方案详情
文
酶辅助湿磨过程中油菜籽衍生蛋白复合物理化性质的变化Changes in the physicochemical properties of rapeseed-derived protein complexes during enzyme-assisted wet milling
方案详情

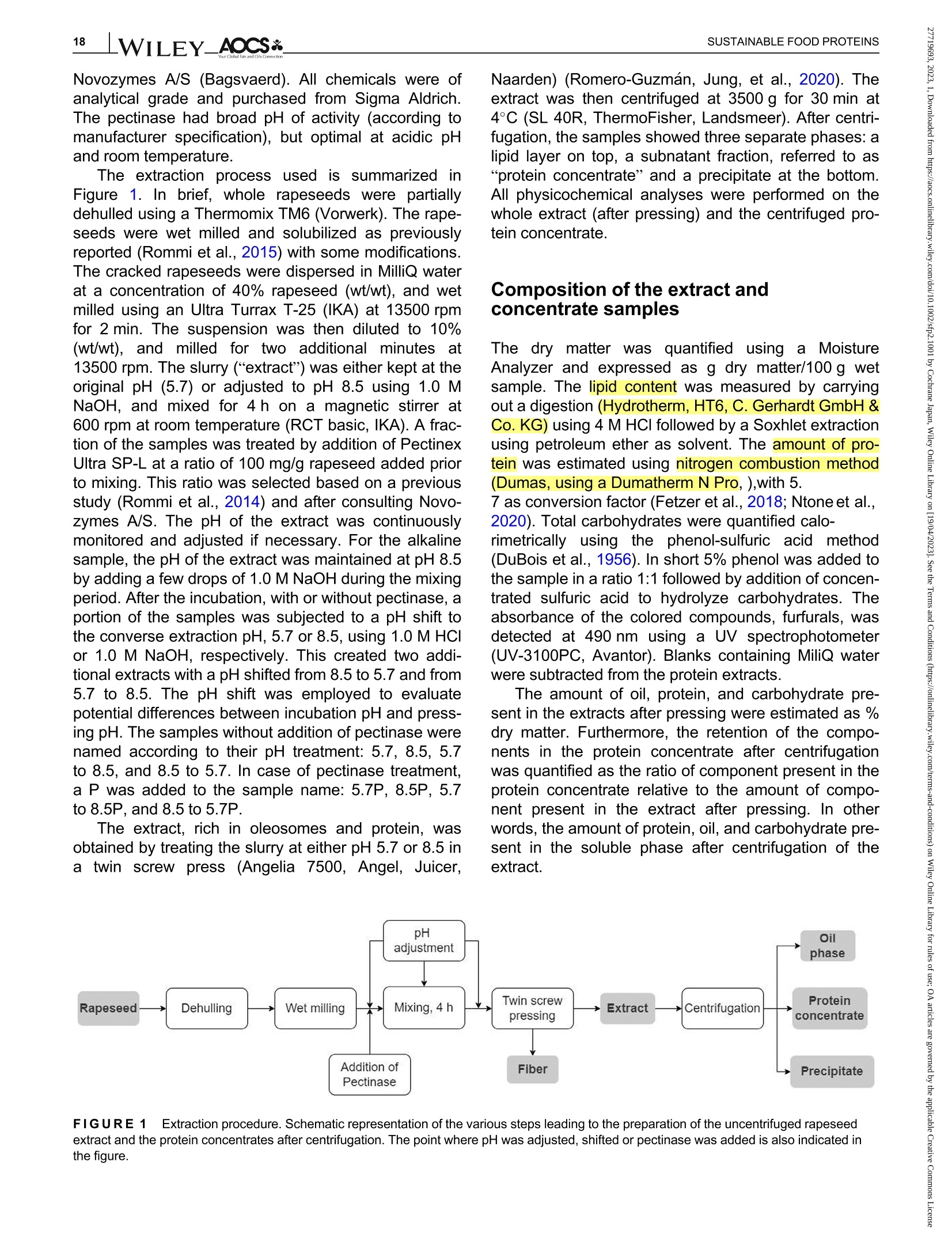
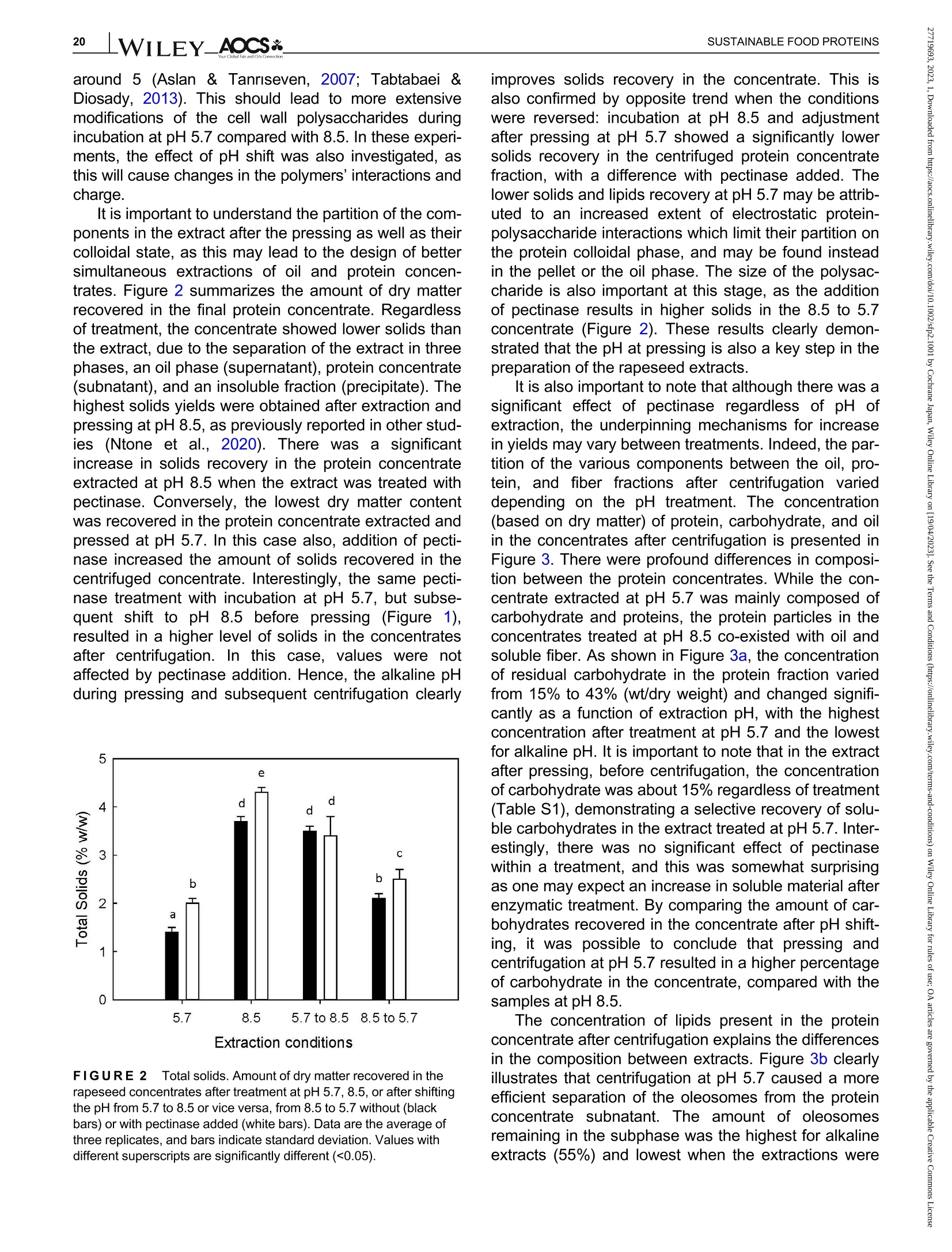
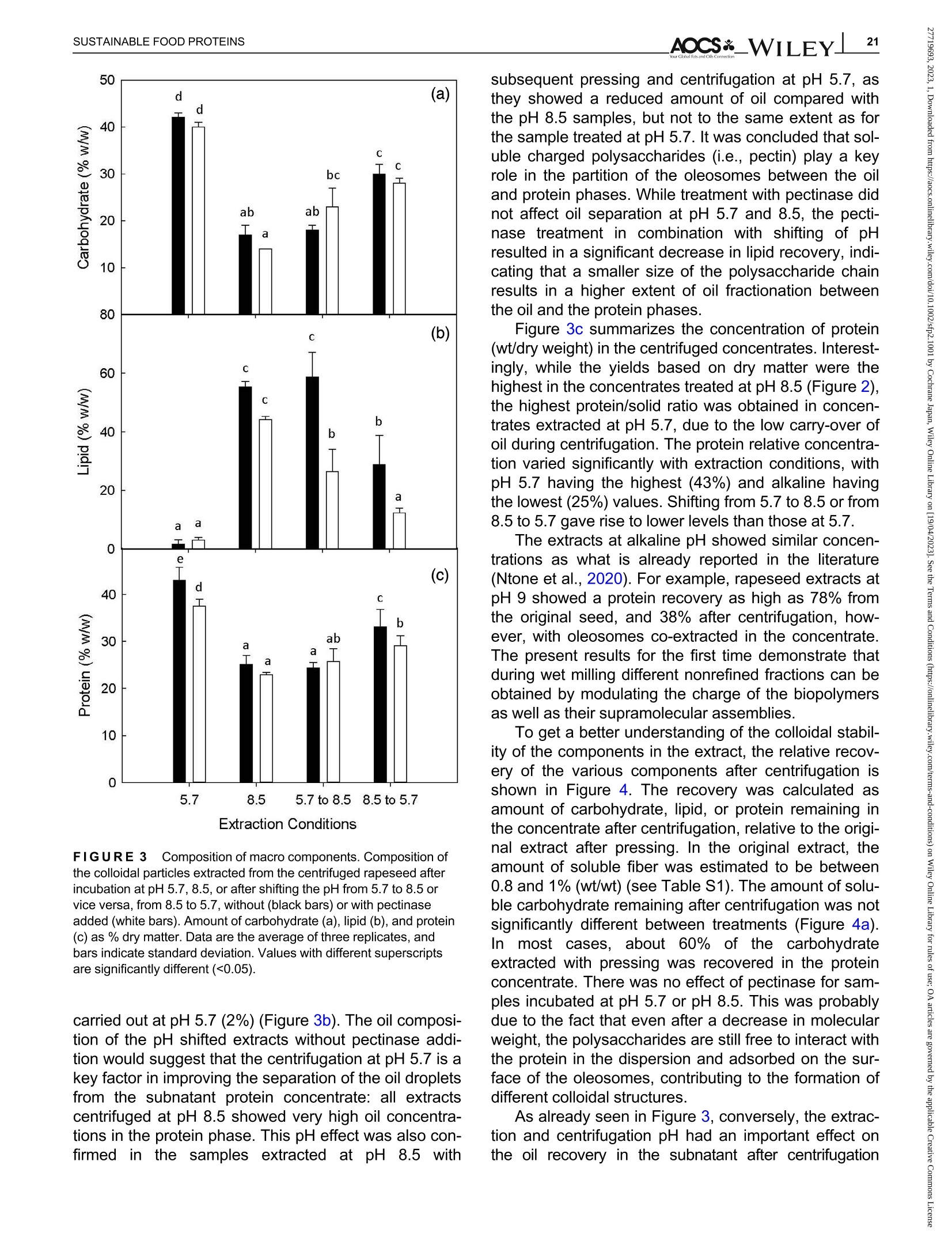
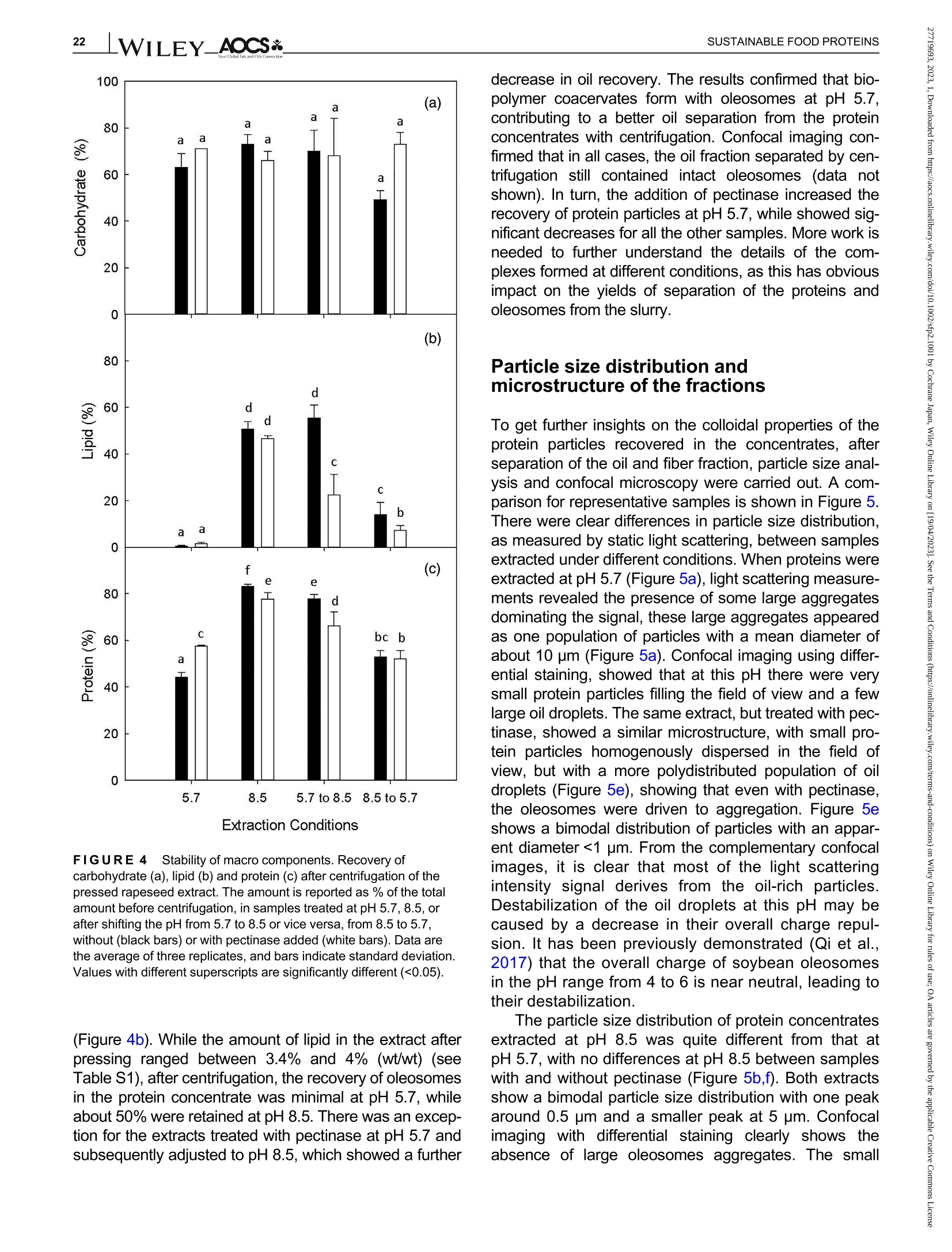
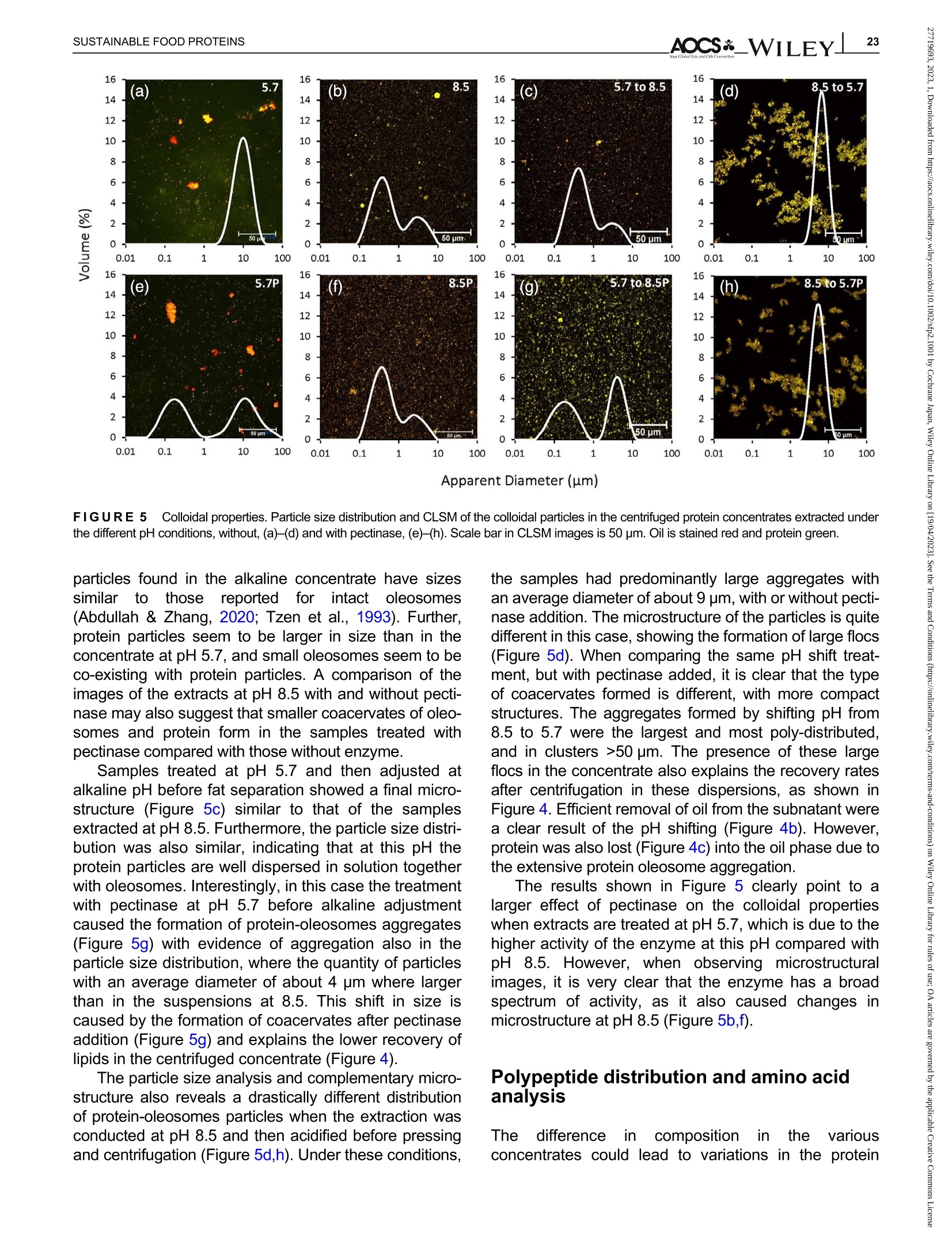
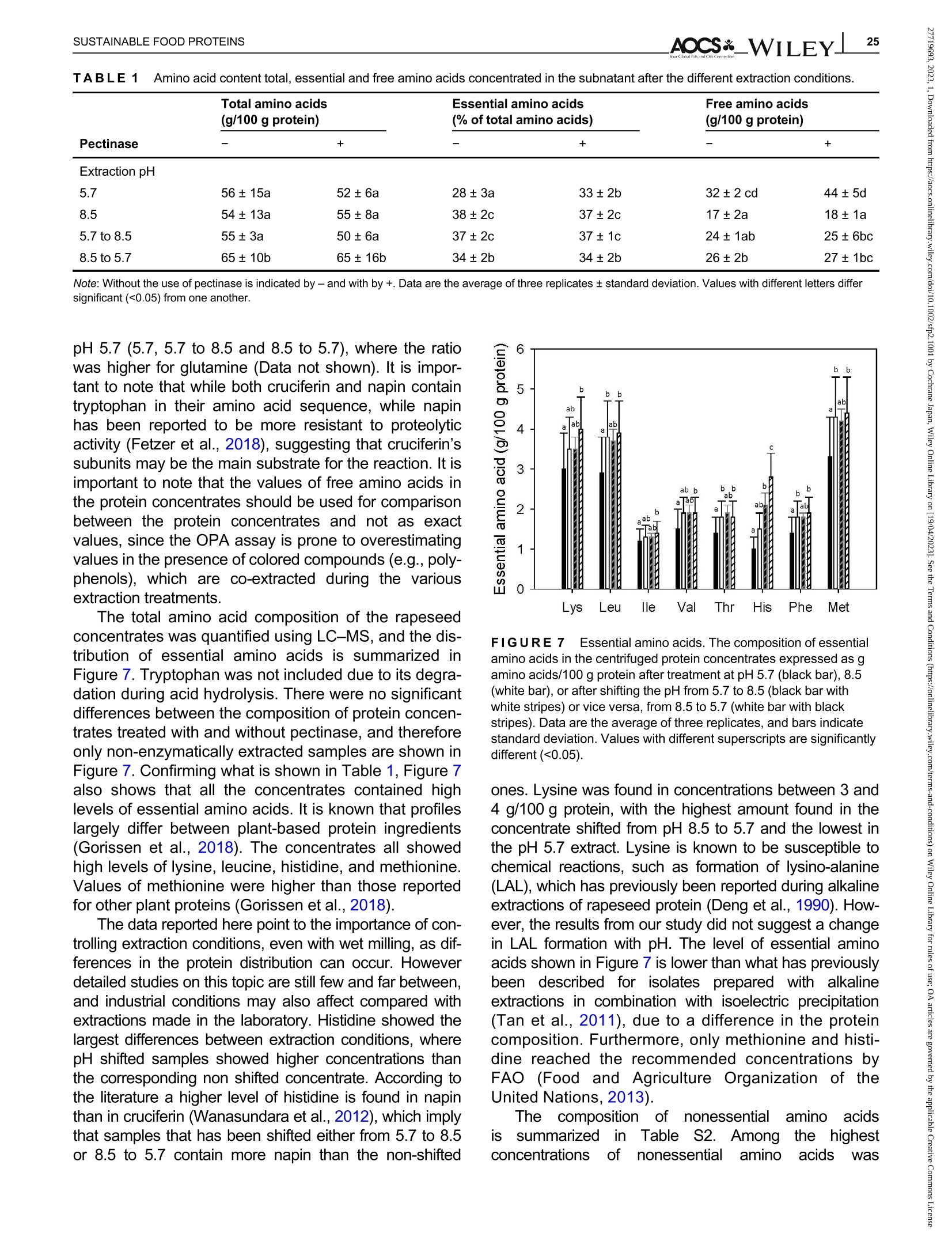
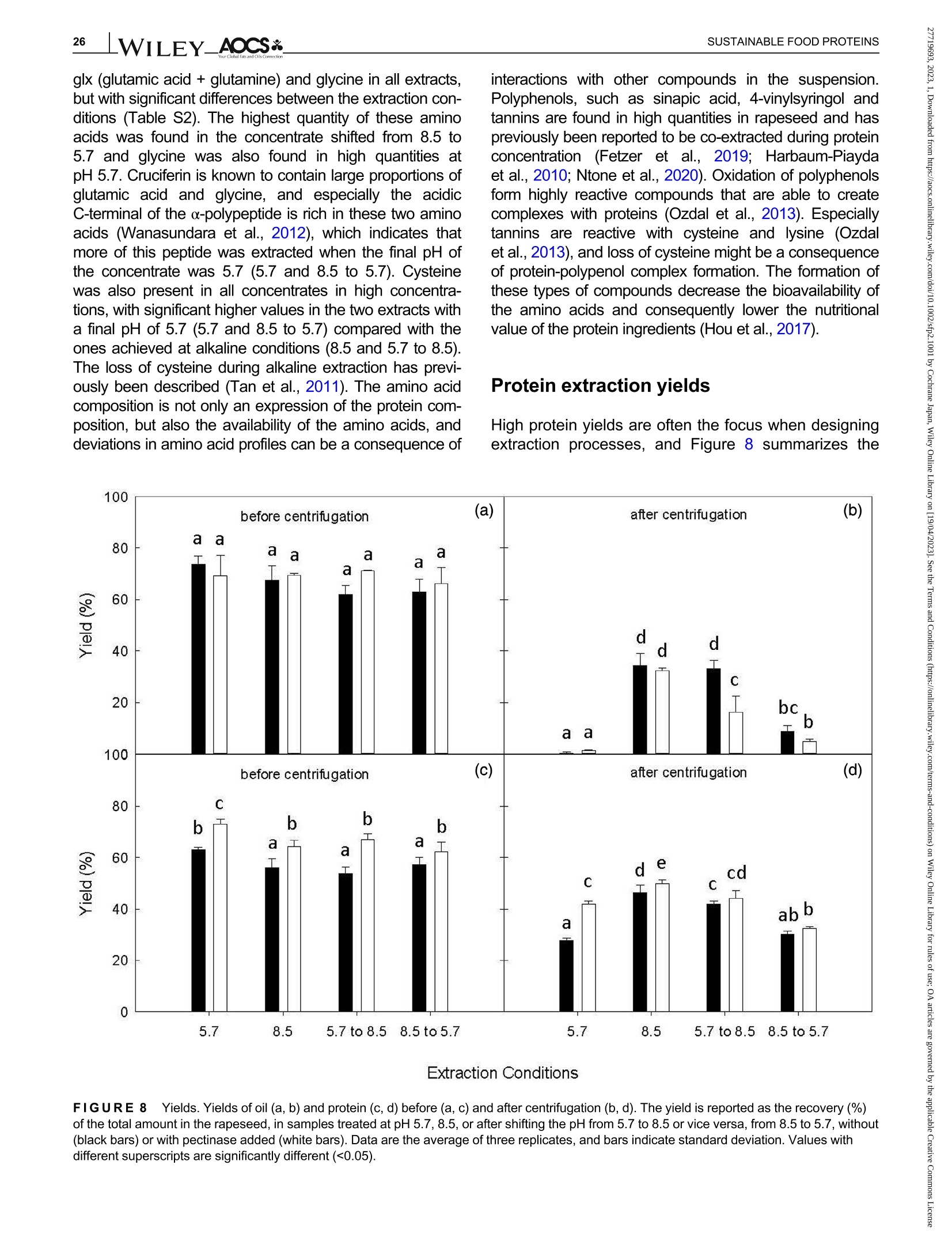
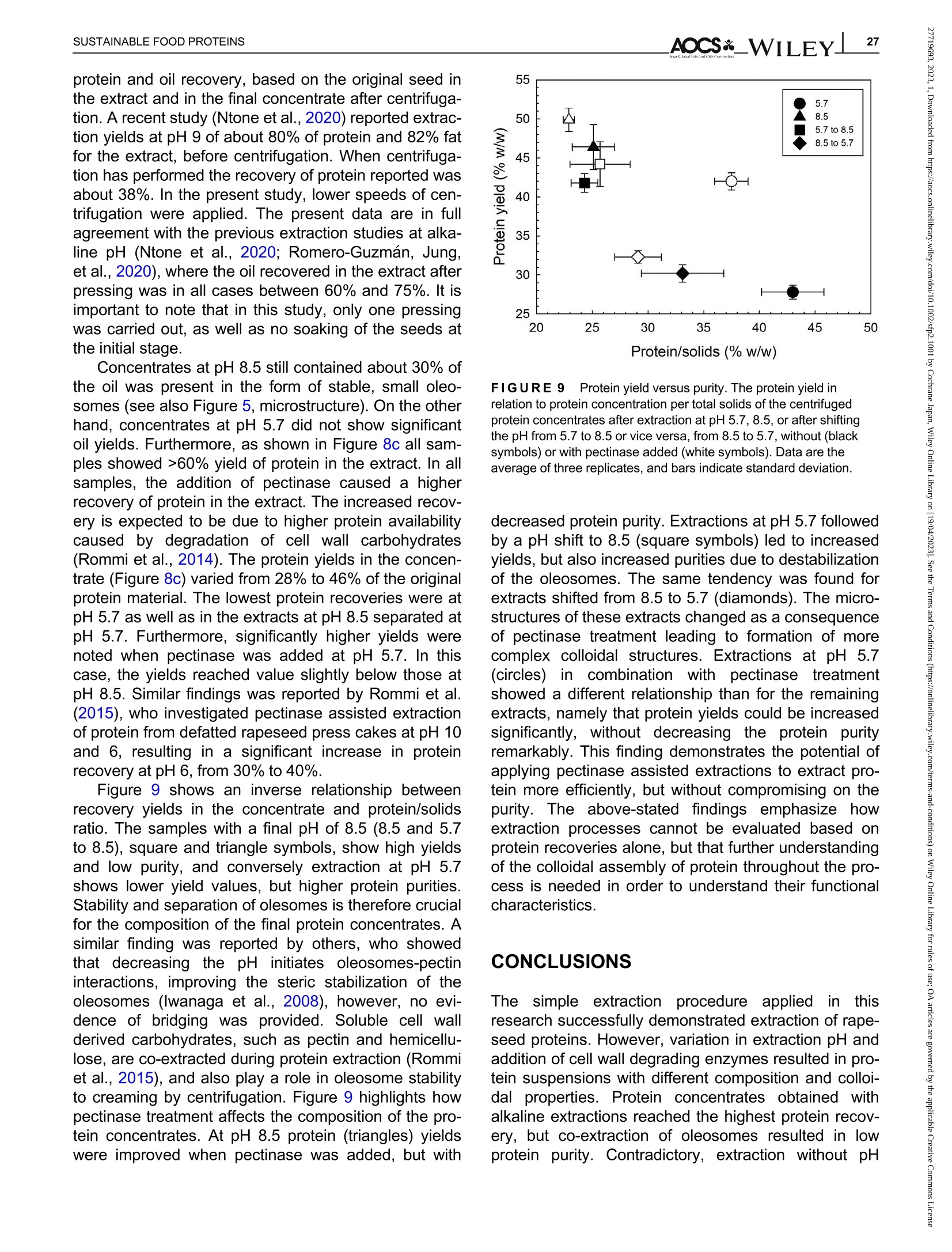
酶辅助湿磨过程中油菜籽衍生蛋白复合物理化性质的变化Changes in the physicochemical properties of rapeseed-derived protein complexes during enzyme-assisted wet millingAccepted: 23 January 2023Revised: 28 December 2022Received: 4 October 2022DOI: 10.1002/sfp2.1001 AOCS&WILEYI17SUSTAINABLE FOOD PROTEINS ORIGINALARTICLE AOCS& WILEY 酶辅助湿磨过程中油菜籽衍生蛋白复合物理化性质的变化 Changes in the physicochemical properties of rapeseed-derived protein complexes during enzyme-assisted wet milling Simone Bleibach Alpiger r D 1Milena Corredig Department of Food Science, ciFood Center,Aarhus University, Skejby, Denmark Correspondence Simone Bleibach Alpiger , Department of Food Science, CiFood Center, Aarhus University,Agro Food Park 48, Skejby, Denmark. Email: sba@food.au.dk Funding information Villum Fonden, Grant/Award Number: 37759;Center for Innovative Foods; CiFood at Aarhus University Abstract Oilseeds need to be exploited not only for the oil but also as a source of f unctional protein-rich ingredients for food. This work evaluated the physicochemical proper-ties of the colloidal fraction resulting from wet milling of rapeseed, used as a model oilseed. The extraction was conducted either at natural pH (5.7) or at alkaline pH (8.5), with or without addition of a commercial pectinase. The slurry was mixed for 4 h at room temperature, and then pressed at the same pH or after shifting to alkaline/acidic pH. The resulting extract, rich in oi l and protein particles,showed differences in particle size, microstructure, and composition. The oi l and protein yields in the extract (in relation to the amount originally in the oilseed) ran-ged between 60% and 70%, with a significant i ncrease in protein recover i es with addition of pectinase, regardless of the pH. A protein concentrate, containing between 25% and 42% protein on dry matter, depending on the treatment was obtained after centrifugation, together with a fiber precipitate and an oi l fraction.The composition of the concentrates varied, with the highest purity found for extracts at pH 5.7, showing a near l y complete physical separation of t he oleo-somes by low-speed centrifugation. All concentrates showed similar protein com-position, as analyzed by gel electrophoresis. The amino acid composition was also similar among the t reatments. This work evidences the cr i tical role played by biopolymers'interactions in forming different colloidal structures, with clear conse-quences on the functionality of the final ingredient. KEYWORDS enzyme assisted extraction, oleosomes, protein concentrates, protein-polysacchar i de interactions,rapeseed protein INTRODUCTION The increasing tendency in the food market towards more environmentally friendly and sustainable food products has led to the need of exploiting not only new plant-derivedprotein sources(Aschemann-Witzel &Peschel, 2019), but also new processing approaches.Soybean, sunflower, and rapeseed are promising food sources, since t hey contain high levels of essential macronutrients, but not without challenges (Dapcevic-Hadnadev et al., 2019). Rapeseed (Brassica napus) is one of the most abundant oil s seeds worldwide (Chmielewska et al., 2020; Ostbring et al., 2019), and,currently mainly utilized for production of vegetable oil and biodiesel. The by-product from the oil industry, the press cake, is rich in protein, but the server processing challenges t he protein quality, and the press cake is therefore mainly used for animal feed (Fetzer et al., 2018). Whole rapeseed contains a significant (<21 wt/wt%) concentration of nutritionally valuable pro-tein (Chmielewska et al., 2020), which makes it a suit-able protein source for human consumption (Mulder et al., 2016). Phytic acids, glucosinolates, and phenolic acids are undesired compounds also found in rapeseed, This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction i n any medium,provided t he original wor k is properly cited. C 2023 The Authors. Sustainable Food Proteins published by American Oil Chemists' Society and Wiley Periodicals LLC. andd their presence challenges Stheuse of whole rapeseed as a food (Malomo & Aluko, 2015). Oilseeds have traditionally been used first and foremost for their oil,and therefore new developments are needed to obtain protein-enriched ingredients from sources less damaged than the traditional defatted and pressed cakes (Fetzer et al., 2018, Fetzer et al., 2019; Ntone et al.,2020). High yields, and high protein or oil purities are usually in focus when designing extraction processes. This comes at a cost to the environment, not only because of the resources used, but also the creation of a number of by-products and side streams. In recent years, more focus has been given to the reduction of the environ-mental footprint. It is becoming clear that a new more sustainable process may result in a shift i n quality and composition, and new ingredients where purity may be less of a priority (Ntone et al., 2020; Romero-Guzman,Jung, et al.,2020). I n other words, there is a need for a better understanding how to reach the right balance between purity, processing history, nutritional quality,and functionality, while minimizing their environmental footprint. The most common industrial practice today for fractionation and purificat i on of plant proteins is a combi-nation of wet extraction (i.e., alkaline, micellar extrac-tion), concentration, for example using isoelectric precipitation or ultrafiltration, followed by drying (Alonso-Miravalles et al., 2019). Depending on the process,co-extraction of polyphenols, phytic acid or other antinu-tritional compounds or precursors of off flavor may also occur (Fetzer et al., 2018). As we move toward the use of less refined ingredient fractions, it is critical to learn how to modify their physical and chemical properties with processing, to create prod-ucts with targeted functionalities and capitalizing on the synergies created by the interactions between various components. The objective of this work was to deter-mine t he changes occurring to the physicochemical properties of the colloidal structures extracted from rape-seed, depending on the processing conditions used.The major protein fractions in rapeseed are the storage proteins (80 wt/wt%), cruciferin, and napin. These pro-teins differ in size, supramolecular structure, isoelectric point, and solubility (Chmielewska et al., 2020, Rommi et al., 2015; Wanasundara et al., 2012), and are located in r membrane-boundproteinbodiesS(Wanasundara et al., 2016). The seed also contains significant amounts of lipids (about 40 wt/wt%), surrounded by a membrane composed of phospholipids and stabilizing proteins,oleosines.These oil structures, called oleosomes,have shown unique processing functionalities (Deleu et al ., 2010; Romero-Guzman, Kollmann, et al., 2020;Tzen & Huang, 1992). Finally, a significant concentration of fiber is also present in the seed, and the water soluble fraction contains mostly celluloses, hemicelluloses, and pectins (Rommi et al., 2015). Recently, it has been demonstrated that extracts rich inF proteins and oleosomes can be obtained from rapeseed by extraction