
方案详情
文
使用烤过和未烤过的可可豆生产的巧克力的氧化稳定性和酚类含量Phenolic content and oxidative stability of chocolates produced with roasted and unroasted cocoa beans
Total fat content:
总脂肪含量:
The total fat content of cocoa beans was determined using the Soxhlet procedure (AOAC, 1995). Fat was extracted with a fully automated extraction system (Gerhardt-Soxtherm, Germany) using petroleum ether as a solvent.
可可豆总脂肪含量使用石油醚做为溶剂由格哈特全自动索氏提取系统-索克森Soxtherm提取。
方案详情

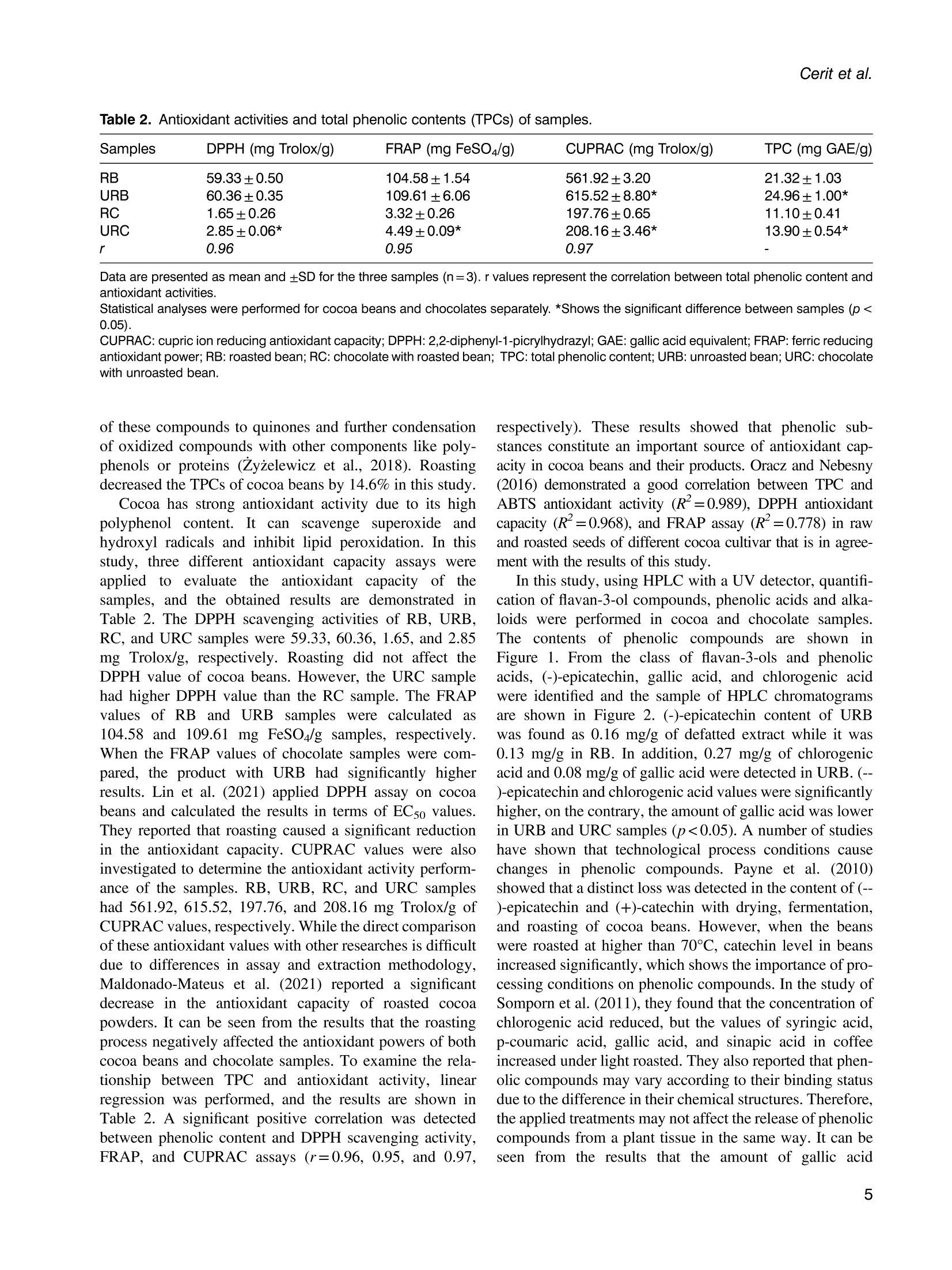
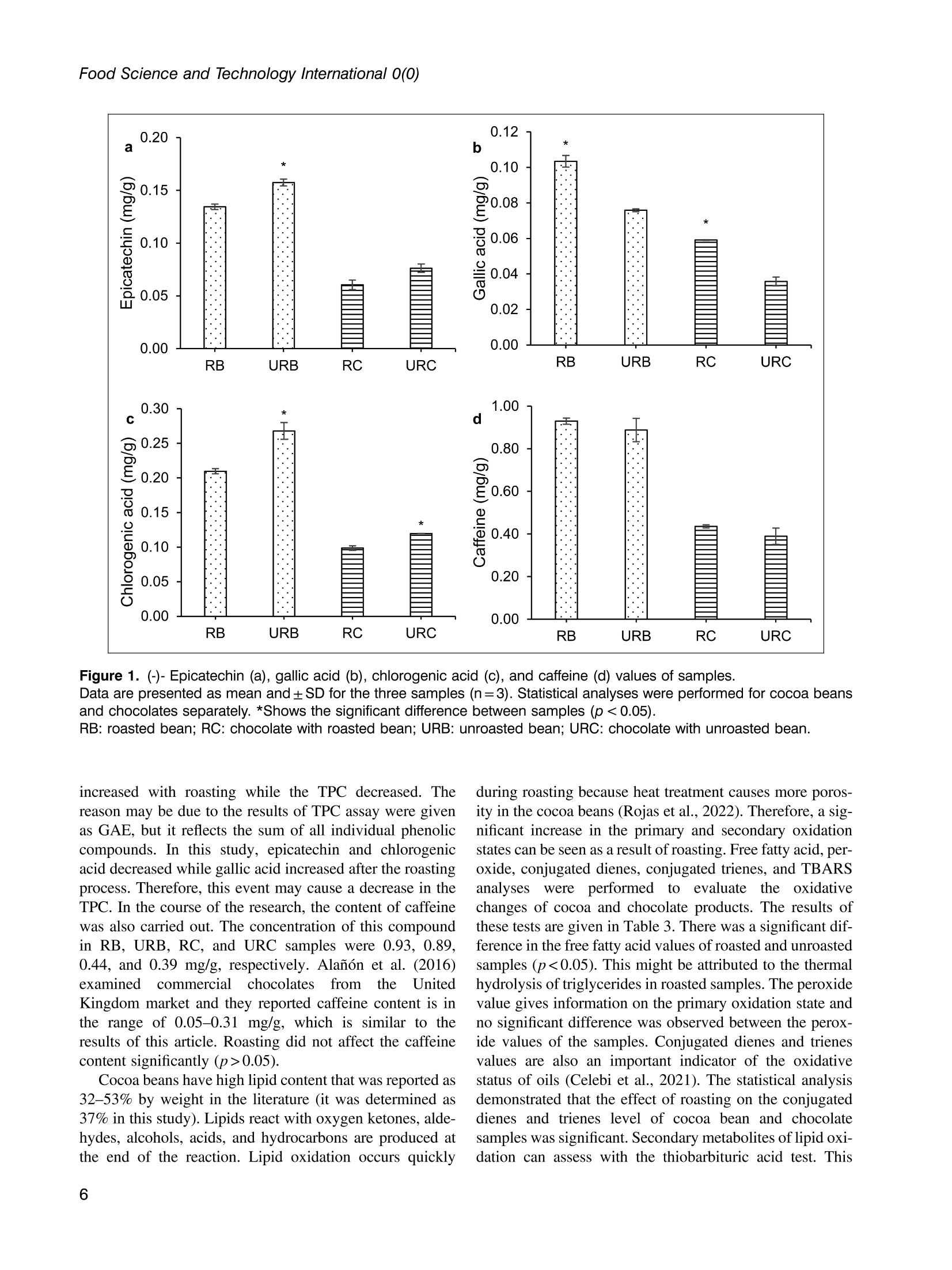
