri-terion Cell combined with PowerPac HC, both Bio-Rad, Hercules (CA), USA) at 200 V, 0.136 A and 300 W for 40–50 min until the protein bands reached 1 cm from the bottom of the gel. The protein in the gel was ixated for 30 min (45.45% methanol; 9.1% acetic acid; 45.45%dist. H2O) and stained with Coomassie Blue for 1 h (0.09% Coomassie blue G250; 9.09% acetic Acid; 45.41% dist. H2O; 45.41% methanol), followed by 15 min cycles of de-staining to remove excess Coomassie Blue (10% acetic acid; 77.5% dist. H2O; 12.5% isopropanol). The gels were photographed (Nikon D3100, Nikon, Minato, Japan) and photos were aligned horizontally, cropped, converted into grey scale images, and brightness and contrast were adjusted (Microsoft Photos, Microsoft, Redmond (WA), USA). The edited gel images were analysed in the data processing software Origin 2020 (OriginLab, Nothampton (MA), USA) using the Gel Analyzer App (GelAnalyzer 19.1 ( www.gelanalyzer.com ) by Istvan Lazar Jr., PhD and Istvan Lazar Sr., PhD, CSc). 2.4.3. Surface hydrophobicity Surface hydrophobicity of the protein extracts was determined according to Kato and Nakai (1980) using 8-anilino-naphthalin-1-sulfonate (ANS) as a luorescent probe. Each protein extract was sol-ubilised and diluted to contain 0.1% protein. This solution was further diluted to ive concentrations of 0.001–0.005% protein and the corre-sponding pH was adjusted to the respective extract pH as indicated by Tang et al. (2023); 5 ml of the solutions were mixed with 20 μ l of ANS on a vortex shaker (MS1 Minishaker, IKA-Werke, Staufen, Germany) immediately before measurement. The samples with ANS and without ANS were exited with 380 nm light (531 V voltage) in the luorescence spectrophotometer (Cary Eclipse & software Advanced Reads, Agilent technologies, Santa Clara (CA), USA) and the luorescence emission was measured at 470 nm (slit widths 5 nm). Surface hydrophobicity deter-mined with the luorescence spectrophotometer method serves only as approximation for the estimation of the hydrophobicity. 2.4.4. Microscopy Protein extracts were dispersed in distilled water for microscopic examination (Axioscope 5, Carl Zeiss, Oberkochen, Germany) and ob-served by transmitted light microscopy at 1000x magniication (objec-tives N-Achroplan 100 x, Carl Zeiss, Oberkochen, Germany). Photos were taken with an attached camera (Axiocam 305 colour, Carl Zeiss, Oberkochen, Germany) and corresponding software (ZEN 3.1 blue edi-tion, Carl Zeiss, Oberkochen, Germany). 2.4.5. Interfacial shear rheology The formation and rheological properties of a viscoelastic protein ilm on the interface between an aqueous solution and medium-chain triglyceride oil (MCT oil) was investigated following the protocol of Brückner-Gühmann et al. (2018) with some modiications. Suspensions containing 0.1% protein were made from protein extracts and distilled water (stirred for 15 min, 500 min − 1 , RT, RT 15 5000, IKA-Werke, Staufen Germany). About 120 g of the suspension was illed into the sample container of the rheometer (Physica MCR 301, Anton Paar, Graz, Austria). Thereafter, the bi-conical test device (68.28 mm diameter, 5 °angle, BI68-5, Anton Paar, Graz, Austria) was half immersed in the sus-pension and the puriied MCT oil was illed on top to cover the upper half of the bi-conus. Three diferent harmonic oscillation deformation measurements were carried out subsequently and recorded by the cor-responding software (RheoCompass, Anton Paar, Graz, Austria): A time sweep to observe the ilm formation (12 h, γ = 0.1%, f = 1 Hz, 144measuring points), a frequency sweep to investigate ilm stability and interactions ( γ = 0.1%, 0.001 < f < 1 Hz, 16 measuring-points), and an amplitude sweep to identify the linear-viscoelastic amplitude range for the interface (0.001 ≤ γ ≤ 100%, f = 0.3 Hz, 20 measuring-points). During the amplitude sweep, detailed information on the sample’s stress response in a large amplitude oscillatory shear setting (LAOS) was col-lected to generate Lissajous plots (shear stress over strain) for the elastic and viscous compounds. All rheological measurements were carried out in triplicate at 20 °C. 2.5. Characterisation of the emulsions 2.5.1. Emulsion bulk rheology The rheological properties of emulsions were examined in three sep-arate measurements. The emulsion was kept at 6°C for at least 12 h before the analysis. A concentric cylinder (CC27/P6, Anton Paar, Graz, Austria) was used as a measuring system. In frequency sweep mode, the shear stress was measured at decreasing oscillation frequency with con-stant deformation ( γ = 0.1%, 0.01 ≤ f ≤ 10 Hz, 16 measuring-points, rheometer Physica MCR 502, Anton Paar, Graz, Austria). In amplitude sweep mode, the deformation was increased at a constant frequency of 1 Hz (0.01 ≤ γ y 1000% in 26 points, rheometer Physica MCR 502, An-ton Paar, Graz, Austria). All rheological measurements were carried out in triplicate at 20 °C. Large amplitude oscillatory shear (LAOS) measurements were per-formed to investigate the emulsion’s behaviour in the non-linear vis-coelastic shear range. The plots were interpreted using the dimensionless parameters in-troduced by Ewoldt et al. (2008) calculated according to Eqs. (1) and (2). S is the strain-stifening ratio (S = 0 for linear elastic response, S > 0for intracycle strain stifening, S < 0 for intracycle strain softening) with G’ M being the tangent of the Lissajous plot at 0 strain, and G’L being the secant at maximum strain. Factor T is the shear thickening ratio (T = 0 for LVE response, T > 0for intracycle shear thickening, T < 0 for intracycle shear thinning) with η M as coeicient of viscous dissipation at zero shear rate (plot tangent) and η L at the largest shear rate (both derived from the loss modulus as G ” = η ’ ω ). In addition to the Lissajous interpretation, the complex shear modulus of the interface was measured and divided into the interfacial storage modulus G’i (elastic modulus) and the interfacial loss modulus G ”i (viscous modulus) at a frequency ω : 2.5.2. ζ -Potential The ζ -potential of emulsions was measured at 25 °C via dynamic laser light scattering (Nano ZS and software Zetasizer, Malvern Panalytical, Malvern, UK). Emulsions had been stored at 6 °C for 24 h and were diluted 1:20 with distilled water before measurement. 2.5.3. Colour Fig. 1. Microscopic images (magniication: 1000x) of RPS protein extracts at ( A ) pH 2, and ( B ) pH 7. 