at alkaline pH. For example,pH 9.0 was recently used, to minimize protein-polyphenols interactions but maximize yields: the final extracts contain oleosomes and protein, with yields of about 80% for both (Ntone et al., 2020). In addition,enzymatic treatments can be employed to assist in t he isolation of plant protein extracts. In particular,I PantE the addi-tion of cell wall-degrading enzymes, has shown to improve protein yields during extraction from rapeseed.A previous study has shown that especially the use of pectinase significantly improved the process (Rommi et al., 2014). Compositional differences of the protein extracts will also vary the technological functionality of the ingredients. While research reports are becoming available on how different unrefined fractions may be employed d as functional i ngredients (Karefyllakis et al., 2019; Romero-Guzman, Kollmann, et al., 2020),studies targeting changes in functionality through the modulation of processing conditions are only at their infancy. For example, it has been shown that controlled use of proteases can modify the functional behavior of plant protein extracts (Klost & Drusch, 2019). More fun-damental studies on the effect of processing on the col-loidal properties of the protein complexes created would lead to a better design and utilization of these ingredi-ents in foods. In this work, it was hypothesized that due to the physicochemical properties (molecular weight, pl, and solubility) of the proteins and the cellular architecture of the oilseed, changes in pH during extraction of rapeseed will affect the complexes formed between proteins, oil droplets (oleosomes) and soluble fiber. Objective of this research was then to study the changes in the composi-tion and solubility occurring to the protein extracts as a function of pH and addition of pectinase enzyme. To evaluate how the soluble fibers (i.e., pectins) may influ-ence protein complex formation, this study also employed a commercial pectinase, to determine how this enzyme may modify the colloidal properties of t he resulting protein particles. The knowledge achieved in this research will serve to improve our fundamental understanding of how to control the structure and stabil-ity of colloidal particles in plant protein extracts, to better target their technological function and create a new gen-eration of plant protein i ngredients from oil seeds. A direct extraction of oilseed protein from seed rather than from press cakes would result in new, more circular, and sustainable value chains. MATERIALS AND METHODS Materials Whole untreated rapeseeds were kindly provided by Lehnsgaard A/S (Aakirkeby). Commercial pectinase,Pectinex Ultra SP-L (3300 units/g) was donated by Novozymes A/S (Bagsvaerd). All chemicals were of analytical grade and purchased from Sigma Aldrich.The pectinase had broad pH of activity (according to manufacturer specification), but optimal at acidic pH and room temperature. The extraction process used i s summarized in Figure 1. In brief, whole rapeseeds were partially dehulled using a Thermomix TM6 (Vorwerk). The rape-seeds were wet milled and solubilized as previously reported (Rommi et al., 2015) with some modifications.The cracked rapeseeds were dispersed in MilliQ water at a concentration of 40% rapeseed (wt/wt), and wet milled using an Ultra Turrax T-25 (IKA) at 13500 rpm for 2 min. The suspension was then diluted to 10%(wt/wt), and milled for two additional minutes a at 13500 rpm. The slurry ("extract") was either kept at the original pH (5.7) or adjusted to pH 8.5 using 1.0 M NaOH, and mixed for 4h on a magnetic stirrer at 600 rpm at room temperature (RCT basic, IKA). A frac-tion of the samples was t reated by addition of Pectinex Ultra SP-L at a ratio of 100 mg/g rapeseed added prior to mixing. This ratio was selected based on a previous study (Rommi et al ., 2014) and after consulting Novo-zymes A/S. The pH of the extract was continuously monitored and adjusted i f necessary. For the alkaline sample, the pH of the extract was maintained at pH 8.5by adding a few drops of 1.0 M NaOH during the mixing period. After the incubation, with or without pectinase, a portion of the samples was subjected to a pH shift to the converse extraction pH, 5.7 or 8.5, using 1.0 M HCI or 1.0 M NaOH, respectively. This created two addi-tional extracts with a pH shifted from 8.5 to 5.7 and from 5.7 to 8.5. The pH shift was employed to evaluate potential differences between incubation pH and press-ing pH. The samples without addition of pectinase were named according to their pH treatment: 5.7,8.5,5.7to 8.5, and 8.5 to 5.7. In case of pectinase treatment,a P was added to the sample name: 5.7P, 8.5P, 5.7to 8.5P, and 8.5 t o 5.7P. The extract, rich in oleosomes and protein, was obtained by treating the slurry at either pH 5.7 or 8.5 i n a t win screwF press S (Angelia 7500, Angel, Juicer, Naarden) (Romero-Guzmán, Jung, et al., 2020). The extract was then centrifuged at 3500 g for 30 min at 4℃ (SL 40R, ThermoFisher, Landsmeer). After centri-fugation, the samples showed three separate phases: a lipid layer on top, a subnatant fraction, referred to as “protein concentrate”and a precipitate at the bottom.All physicochemical analyses were performed on t he whole extract (after pressing) and the centrifuged pro-tein concentrate. Composition of the extract and concentrate samples The dry matter was c quantified using a N Moisture Analyzer and expressed as g dry matter/100 g wet sample. The lipid content was measured by carrying out a digestion (Hydrotherm, HT6, C. Gerhardt GmbH &C o. KG) using 4 M HCI followed by a Soxhlet extraction using petroleum ether as solvent. The amount of pro-tein was estimated using nitrogen combustion method (Dumas, using a Dumatherm N Pro, ),with 5.7 as conversion f actor (Fetzer et al., 2018; Ntone et al.,2020). Total carbohydrates were quantified calo-rimetrically using the phenol-sulfuric acid method (DuBois et al., 1956). In short 5% phenol was added to the sample in a ratio 1:1 followed by addition of concen-trated sulfuric acid to hydrolyze carbohydrates. The absorbance of the colored compounds, furfurals, was detected at 490 nm using a UV spectrophotometer (UV-3100PC, Avantor). Blanks containing MiliQ water were subtracted from the protein extracts. The amount of oil, protein, and carbohydrate pre-sent i n the extracts after pressing were estimated as %dry matter. Furthermore, the retention of the compo-nents i n the protein concentrate after cent r ifugation was quantified as the ratio of component present in the protein concentrate relative to the amount of compo-nent present in the extract after pressing. I n other words, the amount of protein, oil, and carbohydrate pre-sent in the soluble phase after centrifugation of the extract. FIGURE 1 Extraction procedure. Schematic representation of the various steps l eading to the preparation of the uncentrifuged rapeseed extract and the protein concentrates after centrifugation. The point where pH was adjusted, shifted or pectinase was added i s also i ndicated in the figure. The protein and oil yields were estimated both in the extract as well as in the protein concentrate, and they represent the residual amount relative to the origi-nal amount present in t he seed before extraction. Electrophoresis SDS-PAGE under non-reducing conditions was used to determine the protein profile of the protein concentrates.Non-reducing conditions were chosen to better highlight the presence of cruciferin, napin, and oleosin in the vari-ous extracts.The samples were diluted in buffer (NuPAGELDS, ThermoFisher) t o a f inal protein con-centration of 1.0 mg/mL. The gel (NuPAGE Novex@4-12% Bis-Tris Gel, ThermoFisher) was loaded with 5 pL protein marker (PageRulerTM Prestained Protein Ladder, 10-180 kDa, ThermoFisher) and 10 pL protein solution, and the chamber was filled with MES running buffer (NuPAGE MES SDS Running Buffer, Thermo-Fisher) and ran for 35 min at 200 kV. The gels were washed and stained (Coomassie Br i lliant Blue R-250Staining Solution, Bio-Rad Laboratories B.V.) overnight.Bands were analyzed using the software Image Lab 6.1.