2.5.4. Emulsion stability Freshly prepared emulsions were illed into graduated 10 ml test tubes, closed with a rubber lid, and stored at RT (approx. 21 °C) for 7 days. Structural changes and separation of the emulsions were docu-mented after one and seven days. 2.6. Statistical analysis With the exception of the analysis of the surface hydrophobicity, all experiments including the extraction of redcurrant seed protein, the chemical characterisation, the rheological measurements as well as the preparation and characterisation of the emulsions were done in trip-licate. Statistical signiicance was determined via analysis of variance (ANOVA) using SPSS Statistics Version 28 (SPSS Inc., Chicago, USA) ( p < 0.05). 3. Results & discussion 3.1. Characterisation of the redcurrant pomace and its fractions Seeds form the main constituent of the dried pomace (59%), while fruit pulp and skins (pericarp) account for approximately 41% of the weight, Table A.1. This agrees with data published by Sójka and Król (2009) about blackcurrant pomace. The seed fraction contains more protein (15.2% vs. 9.9%, Table A.1) and fat (30.3% vs. 13.8%, Ta-ble A.1) than the pericarp fraction, which is related to the diferent biological functions of the pericarp and endosperm ( Lopez and Bar-clay, 2017 ). In redcurrant seeds, the cellular structure is the matrix, from which the protein needs to be desorbed by the extraction solvent. Aque-ous extraction resulted in a concentration of the protein content from 15.2 ± 0.1% for the seeds to 26.4 ± 0.3% at pH 2 and to 29.4 ± 0.7%at pH 7 (Table A.2). At pH 2 aqueous extraction yielded 16.7 ± 7%protein while at pH 7 the protein extraction yield reached a value of 33.8 ± 13.7%. Nevertheless, due to the high variations this diference in the protein extraction yield was statistically not signiicant (n = 3, p <0.05). Most plant proteins have an isoelectric point between 4 and 5 for example the majority of proteins in wine ( Ferreira et al., 2001 ). Usually, a low solubility in this area in combination with reduced electrostatic repulsion decreases the protein extraction yield ( Sari et al., 2015 ). The proteins remain irmly bound in the cellular matrix and the interaction with the extraction medium remains low. Under neutral conditions, sol-ubility and thus the protein extractability of plant proteins is increased by increasing the proteins’ net charge and by reducing intermolecular disulphide bonds ( Contreras et al., 2019; Sari et al., 2015; Shao et al.,2014 ) generally resulting in higher protein extraction yields. This inlu- ence of the pH-value on the protein extraction yield of the berry proteins was not observed in the present study. Extraction in aqueous medium reduced the fat content from 30.3 ± 1.5% to 8.3 ± 3.9% (pH 2) and 14.7 ± 1.9% (pH 7), Table A.2. Fat and protein together account for 35 to 45% of the extracted mass. Micro-scopic images of the resuspended extracts reveal that the remaining pro-portion is constituted by soluble ibre-rich cell wall material ( Fig. 1 A). These cellular structures form aggregated particles of up to more than 100 um ( Fig. 1 A). Furthermore, irregular shaped structures of approxi-mately 0.5 um occur freely dispersed in all samples, especially at pH 7( Fig. 1 B). Due to the experimental setup in the present study, redcurrant protein-enriched fractions are multicomponent mixtures of protein, fat and ibre. We assume only minor efects of the co-components on the interfacial properties which has already been shown by Karefyllakis et al. (2019) for the interfacial properties of sunlower press cake. 3.2. Molecular characteristics of protein extracted from RPS The molecular weight distributions of the proteins being present in the extracts were analysed via size exclusion chromatography analy-sis (HP-SEC) and are displayed in Fig. 2 A. The proteins extracted at pH 7 have a diferent molecular weight proile than proteins extracted at pH 2 ( Fig. 2 A). In chromatograms of pH 7 samples, protein struc-tures ranging between molecular weights of 350 kDa and 150 kDa (peak between 10.6 and 12.2 ml), 30 kDa and 10 kDa (peak between 15.5and 17.8 ml) and below 6.5 kDa were detected. Compared to proteins extracted at pH 7, proteins extracted at pH 2 reveal only three peaks with smaller molecular weight, starting with a peak between 10 kDa (17.8 ml) and 18 kDa (16.5 ml) with a peak maximum at 13 kDa and two larger peaks with molecular weights below 6.5 kDa. It can be as-sumed, that the fractions between 350 and 150 kDa present in protein extracts at pH 7 can be attributed to hexamers or trimers of a 11S glob-ulin which is an ubiquitous oligomeric plant protein with a molecular weight of 300–350 kDa ( Brückner-Gühmann et al., 2018; Ntone et al.,2020; Wanasundara et al., 2016 ). The hexameric form has a six-stranded beta-barrel shape. Each of the subunits (approx. 55 kDa) consists of an acidic (32–44 kDa) and basic (21–27 kDa) apoprotein connected by one disulphide bridge. The composition of 11S proteins is well conserved across species with shared primary, secondary, tertiary and quaternary structures ( González-Pérez and Vereijken, 2007 ) and the correspond-ing protein molecular weight spectrum appears in many other botanical berries such as grape ( Gazzola et al., 2014; Grimplet et al., 2009 ), cocoa ( Voigt et al., 1993 ), tomato ( Sogi et al., 2002 ), and pumpkin ( Rezig et al.,2013 ), as well as various other crops (e.g. oat ( Brückner-Gühmann et al., 一pH 2 一 pH 7 B 一pH2 --pH2+ DTT —pH7--pH7+DTT Fig. 2. ( A ) Molecular weight distribution analysed by HP-SEC and ( B ) protein migration distance on SDS-PAGE gel and approximate molecular weight distribution with and without DTT of protein extracts from redcurrant pomace seeds (RPS) at pH 2 or pH 7. 