(Bio-Rad Laboratories,Inc.). Amino acid analysis Free amino acids were quantified calorimetrically using the OPA assay (Sousa et al., 2020). I n short, the extracts were diluted with trichloroacetic acid in a ratio of 1:1.6 to precipitate proteins. The soluble fraction was recovered after centrifugation at 13,000 g for 20 min at 4°C using a microcentrifuge (Centrifuge 5417R,Eppendorf). o-phthalaldehyde (OPA) was added to the subnatant to bind free N-terminals, which were detected at 340 nm using a microplate reader (Synergy 2 multi-detection microplate reader, Biotek instruments). A blank sample containing phosphate-buffered saline (PBS) was subtracted from the protein extracts. The concentration of free amino acids was expressed as glutamine equiva-lents using glutamic acid as standard. Imtakt), using 10% methanol in acetonitrile as mobile phase. During hydrolysis cystine, asparagine, and gluta-mine were oxidized, and these are therefore represented as the sum of both the oxidized and the non-oxidized form (Tsochatzis et al., 2017). Particle size distribution The particle size was s measured using static light scattering (Malvern Master Sizer 2000, Malvern Instruments), after extensive dilution in water. Refractive index 1.47 was used for rapeseed oil according to the literature (Ntone et al., 2020) and 1.33 was used for the continuous phase. Each measurement was performed in triplicate and expressed as volumetric particle size distribution (D4,3). Confocal laser scanning microscopy Rapeseed protein concentrates were visualized by CLSM using a Nikon microscope (Nikon C2, Nikon Instrument Inc.) applying a ×60 objective. Oil and pro-tein were f luorescently labeled with 0.1% (wt/wt) Nile Red and 0.1% (wt/wt) Fitc Green solubilized in acetone.Nile red and Fitc Green were excited using a wave-length of 488 and 633 nm, respectively. Statistical analysis All treatments were performed in triplicate, with three independent extractions. Two-way analysis of Variance (ANOVA) and Turkey’s t test were performed to show any significant differences (p< 0.05) using R-studio (version 1.4.1717). RESULTS AND DISCUSSION Composition of the protein concentrate suspensions The chemical composition of the various extracts was measured, as a function of the different pH extraction conditions, with or without the addition of pectinase. As previously reported (Ntone et al., 2020), wet milling ofdehulled rapeseed results in an extract rich in oleo-gen somes and proteins. The rapeseed extract, after press-ing, varied in dry matter content between 5.3% and 6.8% (Table S1) with significantly higher solids with the addition of pectinase, regardless of the pH. The addition of pectinase led to a loosening of the cell wall architec-ture, but not a higher l evel of extracted carbohydrates (Table S1). It is important to note that although the pecti-nase had a broad range of activity, its optimal pH is around 5 (Aslan & Tanriseven, 2007; Tabtabaei &Diosady, 2013). This should lead to more extensive modifications of the cell wall polysaccharides during incubation at pH 5.7 compared with 8.5. In these experi-ments, the effect of pH shift was also i nvestigated, as this will cause changes in the polymers'interactions and charge. Extraction conditions FIGURE2 Total solids. Amount of dry matter recovered i n the rapeseed concentrates after treatment at pH 5.7, 8.5, or after shifting the pH from 5.7 to 8.5 or vice versa, f rom 8.5 to 5.7 without (black bars) or with pectinase added (white bars). Data are the average of three replicates, and bars indicate standard deviation. Values with different superscripts are significantly different (<0.05). improves solids recovery in the concentrate. This is also confirmed by opposite trend when t he conditions were reversed: incubation at pH 8.5 and adjustment after pressing at pH 5.7 showed a significantly lower solids recovery i n the centrifuged protein concentrate fraction, with a difference with pect i nase added. The lower solids and lipids recovery at pH 5.7 may be attrib-uted to an increased extent of electrostatic protein-polysaccharide interactions which limit their partition on the protein colloidal phase, and may be found instead in the pellet or the oil phase. The size of the polysac-charide is also important at this stage, as the addition of pect i nase results in higher solids in the 8.5 to 5.7concentrate (Figure 2). These results clearly demon-strated that the pH at pressing is also a key step in the preparation of the rapeseed extracts. It is also important to note that although there was a significant effect of pectinase regardless of pH of extraction, the underpinning mechanisms for increase in yields may vary between treatments. Indeed, t he par-tition of the various components between the oil , pro-tein, and fiber fractions after centrifugation varied depending on the pH treatment. The concentration (based on dry matter) of protein, carbohydrate, and oil in the concentrates after centrifugation is presented in Figure 3. There were profound differences i n composi-tion between the protein concentrates. While the con-centrate extracted at pH 5.7 was mainly composed of carbohydrate and proteins, the protein particles in the concentrates treated at pH 8.5 co-existed with oil and soluble fiber . As shown in Figure 3a, the concentration of residual carbohydrate in the protein fraction varied from 15% to 43%(wt/dry weight) and changed signifi-cantly as a function of extraction pH, with the highest concentration after treatment at pH 5.7 and the lowest for alkaline pH. I t is i mportant to note that in the extract after pressing, before centrifugation, the concentration of carbohydrate was about 15% regardless of treatment (Table S1), demonstrating a selective recovery of solu-ble carbohydrates in the extract t reated at pH 5.7. I nter-estingly, there was no significant effect of pectinase within a treatment, and this was somewhat surprising as one may expect an increase in soluble material after enzymatic treatment. By comparing the amount of car-bohydrates recovered in the concentrate after pH shift-ing, it was possible to conclude that pressing and cent r ifugation at pH 5.7 resulted in a higher percentage of carbohydrate i n the concentrate, compared with the samples at pH 8.5. atP川 The concentration of lipids present in the protein concentrate after centrifugation explains the differences in the composition between extracts. Figure 3b clearly i llustrates that centrifugation at pH 5.7 caused a more efficient separation of the oleosomes from the protein concentrate subnatant. The amount of oleosomes remaining in the subphase was the highest for alkaline extracts (55%) and lowest when the extractions were FIGURE 33Composition of macro components. Composition of the colloidal particles extracted from the centrifuged rapeseed after incubation at pH 5.7, 8.5, or after shifting the pH from 5.7 t o 8.5 or vice versa, from 8.5 to 5.7, without (black bars) or with pectinase added (white bars). Amount of carbohydrate (a), lipid (b), and protein (c) as % dry matter . Data are the average of t hree replicates, and bars indicate standard deviation. Values with different superscripts are significantly different (<0.05). carried out at pH 5.7 (2%) (Figure 3b). The oil composi-tion of the pH shifted extracts without pectinase addi-tion would suggest that the centrifugation at pH 5.7 i s a key factor in improving the separation of the oil droplets from the subnatant protein concentrate: all extracts centrifuged at pH 8.5 showed very high oil concentra-tions in the protein phase. This pH effect was also con-firmed in the samples extracted at pH 8.5with subsequent pressing and centr i fugation at pH 5.7, as they showed a reduced amount of oil compared with the pH 8.5 samples, but not to the same extent as for the sample treated at pH 5.7. It was concluded that sol-uble charged polysaccharides (i.e., pectin) play a key role in the partition of the oleosomes between t he oil and protein phases. While treatment with pectinase did not affect oil separation at pH 5.7 and 8.5, the pecti-nase treatment in combination with shifting of pH resulted in a significant decrease in lipid recovery, indi-cating that a smaller size of the polysaccharide chain results in a higher extent of oil fractionation between the oil and the protein phases. Figure 3c summarizes the concentration of protein (wt/dry weight) in the centrifuged concentrates. Interest-ingly, while the yields based on dry matter were the highest i n the concentrates treated at pH 8.5 (Figure 2),the highest protein/solid ratio was obtained in concen-trates extracted at pH 5.7, due to t he low carry-over of oil during centrifugation. The protein relative concentra-tion varied significantly with extraction conditions, with pH 5.7 having the highest (43%) and alkaline having the lowest (25%) values. Shifting from 5.7 to 8.5 or from 8.5 to 5.7 gave rise to lower levels than those at 5.7. The extracts at alkaline pH showed similar concen-trations as what is already reported in the literature (Ntone et al., 2020). For example, rapeseed extracts at pH 9 showed a protein recovery as high as 78% from the original seed, and 38% after centrifugation, how-ever, with oleosomes co-extracted in the concentrate.The present results for the first t i me demonstrate that during wet milling different nonrefined fractions can be obtained by modulating the charge of the biopolymers as well as their supramolecular assemblies. To get a better understanding of the colloidal stabil-ity of the components in the extract, the relat i ve recov-ery of the various components after centrifugation is shown i n Figure 4. The recovery was calculated as amount of carbohydrate, lipid, or protein remaining in the concentrate after centrifugation, relative to the origi-nal extract after pressing. In the original extract, the amount of soluble fiber was estimated to be between 0.8 and 1% (wt/wt) (see Table S1). The amount of solu-ble carbohydrate remaining after centrifugation was not significantly different between treatments (Figure 4a).In most cases,about60%of the carbohydrate extracted with pressing was recovered in the protein concentrate. There was no effect of pectinase for sam-ples incubated at pH 5.7 or pH 8.5. This was probably due to the fact that even after a decrease in molecular weight, the polysaccharides are still free to interact with the protein in the dispersion and adsorbed on the sur-face of the oleosomes, contributing to the formation of different colloidal structures. As already seen in Figure 3, conversely, t he extrac-tion and centrifugation pH had an i mportant effect on the oil recovery in the subnatant after centrifugation FIGURE 4Stability of macro components. Recovery of carbohydrate (a), lipid (b) and protein (c) after centrifugation of t he pressed rapeseed extract. The amount is reported as % of t he total amount before centrifugation, in samples t reated at pH 5.7, 8.5, or after shifting the pH from 5.7 to 8.5 or vice versa, from 8.5 t o 5.7,without (black bars) or with pectinase added (white bars). Data are the average of three replicates, and bars indicate standard deviation.Values with different superscripts are signi f icantly different (<0.05). (Figure 4b). While the amount of lipid in t he extract after pressing ranged between 3.4% and 4% (wt/wt) (see Table S1), after centrifugation, the recovery of oleosomes in the protein concentrate was minimal at pH 5.7, while about 50% were retained at pH 8.5. There was an excep-tion for the extracts treated with pectinase at pH 5.7 and subsequently adjusted to pH 8.5, which showed a further Particle size distribution and microstructure of the fractions The particle size distribution of protein concentrates extracted at pH 8.5 was quite different from that at pH 5.7, with no differences at pH 8.5 between samples with and without pectinase (Figure 5b,f ). Both extracts show a bimodal particle size distribution with one peak around 0.5 pm and a smaller peak at 5 pm. Confocal imaging with differential staining clearly shows the absence of large oleosomes aggregates. The small Apparent Diameter (um) FIGURE 55Colloidal properties. Particle size dist r ibution and CLSM of the colloidal particles in the centrifuged protein concentrates extracted under the different pH conditions, without, (a)-(d) and with pectinase,(e)-(h). Scale bar in CLSM images is 50 pm. Oil is stained red and protein green. particles found in the alkaline concentrate have sizes similar to t those reported for r intact oleosomes (Abdullah & Zhang, 2020; Tzen et al., 1993). Further,protein particles seem to be larger in size than in the concentrate at pH 5.7, and small oleosomes seem to be co-existing with protein particles. A comparison of the images of the extracts at pH 8.5 with and without pecti-nase may also suggest that smaller coacervates of oleo-somes and protein form in the samples treated with pectinase compared with those without enzyme. Samples t reated at pH 5.7 and then adjusted at alkaline pH before fat separation showed a final micro-structure (Figure 5c) similar to that of the samples extracted at pH 8.5. Furthermore, t he part i cle size distri-bution was also similar , indicating that at this pH the protein particles are well dispersed in solution together with oleosomes. Interestingly, in this case the treatment with pectinase at pH 5.7 before alkaline adjustment caused the formation of protein-oleosomes aggregates (Figure 5g ) with evidence of aggregation also in the particle size distribution, where the quantity of particles with an average diameter of about 4 pm where larger than in the suspensions at 8.5. This shift in size is caused by the formation of coacervates after pectinase addition (Figure 5g ) and explains the lower recovery of lipids in the centrifuged concentrate (Figure 4). The particle size analysis and complementary micro-structure also reveals a drastically different distribution of protein-oleosomes particles when the extraction was conducted at pH 8.5 and then acidified before pressing and centrifugation (Figure 5d,h). Under these conditions, the samples had predominantly large aggregates with an average diameter of about 9 pm, with or without pecti-nase addition. The microstructure of the particles is quite different in this case, showing the formation of large flocs (Figure 5d). When comparing the same pH shift t reat-ment, but with pectinase added, it is clear t hat t he type of coacervates formed i s different, with more compact structures. The aggregates