2018 ), rapeseed ( Ntone et al., 2020 ), sunlower ( González-Pérez and Vereijken, 2007 ), sesame ( Görgüç et al., 2020 ). The peak group around 30 kDa or the small peak at 17 kDa might also include a thaumatin-like protein. These plant pathogenesis-related proteins occur in many fruits (e.g. kiwi, apple, banana, cherry ( Tamburrini et al., 2005 ), abundantly in grape ( Sarry et al., 2004 )) and are a main cause of fruit allergies ( Leone et al., 2006 ). The 10–13 kDa peak might correspond to 2S al-bumin, a common single chain protein with typical molecular weights between 10 and 18 kDa ( González-Pérez and Vereijken, 2007 ). In rape-seed, the 2S albumin napin weighs around 13 kDa and consists of a 4 kDa and 9 kDa chain connected via disulphide bonds ( Perera et al.,2016 ). Similar to the molecular weight distributions obtained by HP-SEC, in the SDS-PAGE proiles proteins present in protein extracts at pH 7possess on average a higher molecular weight based on the presence of globular proteins than proteins extracted at pH 2 which are primarily composed of albumins ( Fig. 2 B). In detail, SDS-PAGE reveals three main protein types present in RPS protein extracts of pH 7 ( Fig. 2 B, full line). The irst fraction has a molecular weight of 55 kDa and due to the mea-suring principle, it is absent in the HP-SEC results, indicating that these fractions constitute fragments of larger proteins held together via hy-drogen bonds or hydrophobic interactions. The second fraction appears as an overlapping triple peak at 34, 32 and 30 kDa. The third fraction has a molecular weight of 10.5 kDa with a subfraction at 14 kDa. The addition of Dithiothreitol (DTT, Fig. 2 B, dashed lines) to reduce disul-phide bonds demonstrated a separation of protein at 55 kDa into two subunits within the ranges of 32 kDa and 25 kDa. Extracts obtained at pH 2 lack the fraction of around 30 kDa and the protein type with a molecular weight of 55 kDa is almost not present. Likewise, the addition of DTT shows no change ( Fig. 2 B). This indicates that these fractions are poorly extractable or insoluble at pH 2. However, the 10.5 kDa fraction is especially pronounced in protein extracted at pH 2. 3.3. Interfacial properties of redcurrant seed protein 3.3.1. Time-dependent formation of an interfacial protein ilm Proteins that adsorb at oil-water-interfaces form a viscoelastic protein ilm via interactions between each other ( Dickinson, 1998; Yusof and Murray, 2011 ). The interfacial shear rheology in time sweep mode is especially useful to characterise this time-dependent formation of viscoelastic properties via interactions between protein molecules at the interface. Based on the setup of the rheological equipment, the area of an interfacial layer is only changed in shape while its area size remains constant allowing the detection of interactions between protein molecules. For pH 2 samples, the interfacial storage modulus G’i of the ilm demonstrates an earlier increase (sharp increase within the irst 100 min) and higher overall values of the elastic properties (28.5 ± 2.5 mPa · m after 12 h) compared to those of the ilms formed by pH 7 samples (1.5 ± 5 mPa m m after 12 h), Figure A.1. For pH 7 sam-ples, the interfacial storage modulus G’I runs on a low level close to 0 and starts to increase after 10 h reaching the level of the loss mod-ulus. The transition from G’’ > G’ to G’ > G’’ indicates the sol-gel transi-tion in the ilm ( Leick et al., 2009 ). A high value of the interfacial stor-age modulus points at a dominating contribution of the elastic prop-erties, which in turn relects the formation of strong interactions be-tween the protein molecules such as electrostatic and hydrophobic in-teractions, disulphide bonds and/or van der Waals forces ( Bos and van Vliet, 2001; Murray, 1998 ). The attractive forces between the protein molecules result in the formation of a two dimensional interfacial gel layer ( Leick et al., 2009 ). It is consequently assumed that the inter-actions at the oil-water-interface between adsorbed protein molecules of proteins extracted at pH 2 appear to form earlier and in a higher quantity and strength compared to those found for proteins extracted at pH 7. The albumin-rich fraction extracted at pH 2 exhibits a high surface hydrophobicity one order of magnitude higher than the value of the globulin-rich fraction extracted at pH 7, Table A.1. In conse-quence, a high value of the inal interfacial storage modulus after 12 h was observed for the protein ilm formed by the albumin-rich fraction which might be the result of stronger interactions based on hydrophobic forces between the protein molecules at the interface. The formation of protein-protein interactions in interfacial ilms based on hydrophobic in-teractions was also reported for globular proteins in general ( Freer et al.,2004 ) as well as for soy protein isolate and two hydrolysates in particu-lar ( Li et al., 2016 ). Proteins extracted at pH 7 possess a low surface hy-drophobicity, consequently a higher surface charge and thus repel each other at the interface leading to weak protein-protein interactions in the ilm. Similar observations have been reported for faba bean and chick pea protein interfacial ilms at an oil-water-interface. Higher values of the interfacial loss modulus have been observed at pH 2.5 compared to pH 7.5 ( Félix et al., 2019 ). 3.3.2. Interfacial ilm stability against shear deformation and frequency After the interfacial ilm had developed over a period of 12 h, it was stressed by frequency (0.001 < f < 1 Hz) and amplitude (0.001 ≤ γ ≤ 100%) sweeps. Trends observed in the time sweeps are further highlighted by the interfacial storage (G’i ) and loss (G’’i ) moduli mea-sured in frequency and amplitude sweep mode. The strong inverted correlation of pH and strength of the molecular interactions in the in-terfacial layer can be seen in both plots: pH 2 samples have the high-est values of both interfacial moduli, pH 7 has signiicantly lower val-ues while still displaying viscoelastic properties (G’’i < G’i ), Fig. 3 A and Figure A.2. In amplitude sweeps, values of the interfacial storage modulus of the protein ilm at pH 7 are lower and show therefore fewer elas-tic properties over the investigated amplitude range in comparison to pH 2, Fig. 3 A. The linear viscoelastic range (LVE), i.e. the amplitude range where the interfacial ilm reacts to stress with reversible structural changes (molecular movement at the interface, ( Erni and Parker, 2012 )), was found to be comparable for pH 2 and 7 samples at 1.4% shear de-formation. Since both samples display a similar LVE range, it can be assumed that the intermolecular interactions present in both protein ilms are able to resist a similar amplitude range. Plateau values of the elastic moduli within the LVE range were 30.8 ± 1.4 mPa m m for pH 2 and 8.1 ± 4.1 mPa m m for pH 7. Values of the interfacial storage modulus of pH 2 samples are in accordance with results reported for whey protein isolate ilms (0.035…0.041 Pa m, Yang et al., 2020 ), while the values of the elastic modulus of pH 7 samples remain on a lower level. Frequency sweeps of RPS protein ilms are depicted in