formed by shifting pH from 8.5 to 5.7 were the largest and most poly-distributed,and in clusters >50 pm. The presence of these large flocs in t he concentrate also explains the recovery rates after centrifugation in these dispersions, as shown in Figure 4. Efficient removal of oil from the subnatant were a clear result of the pH shifting (Figure 4b). However,protein was also l ost (Figure 4c) into the oil phase due to the extensive protein oleosome aggregation. The results shown in Figure 5 clearly point to a larger effect of pectinase on the colloidal properties when extracts are treated at pH 5.7, which i s due t o t he higher activity of the enzyme at this pH compared with pH 8.5. However, when observing gmicrostructural images, it is very clear that the enzyme has a broad spectrum of activity, as it also caused changes in microstructure at pH 8.5 (Figure 5b,f). Polypeptide distribution and amino acid analysis The ed difference in nc composition in the various concentrates could lead to var i ations in the protein distributions. Hence, SDS-PAGE analysis s was 紡 per-formed under non-reducing conditions (Figure 6).Samples were loaded at equal protein concentration, to compare the treatments. All protein concentrates con-tained the most abundant rapeseed protein, cruciferin.Its subunits migrated between 60 and 20 KDa. Previ-ous reports show cruciferin as associated with oleo-somes at pH 7 (Romero-Guzman, Jung, et al., 2020),however, in this work, there seemed to be no partition-ing of t his protein at particular high or l ow pH values.Napin, the second most abundant protein in rapeseed,migrating at about 18 kDa, was prominent in al l concen-trates after centrifugation. Under reducing conditions (data not shown) this band was no longer present,due to the presence of disulfide bonds linking the two polypeptide (4 and 9 kDa) subunits (Wanasundara et al., 2012). This protein has also been shown to be associated with oleosomes at pH9 (Ntone et al., 2020).The only samples that seemed t o have lower levels of napin were those t reated solely at pH 5.7, with or with-out pectinase (Lanes 1-2). Cruciferin and napin have very distinct isoelectric points (7.25 and 10) and a very different solubility at acidic pH, and their similarity in the composition of the concentrates was somewhat of a surprise, and may be due to their interaction with the naturally present polysaccharides in the extracts. A distinct protein band was also noted at 20 kDa in all extracts indicating the presence of oleosin (Romero-Guzman, Vardaka, et al., 2020; Tzen & Huang, 1992),a protein associated with t he oil bodies in oilseeds, the oleosomes. Similar ratios were found for oleosin in all protein concentrates independent of oil content, which could indicate that, in samples with very l ow oil content (5.7 and 5.7P) oleosin would not only be present on the oleosome surface, but also as soluble protein com-plexes. Another protein associated with the oleosome is steroleosin (Lin et al., 2005), which migrates at about 40 kDa, was also present in all concentrates. Samples with lower oil content showed the lowest band intensity,whereas samples with high co-extraction of oil (8.5,8.5P and 5.7 to 8.5) showed higher band intensity. This indicates that steroleosin was prevalently associated with the oleosomes. It i s important to note that all sam-ples were compared at equal protein concentration,hence, these results would suggest that the partition of proteins between the oil, the subnatant concentrate and the insoluble phase during centrifugation was not specific for a particular protein. To further understand potential differences in the par-名 titioning of the proteins in the various concentrates,values of total, essential, and free amino acids per total protein in the various concentrates are summarized in Table 1. The amount of protein was calculated applying a 5.7 conversion factor (Fetzer et al., 2018). Most protein concentrates contained between 50 and 56 g amino acids/100 g crude protein, whereas only the concentrates extracted at pH 8.5 and shifted to 5.7 i ndependent of FIGURE6 Protein profile SDS-PAGE of protein concentrates after centrifugation under non-reducing conditions. M, molecular weight marker. pectinase treatment had significantly higher values of 65 g amino acids/100 g protein, suggesting a different protein partitioning with t his t reatment. The percentage of essential amino acids in t he rapeseed concentrate was >30% and higher than what is reported for oat, lupin, and wheat (Gorissen et al., 2018), and varied according to extraction pH. Protein concentrates extracted at or shifted to pH 8.5 contained higher values compared with pH 5.7 samples, indicating the partitioning of proteins with pH during centrifugation. The addition of pectinase showed an increase i n the essential amino acid concen-tration only for pH 5.7. These results would point to a dif-ference i n the protein distribution during extraction/wet fractionation. The amount of free amino acids present in the vari-ous concentrates were also measured, to determine potential differences in the hydrolysis. It was clear that there was an effect of hydrolysis for samples t reated at pH 5.7 (Table 1). The hydrolysis was even more pro-nounced using pectinase, albeit both extracts at pH 5.7showed the highest extent of hydrolysis, indicating the effect of endogenous proteases. Some unspecific pro-teolyt i c activity at low pH was expected as t his is a commercially available enzyme (Rommi et al., 2014).Interestingly, samples with pH shift did not show such high extent of hydrolysis, also when the sample was stored at 5.7 but extracted at alkaline pH. These results demonstrate for the first t ime that the distribution of pro-teins and the potential of indigenous enzyme activity may play an important role i n imparting differences in the nutritional profile of t he rapeseed concentrates. The amino acids composition was further analyzed by LC-MS analysis. The population of free amino acids was dominated by glutamine and t ryptophan, and they were present in n:similar ratios (~1:1) apart from when pectinase was added at pH 5.7 or shifted to TABLE 11Amino acid content total, essential and free amino acids concentrated in the subnatant after the different extraction conditions. Total amino acids Essential amino acids Free amino acids (g/100 g protein) (% of total amino acids) (g/100 g protein) Pectinase 一 一 十 一 十 Extraction pH 5.7 56±15a 52±6a 28±3a 33±2b 32±2 cd 44±5d 8.5 54±13a 55±8a 38±2c 37±2c 17±2a 18±1a 5.7 to 8.5 55±3a 50±6a 37±2c 37±1c 24±1ab 25±6bc 8.5 to 5.7 65±10b 65±16b 34±2b 34±2b 26±2b 27±1bc Note: Without the use of pectinase is indicated by-and with by+. Data are t he average of three replicates ± standard deviation. Values with different letters differ signi fi cant (<0.05) from one another. pH 5.7 (5.7, 5.7 to 8.5 and 8.5 to 5.7), where t he ratio was higher for glutamine (Data not shown). It is impor-tant to note that while both cruciferin and napin contain tryptophan in their amino acid sequence, while napin has been reported to be more resistant to proteolytic activity (Fetzer et al., 2018), suggesting that cruciferin’s subunits may be the main substrate for the react i on. It is important to note that the values of free amino acids in the protein concentrates should be used for comparison between the protein concentrates and not as exact values, since the OPA assay is prone to overestimating values in the presence of colored compounds (e.g., poly-phenols), which are co-extracted during the various extraction