Fig. A.2. For the albumin-rich fraction, the interfacial storage modulus slightly in-creases within the frequency range and always exceeds the interfacial loss modulus, which in turn remains constant until an increase at fre-quencies of approximately 0.1 Hz. The storage modulus is signiicantly larger than the loss modulus which is a typical characteristic for gel structures in bulk phases ( Almdal et al., 1993 ) and indicates a predom-inately elastic interfacial behaviour ( Yang et al., 2020 ). Moreover, the continuous slight increase of G’i while G ”i only increases at high fre-quencies after a plateau, also implies that the interfacial network struc-tures possess a wide range of relaxation times ( Yang et al., 2020 ). For the globulin-rich fraction, the interfacial storage modulus slightly increases within the frequency range and exceeds the interfacial loss modulus un-til a frequency of 0.25 Hz. At high frequencies, an abrupt decrease of G’i is detected. Here, the protein network is destructed under the impact of the frequency, thus the elastic properties of the interface are reduced as it becomes more liquid. The liquid character of the ilm is increas-ing with frequency, i.e. the molecular interactions between the proteins are weakened and the mobility of the interfacial structure is increased ( Yang et al., 2020 ). 3.3.3. Non-linear rheological behaviour of the ilms Nonlinear viscoelastic properties of interfacial layers containing RPS protein were examined by large amplitude oscillatory shear (LAOS) ex-periments. At large shear (LAOS), i.e. outside the LVE range, the stress response includes higher harmonics of the oscillation ( Ewoldt et al.,2008 ). The results are displayed in Lissajous plots, where shear stress is plotted against intracycle strain or shear deformation, revealing non-linear stress responses ( Sagis and Fischer, 2014 ). An elliptical shape rep-resents linear viscoelastic behaviour, while distortion of the curve im-plies a nonlinear stress response ( Sridharan et al., 2021 ). The non-linear stress responses of the interfacial layer are displayed in Lissajous plots, Fig. 3 B, C. Overall, protein solutions of pH 2 and 7 show same trends: at low deformation (0.127%), the viscous Lissajous plots ( Fig. 3 B) depict almost circular, wide, symmetrical, elliptic graphs. With increasing de-formation (29.8%) the ellipses become narrower and more tilted, the en-closed area decreases relatively. At high deformation (100%), the curves transform into double sigmoidal shapes with pointed ends for pH 2 sam-ples, indicating strain hardening, and rounded ends for pH 7 samples, indicating strain softening ( Van Den Berg et al., 2018 ). At low shear, pH 2 and 7 samples show linear elastic responses (narrow, symmetri-cal ellipses). With increasing shear deformation, the ellipsoids become broader and more rhomboid shaped. To sum up, the Lissajous plots of the interfaces at pH 2 and 7 show a LVE behaviour at low strain with a mainly elastic response characterised by almost circular viscous and almost linear elastic plots. With increasing deformation, the interface shows increasingly viscous behaviour, marked by a narrowing viscous and widening elastic plot ( Sagis and Fischer, 2014; Van Den Berg et al.,2018 ). 3.3.4. Emulsion rheology Emulsions prepared of the RPS protein extracts difered in the net charge, i.e. the ζ -potential, Table A.1. Oil droplets in emulsions at pH 7 showed average negative surface charges close to ± 30 mV ( − 26.7 ± 3.4 mV, Table A1). Emulsions at pH 2 had very low absolute surface charges ( − 5.1 ± 1.0 mV, Table A1). Therefore, diferent types of interactions between the emulsion droplets can be assumed which are repulsive forces between droplets in pH 7 and hydrophobic interactions between droplets in pH 2 emulsions. The impact of these interactions on rheological properties was analysed by frequency (0.01 ≤ f ≤ 10 Hz) and amplitude (0.01 ≤ γ y 1000%) sweeps. The emulsions were stored over a period of 12 h until analysis to resemble the ilm development which was already observed in time sweep measurements of the inter-facial protein ilm, Fig. A.1. Frequency sweeps can be used to estimate the behaviour of emul-sions or dispersions at rest, the long-term behaviour and the tendency of separation like sedimentation or syneresis (I b anescu et al., 2010 ). In frequency sweeps, Fig. 4, pH 2 emulsions possess the highest values of the storage and loss moduli at all frequencies. At both pH values, only a slight increase in the storage modulus with increasing frequency could be detected. In consequence, slopes of the storage modulus G’(dlogG’/dlogf) are small (0.05 ± 0.02 for pH 2, -0.01 ± 0.05 for pH 7). Nevertheless, at pH 7, G’ decreases abruptly at a frequency above 4 Hz. With increasing frequency, G ” increases exponentially at both pH lev-els, indicating a frequency-induced structural damage that increases the emulsions’ liquid-like properties. Nevertheless, the elastic proportion of the complex moduli outweighs the viscous proportion in all samples in the investigated frequency range, thus all emulsions show viscoelastic, gel-like behaviour (G’ > G’’). In amplitude sweeps, at low deformation the values for the storage modulus (G’) and the loss modulus (G ”) remain at a constant plateau level (LVE range) in all emulsions with G’ about one order of mag-nitude larger than the corresponding G ”, Fig. 5. Emulsions of protein extracts at pH 2 have the highest moduli level (G’: 80.7 ± 19 Pa, G’’:9.1 ± 0.4 Pa) while pH 7 samples have lower values (G’: 16.4 ± 8.5 Pa, G ”: 2.1 ± 0.7 Pa). In both interfacial and emulsion amplitude sweeps, extracts of pH 2 achieve the highest plateau values. For both emulsions, the LVE ends at a deformation of 1%. The crossover point (G ”> G’) after the linear regime suggests yielding of the emulsion structure. The difer-ences in the strain at the crossover do not signiicantly difer between both samples (pH 2: 33.3 ± 17.8%, pH 7: 23.0 ± 3.9%). In the amplitude sweeps of the emulsions the loss modulus re-veals a peak, or overshoot, at the yield strain (crossover of G’ and G’’)( Fig. 5 A, B). The small increase at the beginning of the irreversible sys-tem destruction indicates the presence of strong droplet–droplet inter-actions and an interconnected network (gel) ( Sridharan et al., 2021 ), underlining the elastic or jammed nature of the emulsion. In detail, both emulsions can be classiied as type III LAOS behaviour: weak strain overshoot (G’ decreasing, G’’ increasing followed by decreasing)( Hyun et al., 2002 ). For ß-lactoglobulin interfacial ilms, the occurrence of this overshoot, a strain hardening behaviour, was associated with the crosslinking of the molecules via cysteine peptides that occurred with increasing ilm ageing ( Sagis and Fischer, 2014 ). When the deformation is increased, the structure resists against deformation up to a certain strain, G’’ increases. Upon further increase in deformation over the crit-ical strain, the complex structure is destroyed, after which the particles align with the low ield, G’’ decreases. The presence of this peak results from loss of contact between neighbouring particles or droplets and at A ◆G'pH22号-G"pH2 O G'pH7 -—-G" pH 7 C Fig. 3. ( A ) Amplitude sweeps (γ = 0.01–100%) of 0.1% protein extracted from redcurrant pomace seeds (RPS) at pH 2 (black) or pH 7 (grey) with interfacial storage modulus G’i (circles) and loss modulus G’’i (squares) (n = 3) analysed by interfacial shear rheology. The frequency was kept constant at ƒ = 0.3 Hz. ( B ) Viscous and ( C ) elastic Lissajous plots, calculated by shear stress against either strain or strain rate at the amplitudes 0.127%, 29.8% and 100%. A Fig. 4. Frequency sweep of emulsions (1% RPS protein,10% oil, 300 bar, 2 runs), ( A ) pH 2, ( B ) pH 7 (n = 3, ± standard deviation). Fig. 5. ( A ) Amplitude sweep (pH 2), ( B ) amplitude sweep (pH 7), ( C ) shear thickening factor (T) and ( D ) strain stifening factor (S) and of emulsions (1% RPS protein, 10% oil, 300 bar, 2 runs), (n = 3, ± standard deviation). B Fig. 6. ( A ) Viscous and ( B ) elastic Lissajous plots of pH 2 (black) or pH 7 (grey) emulsions obtained at a deformation of 1.58, 25.1, 99.7 and 630% ( n = 3). the same time rearrangements and direct low ( Knowlton et al., 2014; Mason et al., 1995 ). Based on the ζ -potential measurement results (Ta-ble A.1), we assume repulsive forces between droplets in pH 7 emulsions and weak attractive forces between droplets in pH 2 emulsions. Conse-quently, the absolute values of G’ were higher for pH 2 samples than for pH 7 samples due to increased network formation as indicated by Fuhrmann et al. (2022). 3.3.4. Non-linear rheological behaviour of the emulsions The non-linear rheological behaviour detected for interfacial ilms corresponds well to the behaviour of emulsions. The emulsion systems show congruent rheological behaviour at all pH levels ( Fig. 6 A, B):round viscous plots narrowing with shear to become curved double sig-moidal, and almost linear elastic plots widening into a roundish rhom-boid shape, indicating a strain-softening behaviour with higher har-monic responses at high shear ( Fuhrmann et al., 2022; Van Den Berg et al., 2018 ). The dimensionless T and S factor are similar at both pH-levels. The T-factor, deined as shear thickening factor ( Fig. 5 C), displays a local maximum at pH 2 sample (0.25 ± 0.09 at 0.997 1/s), which indi-cates intra-cycle shear thickening behaviour, followed by shear thinning ( Fuhrmann et al., 2022 ). Due to strong variations in the pH 7 samples, the local maximum is not clearly detectable. Within the LVE range, S is close to 0 (below 1% deformation, Fig. 5 D) indicating linear viscoelas-ticity ( Ewoldt et al., 2010 ). With increasing deformation, S increases in both samples and exceeds 1 at very high shear deformations. High values of the S-factor imply intracycle strain stifening which is in con-tradiction to the results derived from the rhomboid-shaped elastic Lis-sajous plots as well as results obtained for mayonnaise-type emulsions ( Duvarci et al., 2017 ). At high deformation and as a consequence of the rhomboid-shaped Lissajous plots, G’ M (refer to Eq. 1 ) tend to approach zero and S exceeds 1. Mermet-Guyennet et al. (2015) already discussed the strain softening/strain hardening paradox. As a consequence, it is more likely that both samples exhibit strain softening behaviour as in-dicated by Fuhrmann et al. (2022). 3.3.5. Colour, structure and stability of emulsions containing redcurrant seed extracts Table 1 displays the colour values of RPS protein extracts as well as of the emulsions in the CIELAB colour space. The emulsions’ colours are dominated by high L-values, indicating a typical cream white colour. With higher a-values, emulsion of pH 2 still have a light red hue which was already indicated by the intensive reddish-brown colour of the pro-tein extracts at pH 2 demonstrated by a lower brightness level and in-creased a-value. Extracts obtained at pH 7 are of beige colour. The dif-ferences in colour of the protein extracts also leads to perceivable dif-ferences within the samples ( A E pH2/7 protein extracts : 26.7), while colour diferences between the emulsions are levelled ( A E pH2/7 emulsions : 13.0). From rheological measurement results we conclude that proteins present in both emulsions are able to build up an interfacial ilm dif-fering in its mechanical strength. Consequently, in emulsions contain- Colour properties of protein extracts and emulsions (n = 3, ± standard deviation). Diferent letters within columns represent signiicant diferences (α = 0.05) derived from ANOVA. L* a* b* Colour (RGB) pH 2protein extracts 33.3±6.2 12.0±2.8 13.7°±4.6 pH 7 protein extracts 58.5°±2.1 3.1±1.5 14.3±3.8 pH 2emulsion 66.6^±5.6 11.3^±3.6 18.1^±2.6 pH 7emulsion 73.8^±2.6 2.6°±0.4 11.8±0.4 ing RPS protein extracts as emulsiier no separation of an oil phase was observed within seven days of storage, Fig. 7. Nevertheless, emul-sions were not fully stable over the course of one week with stronger phase separation efects being present in pH 7 samples than in pH 2emulsions ( Fig. 7 ). In the course of the ageing of the pH 7 emulsions, networks tightened as large locculates ( > 5 mm) separated from clear serum-illed gaps. After 7 days of storage, a clear serum phase (approx.10–15% of the total volume) and a sediment from insoluble solids was formed at the bottom of the test tube ( Fig. 7 ). As already indicated by the results of the frequency sweeps, the value of the elastic modulus G’ pH 7 emulsions (12 ± 7 Pa at 0.01 Hz) was low and even closer to the critical stability limit of G’ > 10 Pa reported by Ib anescu et al. (2010). Furthermore, at pH 7 phase separation could be enforced by complex-ation reactions between remaining polyphenolic compounds present in the extracts with the proteins adsorbed at the oil droplet interface. Co-valent