treatments. The total amino acid composition of the rapeseed concentrates was quantified using LC-MS, and the dis-tribution of essential amino acids is summarized in Figure 7. Tryptophan was not i ncluded due to its degra-dation during acid hydrolysis. There were no significant differences between the composition of protein concen-trates treated with and without pectinase, and therefore only non-enzymatically extracted samples are shown in Figure 7. Confirming what is shown in Table 1, Figure 7also shows that all the concentrates contained high levels of essential amino acids. I t is known that profiles largely differ between plant-based protein ingredients (Gorissen et al., 2018). The concentrates all showed high levels of lysine, l eucine, histidine, and methionine.Values of methionine were higher than those reported for other plant proteins (Gorissen et al., 2018). The data reported here point to the importance of con-trolling extraction conditions, even with wet milling, as dif-ferences in the protein distribution can occur. However detailed studies on this topic are stil l few and far between,and industrial conditions may also affect compared with extractions made in the laboratory. Histidine showed the largest differences between extraction conditions, where pH shifted samples showed higher concentrations than the corresponding non shifted concentrate. According to the literature a higher level of histidine is found in napin than in cruciferin (Wanasundara et al., 2012), which imply that samples that has been shifted either f rom 5.7 to 8.5or 8.5 to 5.7 contain more napin than the non-shifted FIGURE7 Essential amino acids. The composition of essential amino acids i n the centrifuged protein concentrates expressed as g amino acids/100 g protein after t reatment at pH 5.7 (black bar), 8.5(white bar), or after shifting t he pH from 5.7 to 8.5 (black bar with white stripes) or vice versa , from 8.5 to 5.7 (white bar with black stripes). Data are the average of three replicates, and bars indicate standard deviation. Values with di f ferent superscripts are significantly different (<0.05). ones. Lysine was found in concentrations between 3 and 4 g/100 g protein, with the highest amount found in the concentrate shifted from pH 8.5 to 5.7 and the lowest in the pH 5.7 extract. Lysine is known to be susceptible to chemical reactions, such as formation of lysino-alanine (LAL), which has previously been reported during alkaline extractions of rapeseed protein (Deng et al., 1990). How-ever, the results from our study did not suggest a change in LAL formation with pH. The level of essential amino acids shown in Figure 7 is lower than what has previously been described for isolates prepared with alkaline extractions in combination with isoelectric precipitation (Tan et al., 2011), due to a difference in the protein composition. Furthermore, only methionine and histi-dine reached the recommended concentrations by FAO (Food and Agriculture Organization of the United Nations,2013). The e compositionn of nonessentialamino acids is summarized inTables S2. Among the e highest concentrationss ofnonessential amino acids was glx (glutamic acid + glutamine) and glycine in all extracts,but with significant differences between the extraction con-ditions (Table S2). The highest quantity of these amino acids was found in the concentrate shifted from 8.5 to 5.7 and glycine was also found in high quantities at pH 5.7. Cruciferin is known to contain large proportions of glutamic acid and glycine, and especially the acidic C-terminal of the o-polypeptide is rich in these two amino acids (Wanasundara et al., 2012), which indicates that more of this peptide was extracted when the final pH of the concentrate was 5.7 (5.7 and 8.5 to 5.7). Cysteine was also present in all concentrates in high concentra-tions, with significant higher values in the two extracts with a final pH of 5.7 (5.7 and 8.5 to 5.7) compared with the ones achieved at alkaline conditions (8.5 and 5.7 to 8.5).The l oss of cysteine during alkaline extraction has previ-ously been described (Tan et al., 2011). The amino acid composition i s not only an expression of the protein com-position, but also the availability of the amino acids, and deviations in amino acid profiles can be a consequence of interactions with other compounds i n the suspension.Polyphenols, such as sinapic acid, 4-vinylsyringol and tannins are found in high quantities in rapeseed and has previously been reported to be co-extracted during protein concentration (Fetzer et al ., 2019; Harbaum-Piayda et al., 2010; Ntone et al.,2020). Oxidation of polyphenols form highly reactive compounds that are able to create complexes with proteins (Ozdal et al., 2013). Especially tannins are reactive with cysteine and lysine (Ozdal et al., 2013), and loss of cysteine might be a consequence of protein-polypenol complex formation. The formation of these types of compounds decrease the bioavailability of the amino acids and consequently lower the nutritional value of the protein ingredients (Hou et al.,2017). Protein extraction yields High protein yields are often the focus when designing extraction processes, and Figure 8 s summarizes t he Extraction Conditions FIGURE8 Yields. Yields of oil (a, b) and protein (c, d) before (a, c) and after centrifugation (b, d). The yield is reported as t he recovery (%)of the total amount i n the rapeseed, in samples treated at pH 5.7, 8.5, or after shifting the pH from 5.7 to 8.5 or vice versa, f rom 8.5 to 5.7, without (black bars) or with pectinase added (white bars). Data are t he average of three replicates, and bars indicate standard deviation. Values with different superscripts are significantly different (<0.05). protein and oil recovery, based on t he original seed in the extract and in t he final concentrate after centrifuga-tion. A recent study (Ntone et al., 2020) reported extrac-tion yields at pH 9 of about 80% of protein and 82% fat for the extract, before centrifugation. When centrifuga-tion has performed the r ecovery of protein reported was about 38%. In the present study, lower speeds of cen-trifugation were applied. The present data are in full agreement with the previous extraction studies at alka-line pH (Ntone et al., 2020; Romero-Guzman, Jung,et al., 2020), where the oil recovered in the extract after pressing was in all cases between 60% and 75%. It is important to note that in this study, only one pressing was carried out, as well as no soaking of the seeds at the initial stage. Concentrates at pH 8.5 still contained about 30% of the oil was present i n the form of stable, small oleo-somes (see also Figure 5, microstructure). On the other hand, concentrates at pH 5.7 did not show significant oil yields. Furthermore, as shown in Figure 8c all sam-ples showed >60% yield of protein i n the extract. In all samples, the addition of pectinase caused a higher recovery of protein in the extract. The increased recov-ery is expected to be due to higher protein availability caused by degradation of cell wall carbohydrates (Rommi et al., 2014). The protein yields in t he concen-trate (Figure 8c) varied f rom 28% t o 46% of t he original protein material. The lowest protein recoveries were at pH 5.7 as well as i n the extracts at pH 8.5 separated at pH 5.7. Furthermore, significantly higher yields were noted when pectinase was added at pH 5.7. In this case, the yields reached value slightly below those at pH 8.5. Similar f indings was reported by Rommi et al.