interactions of polyphenolic compounds and protein are induced at pH values > 7 while acidic pH values slow the polyphenol oxidation ( Keppler et al., 2020 ) and consequently under acidic conditions interac-tions occur to a lesser extent and only by non-covalent interactions. On the other hand, the value of the elastic modulus of the pH 2 emulsion (60 ± 18 Pa at 0.01 Hz) taken from frequency sweeps ( Fig. 4 A) implied good mechanical stability. We assume that proteins present in extracts at pH 2 are able to form an interfacial ilm via hydrophobic interactions which is stable against coalescence. However, due to the low surface charge (low ζ -potential, Table A1) locculation occurred and intensi-ied by the density diferences a concentration of the emulsion droplets at the top of the test tube was observed within one week of storage, Fig. 7 . ■ sed im ent口 c lear s erum 口 co nt in uo us em ul si on 1E 圆 f l o cc ulate d em u lsio n 日 c ream Fig. 7. Phase separation of emulsions (1% RPS protein, 10% oil, 300 bar, 2runs) one and seven days after preparation. 4. Conclusion In this paper we have shown that redcurrant pomace seeds (RPS), containing a high crude protein content, are a potential source for func-tional protein. Through variation of the pH-value during extraction, two protein extracts difering in their protein composition were obtained. Under acidic conditions, predominantly albumins with high molecular mobility and surface hydrophobicity were extracted, while a broad spec-trum of proteins (mainly storage globulins) with higher surface charge were extracted at neutral pH-value. As hypothesized, albumins being smaller in size and having a higher surface hydrophobicity formed sta-ble interfacial ilms at an oil–water-interface due to increased protein–protein-interactions. The interfacial ilm formed by the globulin-rich ex-tract was not as stable as the ilm formed by the albumin-rich fraction. In consequence, the protein network was impacted by frequency. The liquid character of the ilm increased, i.e. the molecular interactions between the proteins were weakened and the mobility of the interfa-cial structure increased. However, the observed values of the interfacial storage modulus of the albumin-rich fraction were even comparable to results reported for whey protein isolate ilms pointing at the potential of berry protein as an emulsiier in food systems. For emulsions containing both fractions, the presence of strong droplet–droplet interactions and an interconnected network (gel) was found. Those gel-like properties of the interconnected emulsion of-fer interesting alternative application prospects, e.g. in cream desserts, mayonnaise-type sauces or even vegan sausage alternatives. The results suggest that extraction of RPS protein serves as a practicable starting point to obtain functional proteins suitable for the stabilisation of high-viscous emulsions. Ethical statement The authors declare, that the presented research did not involve hu-mans or living animals. Declaration of Competing Interest The authors declare that they have no known competing inancial interests or personal relationships that could have appeared to inluence the work reported in this paper. CRediT authorship contribution statement L.V. Sankowski: Conceptualization, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Vi-sualization. L. Hennig: Formal analysis, Investigation, Data curation. S. Drusch: Resources, Writing –review & editing, Supervision. M. Brückner-Gühmann: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition. Data availability Data will be made available on request. Funding This IGF Project ( AiF 20917 BG ) of the FEI is supported via AiF within the programme for promoting the Industrial Collective Research (IGF) of the Federal Ministry of Economic Afairs and Climate Action (BMWK), based on a resolution of the German Parliament. Acknowledgment The authors gratefully acknowledge the expertise of S. Struck, A.-M. Reißner and H. Rohm from Chair of Food Engineering, Technische Uni-versität Dresden, Germany as project partners of this IGF Project for the organisation, provision and preparation of the raw material redcurrant pomace. Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fufo.2023.100232. References Almdal, K., Dyre, J., Hvidt, S., Kramer, O., 1993. Towards a phenomenological deinition of the term “gel. Polym. Gels Netw. 1 (1), 5–17. doi:10.1016/0966-7822(93)90020-I. Bos, M.A., van Vliet, T., 2001. Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv. Colloid Interface Sci. 91 (3), 437–471. doi:10.1016/S0001-8686(00)00077-4. Brückner-Gühmann, M., Heiden-Hecht, T., Sözer, N., Drusch, S., 2018. Foaming charac-teristics of oat protein and modiication by partial hydrolysis. Eur. Food Res. Technol.244 (12), 2095–2106. doi:10.1007/s00217-018-3118-0. Chéreau, D., Videcoq, P., Ruieux, C., Pichon, L., Motte, J.C., Belaid, S., Ventureira, J., Lopez, M., 2016. Combination of existing and alternative technologies to promote oilseeds and pulses proteins in food applications. OCL - Oilseeds Fats, Crops Lipids 41(1). doi:10.1051/ocl/2016020. Contreras, M.del M., Lama-Muñoz, A., Manuel Gutiérrez-Pérez, J., Espínola, F., Moya, M., Castro, E., 2019. Protein extraction from agri-food residues for integration in biore-inery: potential techniques and current status. Bioresource Technol. 280, 459–477. doi:10.1016/j.biortech.2019.02.040, December 2018. Dickinson, E., 1998. Proteins at interfaces and in emulsions Stability, rheology and inter- actions. J. Chem. Soc., Faraday Trans. 94 (12), 1657–1669. doi:10.1039/a801167b. Dienaite, L., Pukalskiene, M., Pereira, C.V., Matias, A.A., Venskutonis, P.R., 2020. Valorization of european cranberry bush (viburnum opulus l.) berry pomace ex-tracts isolated with pressurized ethanol and water by assessing their phyto-chemical composition, antioxidant, and antiproliferative activities. Foods 9 (10). doi:10.3390/foods9101413. Duvarci, O. C., Yazar, G., Kokini, J. L., 2017. The comparison of LAOS behavior of struc-tured food materials (suspensions, emulsions and elastic networks). Trends Food Sci. Technol. 60, 2–11. doi:10.1016/j.tifs.2016.08.014. Erni, P., Parker, A., 2012. Nonlinear viscoelasticity and shear localization at complex luid interfaces. Langmuir 28 (20), 7757–7767. doi:10.1021/la301023k. Ewoldt, R.H., Hosoi, A.E., McKinley, G.H., 2008. New measures for characterizing nonlin-ear viscoelasticity in large amplitude oscillatory shear. J. Rheol. 52 (6), 1427–1458. doi:10.1122/1.2970095. Ewoldt, R. H., Winter, P., Maxey, J., McKinley, G. H., 2010. Large amplitude oscillatory shear of pseudoplastic and elastoviscoplastic materials. Rheologica Acta 49 (2), 191–212. doi:10.1007/s00397-009-0403-7. Freer, E.M., Yim, K.S., Fuller, G.G., Radke, C.J., 2004. Interfacial rheology of globular and lexible proteins at the hexadecane/water interface: comparison of shear and dilata-tion deformation. J. Phys. Chem. B 108 (12), 3835–3844. doi:10.1021/jp037236k. Fuhrmann, P.L., Breunig, S., Sala, G., Sagis, L., Stieger, M., Scholten, E., 2022. Rheological behaviour of attractive emulsions difering in droplet-droplet interaction strength. J. Colloid Interface Sci. 607, 389–400. doi:10.1016/j.jcis.2021.08.124. Gazzola, D., Vincenzi, S., Gastaldon, L., Tolin, S., Pasini, G., Curioni, A., 2014. The proteins of the grape (Vitis vinifera L.) seed endosperm: fractionation and identiication of the major components. Food Chem. 155, 132–139. doi:10.1016/j.foodchem.2014.01.032. Ghumman, A., Kaur, A., Singh, N., 2016. Functionality and digestibility of albumins and globulins from lentil and horse gram and their efect on starch rheology. Food Hydro-coll. 61, 843–850. doi:10.1016/j.foodhyd.2016.07.013. González-Pérez, S., Vereijken, J.M., 2007. Sunlower proteins: overview of their physico-chemical, structural and functional properties. J. Sci. Food Agric. 87 (12), 2173–2191. doi:10.1002/jsfa.2971. Görgüç, A., Özer, P., Yılmaz, F. M., 2020. Microwavemassisted enzymatic extraction of plant protein with antioxidant compounds from the food waste sesame bran: Com-parative optimization study and identiication of metabolomics using LC/Q-TOF/MS. J. Food Process. Preserv. 44 (1). doi:10.1111/jfpp.14304. Grimplet, J., Wheatley, M.D., Jouira, H.Ben, Deluc, L.G., Cramer, G.R., Cushman, J.C,2009. Proteomic and selected metabolite analysis of grape berry tissues under well-watered and water-deicit stress conditions. Proteomics 9 (9), 2503–2528. doi:10.1002/pmic.200800158. Hyun, K., Kim, S.H., Ahn, K.H., Lee, S.J., 2002. Large amplitude oscillatory shear as a way to classify the complex luids. J. Non-Newton. Fluid Mech. 107 (1–3), 51–65. doi:10.1016/S0377-0257(02)00141-6. Ibanescu, C., Danu, M., Nanu, A., Lungu, M., Simionescu, B.C., 2010. Stability of disperse systems estimated using rheological oscillatory shear tests. Revue Roumaine de Chim.55 (11–12), 933–940. Jukić, M., Lukinac, J., Čuljak, J., Pavlović, M., Šubarić, D., Koceva Komlenić, D.,2019. Quality evaluation of biscuits produced from composite blends of pumpkin seed oil press cake and wheat lour. Int. J. Food Sci. Technol. 54 (3), 602–609. doi:10.1111/ijfs.13838. Karefyllakis, D., Altunkaya, S., Berton-Carabin, C.C., van der Goot, A.J., Nikiforidis, C.V.,2017. Physical bonding between sunlower proteins and phenols: Impact on interfacial properties. Food Hydrocoll. 73, 326–334. doi:10.1016/j.foodhyd.2017.07.018. Karefyllakis, D., Octaviana, H., van der Goot, A.J., Nikiforidis, C.V., 2019. The emulsifying performance of mildly derived mixtures from sunlower seeds. Food Hydrocoll. 88,75–85. doi:10.1016/j.foodhyd.2018.09.037, June 2018. Kato, A., Nakai, S., 1980. Hydrophobicity determined by a luorescence probe method and its correlation with surface properties of proteins. Biochim. Biophys. Acta 624(1), 13–20. doi:10.1016/0005-2795(80)90220-2. Keppler, J.K., Schwarz, K., van der Goot, A.J., 2020. Covalent modiication of food proteins by plant-based ingredients (polyphenols and organosulphur compounds): a common-place reaction with novel utilization potential. Trends Food Sci. Technol. 101, 38–49. doi:10.1016/j.tifs.2020.04.023, March . Knowlton, E.D., Pine, D.J., Cipelletti, L., 2014. A microscopic view of the yield-ing transition in concentrated emulsions. Soft Matter 10 (36), 6931–6940. doi:10.1039/c4sm00531g. Kroll, J., Rawel, H.M., Rohn, S., 2003. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci. Technol. Res. 9 (3),205–218. doi:10.3136/fstr.9.205. Laemmli, U. K., 1970. 227680a0. Nature 227, 680–685. Laguna, O., Barakat, A., Alhamada, H., Durand, E., Baréa, B., Fine, F., Villeneuve, P., Citeau, M., Dauguet, S., Lecomte, J., 2018. Production of proteins and phe-nolic compounds enriched fractions from rapeseed and sunlower meals by dry fractionation processes. Ind. Crops Prod. 118, 160–172. doi:10.1016/j.indcrop.2018.03.045. Leick, S., Degen, P., Köhler, B., Rehage, H., 2009. Film formation and surface gelation of gelatin molecules at the water/air interface. Phys. Chem. Chem. Phys. 11 (14), 2468. doi:10.1039/b819708c. Leone, P., Menu-Bouaouiche, L., Peumans, W.J., Payan, F., Barre, A., Roussel, A., Van Damme, E.J.M., Rougé, P., 2006. Resolution of the structure of the allergenic and antifungal banana fruit thaumatin-like protein at 1.7-Å. Biochimie 88 (1), 45–52. doi:10.1016/j.biochi.2005.07.001. Li, W., Wang, Y., Zhao, H., He, Z., Zeng, M., Qin, F., Chen, J., 2016. Im-provement of emulsifying properties of soy protein through selective hydroly-sis: interfacial shear rheology of adsorption layer. Food Hydrocoll. 60, 453–460. doi:10.1016/j.foodhyd.2016.04.019. Li, X., Dai, T., Hu, P., Zhang, C., Chen, J., Liu, C., Li, T., 2020. Characterization the non-covalent interactions between beta lactoglobulin and selected phenolic acids. Food Hydrocoll. 105, 105761. doi:10.1016/j.foodhyd.2020.105761. Liang, S., Were, L.M., 2018a. Chlorogenic acid oxidation-induced greening of sunlower butter cookies as a function of diferent sweeteners and storage conditions. Food Chem. 241, 135–142. doi:10.1016/j.foodchem.2017.08.084, August 2017. Liang, S., Were, L.M., 2018b. Chlorogenic acid induced colored reactions and their efect on carbonyls, phenolic content, and antioxidant capacity in sunlower butter cookies. LWT 87, 16–22. doi:10.1016/j.lwt.2017.08.069. Lopez, F.B., Barclay, G.F., 2017. Plant Anatomy and Physiology. In: Pharma-cognosy: Fundamentals, Applications and Strategy. Elsevier Inc, pp. 45–60. doi:10.1016/B978-0-12-802104-0.00004-4. Mason, T.G., Bibette, J., Weitz, D.A., 1995. Elasticity of compressed emulsions. Phys. Rev. Lett. 75 (10), 2051–2054. doi:10.1103/PhysRevLett.75.2051. Murray, B. S. (1998). Interfacial rheology of mixed food protein and surfactant adsorption layers with respect to emulsion and foam stability (pp. 179-220). https://doi.org/10.1016/S1383-7303(98)80052-5 Mermet-Guyennet, M. R. B., Gianfelice de Castro, J., Habibi, M., Martzel, N., Denn, M. M., Bonn, D., 2015. LAOS: The strain softening/strain hardening paradox. J. Rheol 59(1), 21–32. doi:10.1122/1.4902000. Ntone, E., Bitter, J.H., Nikiforidis, C.V., 2020. Not sequentially but simultaneously: facile extraction of proteins and oleosomes from oilseeds. Food Hydrocoll. 