(2015), who invest i gated pectinase assisted extraction of protein from defatted rapeseed press cakes at pH 10and 6, resulting in a significant increase in protein recovery at pH 6, from 30% to 40%. Figure 9 shows an i nverse relationship between recovery yields in the concentrate and protein/solids ratio. The samples with a final pH of 8.5 (8.5 and 5.7to 8.5), square and triangle symbols, show high yields and low purity , and conversely extraction at pH 5.7shows l ower yield values, but higher protein purities.Stability and separation of olesomes is therefore crucial for the composition of the final protein concentrates. A similar finding was reported by others, who showed that decreasing the pH initiates oleosomes-pectin interactions, improving t he steric stabilization of the oleosomes (Iwanaga et al., 2008), however, no evi-dence of bridging was provided. Soluble cell wall derived carbohydrates, such as pectin and hemicellu-lose, are co-extracted during protein extraction (Rommi et al., 2015), and also play a role in oleosome stability to creaming by centrifugation. Figure 9 highlights how pectinase treatment affects the composition of the pro-tein concentrates. At pH 8.5 protein (triangles) yields were i mproved when pect i nase was added, but with FIGURE9 Protein yield versus purity. The protein yield in relation to protein concentration per total solids of t he centrifuged protein concentrates after extraction at pH 5.7, 8.5, or after shifting the pH from 5.7 to 8.5 or vice versa, from 8.5 to 5.7, without (black symbols) or with pectinase added (white symbols). Data are the average of t hree replicates, and bars indicate standard deviation. decreased protein purity. Extractions at pH 5.7 f ollowed by a pH shift to 8.5 (square symbols) led to increased yields, but also increased purities due to destabilization of the oleosomes. The same tendency was found for extracts shifted from 8.5 to 5.7 (diamonds). The micro-structures of these extracts changed as a consequence of pectinase treatment leading to formation of more complex colloidal structures. Extractions at pH 5.7(circles)in combination with pectinase treatment showed a different relationship than for the remaining extracts, namely that protein yields could be increased significantly, without decreasing the protein purity remarkably. This f inding demonstrates the potential of applying pectinase assisted extractions to extract pro-tein more efficiently, but without compromising on the purity. The above-stated findings emphasize how extraction processes cannot be evaluated based on protein recoveries alone, but that further understanding of the colloidal assembly of protein throughout the pro-cess is needed i n order to understand their functional characteristics. CONCLUSIONS Thesimple extractionprocedure a applied in this research successfully demonstrated extraction of rape-seed proteins. However, variation in extraction pH and addition of cell wall degrading enzymes resulted in pro-tein suspensions with different composition and colloi-dal properties. Protein concentrates obtained with alkaline extractions reached the highest protein recov-ery, but co-extraction of oleosomes resulted in low protein purity. Contradictory, extraction without pH adjustment (pH 5.7) gave rise to lower protein yield, but also higher protein purity due to more efficient separa-tion of oleosomes from the protein rich suspension.Pectinase treatment successfully increased dprotein recovery at natural pH resulting in yields comparable to the ones achieved under alkaline conditions. Further-more, the assembly and stability of oleosomes were influenced by degradation of the cell wall carbohydrates resulting in the formation of oleosome-protein aggre-gates with different characteristics. Quantification of free and total amino acids revealed that the composi-tion of the protein and the nutritional quality may be affected by the process conditions. Proteolytic activity was more prone at pH 5.7 and when pectinase was added resulting in more severe protein hydrolysis.These findings highlight the i mportance of process con-ditions in i mparting different physiochemical properties to the wet milled concentrates, resulting in different par-titions of proteins in the various phases. New proces-sing approaches will then arise from understanding the supramolecular details and their influence on the func-tional properties. AUTHORCONTRIBUTIONS Simone Bleibach Alpiger has designed and performed the experiment, analyzed the data, performed data visualization, and prepared the initial draft. Milena Corredig has designed the experimental set up and supervised Simone Bleibach Alpiger during the experi-mental work. Further, Milena Corredig has been editing the final draft and contributing to the visuals. Both authors have read the f inal version of the manuscript. ACKNOWLEDGMENTS The ea authors gratefully acknowledge Emmanouil Tsochatzis for his expert advice and analytical help on LC-MS analysis, Hans Heldt-Hansen for his f ruitful dis-cussions on enzyme assisted extractions, Novozymes A/S for donating the enzyme, and Lehnsgaard A/S for donating the rapeseeds. FUNDINGINFORMATION The research was partly f unded by the Center for Innovative Foods, CiFood at Aarhus University, and the Villum Foundation (award number 37759). CONFLICTOFINTEREST STATEMENT The authors declare that they have no conflict of interest. ETHICS STATEMENT No human or animal subjects were used di in this research. ORCID Abdullah JW, Zhang H. Recent advances i n the composition, extrac-tion and food applications of plant-derived oleosomes. Trends Food Sci Technol. 2020;2020(106):322-32. htt ps://doi.org /10.1016/j.t ifs.2020.10.029 Alonso-Miravalles L, Jeske S, Bez J, Detzel A, Busch M, Krueger M,et al . Membrane filtration and isoelectric precipitation techno-logical approaches for the preparation of novel, functional and sustainable protein isolate from lentils. Eur Food Res Technol.2019;245:1855-69.h tt ps://doi.org /10.1007/s00217-019-03296-y Aschemann-Witzel J, Peschel AO. Consumer perception of plant-based proteins: the value of source transparency for alternative protein ingredients. Food Hydrocoll. 2019;96:20-8. https://doi.org /10.1016/j.f oodh yd .2019.05.006 Aslan Y, Tanriseven A. Immobilization of Pectinex ultra SP-L to pro-duce galactooligosaccharides. J Mol Catal B Enzym. 2007;45:73-7. h tt ps://doi.o rg/10.1016/j.molcatb.2006.12.005 Chmielewska A. Koztowska M, Rachwal D, Wnukowski P,Amarowicz R, Nebesny E, et al. Canola/rapeseed protein -nutritional value, functionality and food application: a review. Crit Rev Food Sci Nutr. 2020;61:3836-56. htt ps://doi.org /10.1080/10408398.2020.1809342 Dapcevic-HadnadevT, Dizdar M,F Pojic M,Krstonosic V,Zychowski LM, Hadnadev M. Emulsifying proper t ies of hemp proteins: effect of isolation technique. Food Hydrocoll . 2019;89:912-20. https://doi.or g/10.1016/j.foodhyd.2018.12.002 Deleu M, Vaca-Medina G, Fabre JF, Roiz J, Valentin R,Mouloungui Z. Interfacial properties of oleosins and phospho-lipids from rapeseed for t he stability of oil bodies in aqueous medium. Colloids Surf B Biointerfaces. 