102, 105598. doi:10.1016/j.foodhyd.2019.105598. Ozdal, T., Capanoglu, E., Altay, F., 2013. A review on protein-phenolic interactions and associated changes. Food Res. Int. 51 (2), 954–970. doi:10.1016/j.foodres.2013.02.009. Perera, S.P., McIntosh, T.C., Wanasundara, J.P.D., 2016. Structural properties of cruciferin and napin of Brassica napus (canola) show distinct responses to changes in pH and temperature. Plants 5 (3), 64–74. doi:10.3390/plants5030036. Pham, T.T., Tran, T.T.T., Ton, N.M.N., Le, V.V.M, 2017. Efects of pH and salt concen-tration on functional properties of pumpkin seed protein fractions. J. Food Process. Preserv. 41 (4). doi:10.1111/jfpp.13073. Pieszka, M., Gogol, P., Pietras, M., Pieszka, M., 2015. Valuable components of dried po-maces of chokeberry, black currant, strawberry, apple and carrot as a source of natural antioxidants and nutraceuticals in the animal diet. Ann. Anim. Sci. 15 (2), 475–491. doi:10.2478/aoas-2014-0072. Prigent, S. V. E., Gruppen, H., Visser, A. J. W. G., van Koningsveld, de Jong, Voragen, A. G. J., 2003. Efects of non-covalent interactions with 5- O -cafeoylquinic acid (Chloro-genic Acid) on the heat denaturation and solubility of globular proteins. J. Agric. Food Chem. 51 (17), 5088–5095. doi:10.1021/jf021229w. Reißner, A.-M., Al-Hamimi, S., Quiles, A., Schmidt, C., Struck, S., Hernando, I., Turner, C., Rohm, H., 2019. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 99 (3), 1284–1293. doi:10.1002/jsfa.9302. Rezig, L., Chibani, F., Chouaibi, M., Dalgalarrondo, M., Hessini, K., Guéguen, J., Hamdi, S., 2013. Pumpkin (cucurbita maxima) seed proteins: Sequential extraction processing and fraction characterization. J. Agric. Food Chem. 61 (32), 7715–7721. doi:10.1021/jf402323u. Sabir, M.a, Sosulski, F.W., Finlayson, A.J., 1974. Chlorogenic acid-protein interactions in sunlower. J. Agric. Food Chem. 22 (4), 575–578. Sagis, L.M.C., Fischer, P., 2014. Nonlinear rheology of complex luid-luid interfaces. Curr. Opin. Colloid Interface Sci. 19 (6), 520–529. doi:10.1016/j.cocis.2014.09.003. Sari, Y.W., Mulder, W.J., Sanders, J.P.M., Bruins, M.E., 2015. Towards plant protein re-inery: review on protein extraction using alkali and potential enzymatic assistance. Biotechnol. J. 10 (8), 1138–1157. doi:10.1002/biot.201400569. Sarry, J.E., Sommerer, N., Sauvage, F.X., Bergoin, A., Rossignol, M., Albagnac, G., Romieu, C., 2004. Grape berry biochemistry revisited upon proteomic analysis of the mesocarp. Proteomics 4 (1), 201–215. doi:10.1002/pmic.200300499. Shao, D., Atungulu, G.G., Pan, Z., Yue, T., Zhang, A., Fan, Z., 2014. Characteristics of isolation and functionality of protein from tomato pomace produced with dif-ferent industrial processing methods. Food Bioprocess Technol. 7 (2), 532–541. doi:10.1007/s11947-013-1057-0. Sogi, D.S., Arora, M.S., Garg, S.K., Bawa, A.S., 2002. Fractionation and elec-trophoresis of tomato waste seed proteins. Food Chem. 76 (4), 449–454. doi:10.1016/S0308-8146(01)00304-1. Sójka, M., Król, B., 2009. Composition of industrial seedless black currant pomace. Eur. Food Res. Technol. 228 (4), 597–605. doi:10.1007/s00217-008-0968-x. Sridharan, S., Meinders, M.B.J., Sagis, L.M., Bitter, J.H., Nikiforidis, C.V., 2021. Jammed emulsions with adhesive pea protein particles for elastoplastic edible 3D printed ma-terials. Adv. Funct. Mater. 31 (45), 1–11. doi:10.1002/adfm.202101749. Struck, S., Plaza, M., Turner, C., Rohm, H., 2016. Berry pomace - A review of processing and chemical analysis of its polyphenols. In: International Journal of Food Science and Technology, 51. Blackwell Publishing Ltd, pp. 1305–1318. doi:10.1111/ijfs.13112. Takefuji, Y., 2021. Sustainable protein alternatives. Trends Food Sci. Technol. 107, 429–431. doi:10.1016/j.tifs.2020.11.012, October 2020. Tamburrini, M., Cerasuolo, I., Carratore, V., Stanziola, A.A., Zofra, S., Romano, L., Ca-mardella, L., Ciardiello, M.A., 2005. Kiwellin, a novel protein from kiwi fruit. Pu-riication, biochemical characterization and identiication as an allergen. Prot. J. 24(7–8), 423–429. doi:10.1007/s10930-005-7638-7. Tang, Q., Roos, Y.H., Miao, S., 2023. Plant protein versus dairy proteins: A pH-dependency investigation on their structure and functional properties. Foods 12 (2),368. doi:10.3390/foods12020368. Van Den Berg, M.E.H., Kuster, S., Windhab, E.J., Sagis, L.M.C., Fischer, P, 2018. Nonlinear shear and dilatational rheology of viscoelastic interfacial layers of cellulose nanocrys-tals. Phys. Fluids 30 (7). doi:10.1063/1.5035334. Venskutonis, P. R., 2020. Berries. In: Valorization of Fruit Processing By-products. Elsevier, pp. 95–125. doi:10.1016/B978-0-12-817106-6.00005-8. Voigt, J., Biehl, B., Wazir, S.K.S., 1993. The major seed proteins of Theobroma cacao L. Food Chem. 47 (2), 145–151. doi:10.1016/0308-8146(93)90236-9. Wanasundara, J.P.D., Tan, S., Alashi, A.M., Pudel, F., Blanchard, C., 2016. Proteins from canola/rapeseed: current status. Sustainable Protein Sources. Elsevier Inc doi:10.1016/B978-0-12-802778-3.00018-4. Wildermuth, S.R., Young, E.E., Were, L.M., 2016. Chlorogenic acid oxidation and its reac-tion with sunlower proteins to form green-colored complexes. Compreh. Rev. Food Sci. Food Saf. 15 (5), 829–843. doi:10.1111/1541-4337.12213. Yang, J., Sagis, L.M.C., 2021. Interfacial behavior of plant proteins — novel sources and extraction methods. Curr. Opin. Colloid Interface Sci. 56, 101499. doi:10.1016/j.cocis.2021.101499. Yang, J., Thielen, I., Berton-Carabin, C.C., van der Linden, E., Sagis, L.M.C., 2020. Nonlinear interfacial rheology and atomic force microscopy of air-water interfaces stabilized by whey protein beads and their constituents. Food Hydrocoll. 101. doi:10.1016/j.foodhyd.2019.105466, October 2019. Yusof, A., Murray, B.S., 2011. Modiied starch granules as particle-stabilizers of oil-in-water emulsions. Food Hydrocoll. 25 (1), 42–55. doi:10.1016/j.foodhyd.2010.05.004.
确定









还剩10页未读,是否继续阅读?
中国格哈特为您提供《红醋栗浆果渣及其提取蛋白中蛋白质和脂肪含量的检测》,该方案主要用于其他水果制品中营养成分检测,参考标准《GB 5009.5 食品安全国家标准 食品中蛋白质的测定》,《红醋栗浆果渣及其提取蛋白中蛋白质和脂肪含量的检测》用到的仪器有格哈特杜马斯定氮仪DT N Pro、格哈特传统经典索氏提取/萃取仪EV6 AII16、格哈特快速干燥仪STL56、格哈特全自动快速索氏提取SOXTHERM、滤纸筒、德国移液器MM
相关方案
更多
该厂商其他方案
更多