2010;80:125-32. https://doi.or g/10.1016/j.colsurfb.2010.05.036 Deng QY, Barefoot RR, Diosady LL, Rubin LJ, Tzeng YM. Lysinoala-nine concentrations in rapeseed protein meals and isolates. Can Inst Food Technol J. 1990;23:140-2. htt ps ://doi.org/10.1016/S0315-5463(90)70218-0 DuBois M 1,,Gilles KA, Hamilton JK, Rebers F PA, Smith F Colorimetric method for determination of sugars and related sub-stances. Anal Chem. 1956;28:350-6. htt ps ://doi.org/10.1021/ac60111a017 Fetzer A, Herfellner T, Eisner P. Rapeseed protein concentrates for non-food applications prepared from pre-pressed and cold-pressed press cake via acidic precipitation and ultrafiltration. Ind Crop Prod. 2019;132:396-406. ht tps ://doi.org /10.1016/j .indcrop .2019.02.039 Fetzer A, Herfellner T , Stabler A, Menner M, Eisner P. Influence of process conditions during aqueous protein extraction upon yield from pre-pressed and cold-pressed rapeseed press cake. Ind Crop Prod. 2018;112:236-46. https://doi.org/10.1016/j.indcrop.2017.12.011 Food and Agriculture Organization of the United Nations, editor. Die-tary protein quality evaluation in human nutrition: Report of an FAO expert consultation, 31 March-2 April, 2011. Auckland,New Zealand. Rome: Food and Agriculture Organization of the United Nations; 2013. Gallego M, Mora L, Escudero E, Toldrá F. Bioactive peptides and f ree amino acids profiles in different types of European dry-fermented sausages. Int J Food Microbiol. 2018;276:71-8.https://doi.org/10.1016/j.i jfoodmicro.2018.04.009 Gorissen SHM, Crombag JJR, Senden JMG, Waterval WAH,Bierau J, Verdijk LB, et al. Protein content and amino acid com-position of commercially available plant-based protein isolates.Amino Acids. 2018;50:1685-95.htt p s://doi.or g/10.1007/s00726-018-2640-5 Harbaum-Piayda B, Oehlke K, Sonnichsen FD, Zacchi P, Eggers R,Schwarz K. New polyphenolic compounds in commercial deo-distillate and rapeseed oils. Food Chem. 2010;123:607-15.https://doi.org/10.1016/j.foodchem.2010.04.078 Hou F, Ding W, Qu W, Oladejo AO, Xiong F, Zhang W, et al. Alkali solution extraction of rice residue protein isolates: i nfluence of alkali concentrat i on on protein functional, structural properties and lys i noalanine formation. Food Chem. 2017;218:207-15.h t tps://doi.o rg/10.1016/j .foodchem.2016.09.064 Iwanaga D, Gray D, Decker EA, Weiss J, McClements DJ. Stabiliza-tion of soybean oil bodies using protective pectin coatings formed by electrostatic deposition. J Agric Food Chem. 2008;56:2240-5. ht t ps://doi.or g/10.1021/jf073060y Karefyllakis D, Octaviana H, van der Goot AJ, Nikiforidis CV. The emulsifying performance of mildly derived mixtures from sun-flower seeds. Food Hydrocoll. 2019;88:75-85. http s://doi.org/10.1016/j.foodh yd .2018.09.037 Klost M, Drusch S. Functionalisation of pea protein by tryptic hydrolysis - characterisation of interfacial and f unctional proper-ties. Food Hydrocoll . 2019;86:134-40. ht t ps://doi .org /10.1016/j.food hy d.2018.03.013 L i n LJ, Liao PC, Yang HH, Tzen JTC. Determination and analyses of the N-termini of oil-body proteins, steroleosin, caleosin and oleosin. Plant Physiol Biochem. 2005;43:770-6. http s://doi.org /10.1016/j.plap h y.2005.07.008 Malomo SA, Aluko RE. Conversion of a low protein hemp seed meal into a functional protein concentrate through enzymatic digestion of f i bre coupled with membrane ultrafiltration. Innovative Food Sci Emerg Technol. 2015;31:151-9. htt ps ://doi.org/10.1016/j.ifset.2015.08.004 Mulder W, van der Peet-Schwering C, Hua NP, van Ree R. Proteins for food, feed and biobased applications: biorefining of protein containing biomass. IEA Bioenergy Task 42. 2016. Ntone E, Bitter JH, Nikiforidis CV. Not sequentially but simulta-neously: facile extraction of proteins and oleosomes from oil-seeds. Food Hydrocoll . 2020;102:105598.h tt ps://doi.org /10.1016/j.foodh yd .2019.105598 Ostbring K, Malmqvist E, Nilsson K, Rosenlind I, Rayner M. The effects of oil extraction methods on recovery yield and emulsify-ing properties of proteins from rapeseed meal and press cake.Foods.2019;9:19.h ttps ://doi.org /10.3390/foods9010019 Ozdal T, Capanoglu E, Altay F. A review on protein-phenolic interac-tions and associated changes. Food Res Int. 2013;51:954-70.h ttp s://doi.or g /10.1016/j.f oodres.2013.02.009 Qi B, Ding J, Wang Z, Li Y, Ma C, Chen F, et al . Deciphering the char-acteristics of soybean oleosome-associated protein in maintain-ing the stability of oleosomes as affected by pH. Food Res Int.2017;100:551-7. https://doi.org /10.1016/j.foodres.2017.07.053 Romero-Guzman MJ, Jung L, Kyriakopoulou K, Boom RM,Nikiforidis CV. Efficient single-step rapeseed oleosome extrac-tion using twin-screw press. J Food Eng. 2020;276:109890.ht tps://doi.or g /10.1016/j .jfoodeng .2019.109890 Romero-Guzman MJ, Kollmann N, Zhang L, Boom RM, Nikiforidis CV. Controlled oleosome extraction to produce a plant-based mayonnaise-like emulsion using solely rapeseed seeds. LWT.2020;123:109120.https://doi.or g/10.1016/j.lwt.2020.109120 Romero-Guzman MJ, Vardaka E, Boom RM, Nikiforidis CV. Influence of soaking time on the mechanical properties of rapeseed and their effect on oleosome extraction. Food Bioprod Process.2020;121:230-7. ht tp s://doi.or g/10.1016/jfbp .2020.03.006 Rommi K, Hakala TK, Holopainen U, Nordlund E, Poutanen K,Lantto R. Effect of enzyme-aided cell wall disintegration on pro-tein extractabi l ity from intact and dehulled rapeseed (Brassica rapa L. and Brassica napus L.) press cakes. J Agric Food Chem.2014;62:7989-97. https://doi.org /10.1021/jf501802e Rommi K, Holopainen U, Pohjola S, Hakala TK, Lantto R, Poutanen K, et al. Impact of particle size reduction and carbohydrate-hydrolyzing enzyme t reatment on protein recovery from rape-seed (Brassica rapa L.) press cake. Food Bioprocess Technol.2015;8:2392-9. https://doi.or g/10.1007/s11947-015-1587-8 Sousa R, Portmann R, Dubois S, Recio I, Egger L. Protein digestion of different protein sources using the INFOGEST static digestion model . Food Res Int. 2020;130:108996. htt ps ://doi.org /10.1016/j.foodres.2020.108996 Tabtabaei S, Diosady LL. Aqueous and enzymatic extraction pro-cesses for the production of food-grade proteins and industr i al oil from dehulled yellow mustard flour. Food Res Int. 2013;52:547-56. h tt ps://doi.or g/10.1016/j.foodres.2013.03.005 Tan SH, Mailer RJ, Blanchard CL, Agboola SO. Canola proteins for human consumption: extraction, profile, and functional proper-ties. J Food Sci. 2011;76:R16-28. https://doi.org/10.1111/j.1750-3841.2010.01930.x Tsochatzis ED, Begou o, Gika HG, Karayannakidis PD,Kalogiannis S. A hydrophilic interact i on chromatography-tandem mass spectrometry method for amino acid profiling in mussels.J Chromatogr B. 2017;1047:197-206. htt ps ://doi.org /10.1016/j.jchromb.2016.05.018 Tzen JTC, Cao YZ, Laurent P, Ratnayake C, Huang AHC. L i pids, pro-teins, and structure of seed oil bodies from diverse species.Plant Physiol . 1993;101:267-76. https://doi .org/10.1104/pp .101.1.267 Tzen JTC, Huang AHC. Surface structure and properties of plant seed oil bodies. J Cell Biol . 1992;117:9-335. Wanasundara JPD, Abeysekara SJ, Mclntosh TC, Falk KC. Solubility differences of major storage proteins of Brassicaceae oilseeds.J Am Oil Chem Soc. 2012;89:869-81. htt ps://doi.org /10.1007/s11746-011-1975-9 Wanasundara JPD, Mclntosh TC, Perera SP, Withana-Gamage TS,Mitra P. Canola/rapeseed protein-functionality and nutrition.OCL. 2016;23:D407.ht tps ://doi.or g/10.1051/ocl/2016028 SUPPORTINGINFORMATION Additional supporting information can be found online in the Supporting Information section at the end of this article. How to cite this article: Alpiger SB, Corredig M.Changes in the physicochemical proper t ies of rapeseed-derived protein complexes during enzyme-assisted wet milling. Sust Food Prot.2023;1(1):16-29. h t tps://doi.or g /10.1002/s f p 2.1001
确定






还剩12页未读,是否继续阅读?
中国格哈特为您提供《油菜籽衍生蛋白复合物中蛋白质和油脂含量的检测》,该方案主要用于生干坚果与籽类食品中营养成分检测,参考标准《GB 5009.6 食品中脂肪的测定》,《油菜籽衍生蛋白复合物中蛋白质和油脂含量的检测》用到的仪器有格哈特全自动超级总脂肪测定系统、格哈特快速干燥仪STL56、格哈特杜马斯定氮仪DT N Pro、德国加液器MM、滤纸筒
相关方案
更多
该厂商其他方案
更多