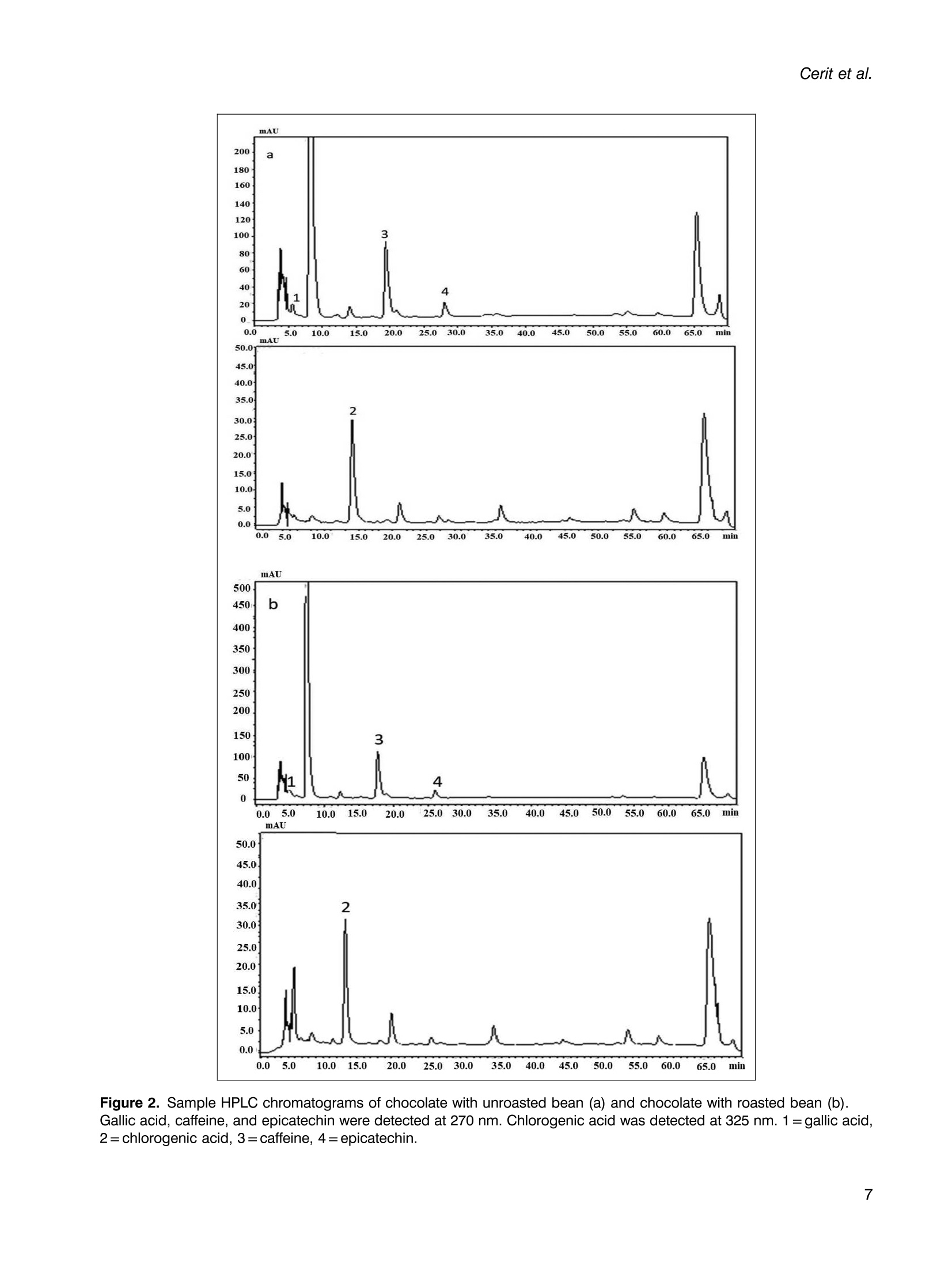
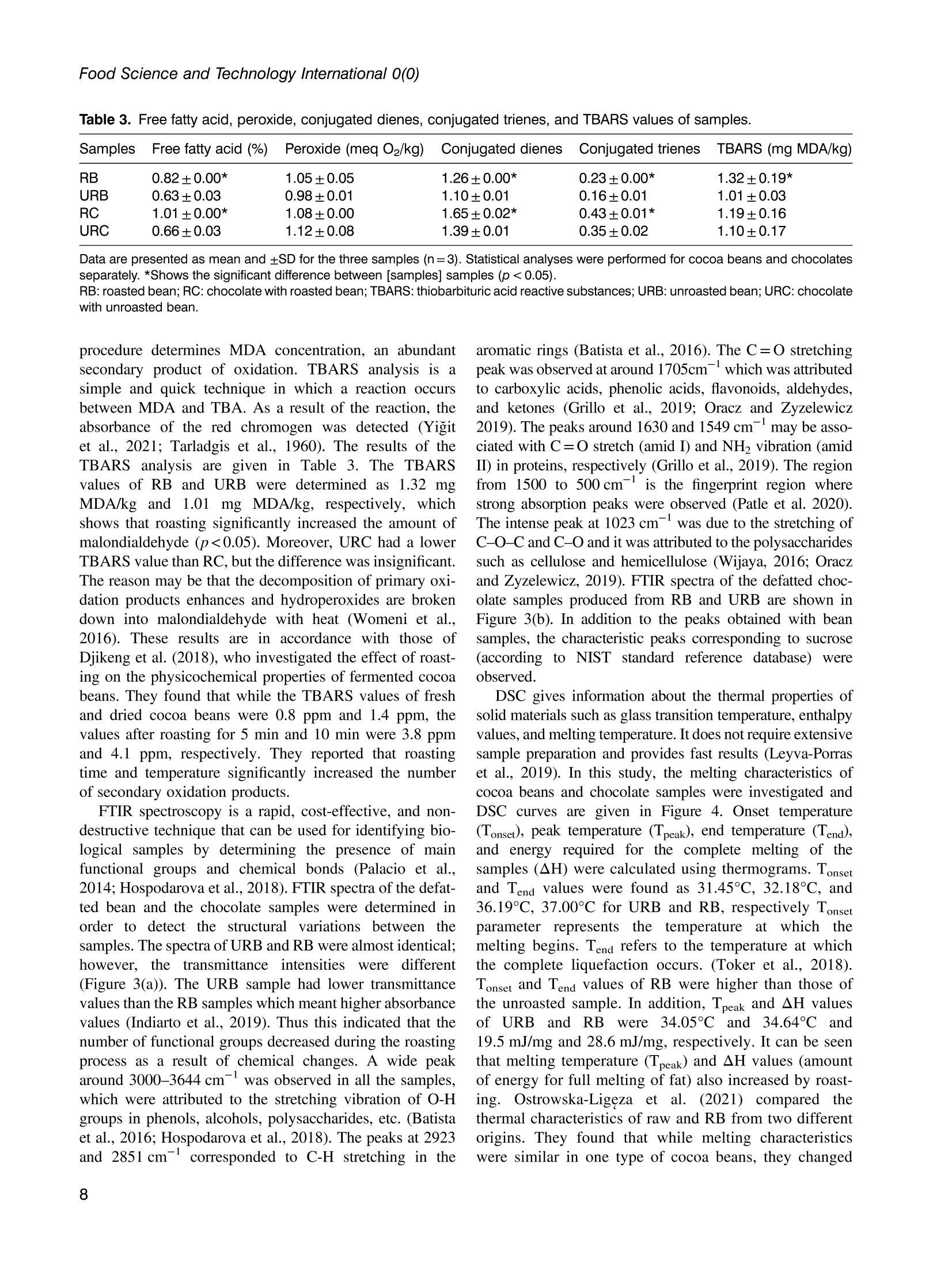
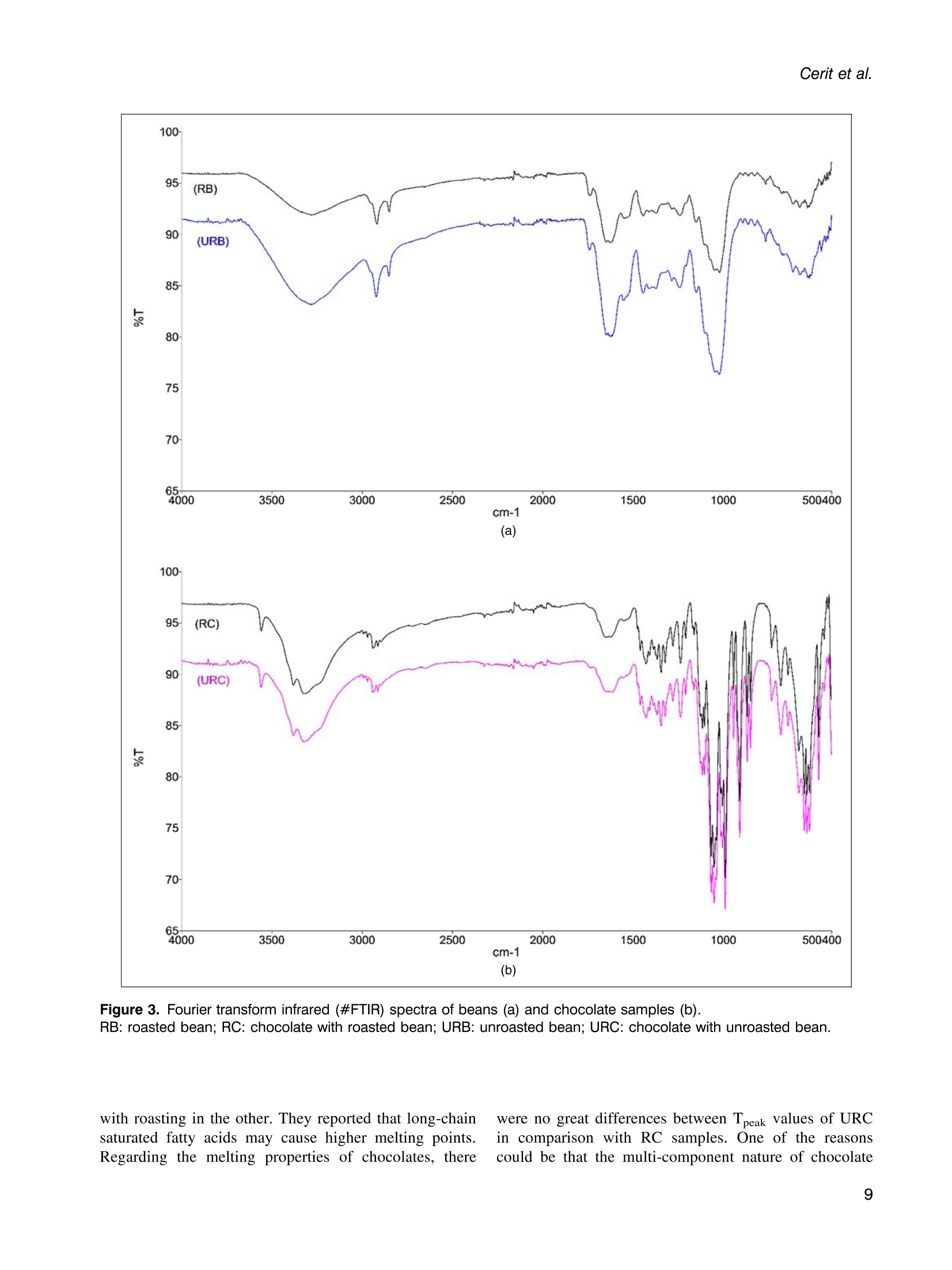
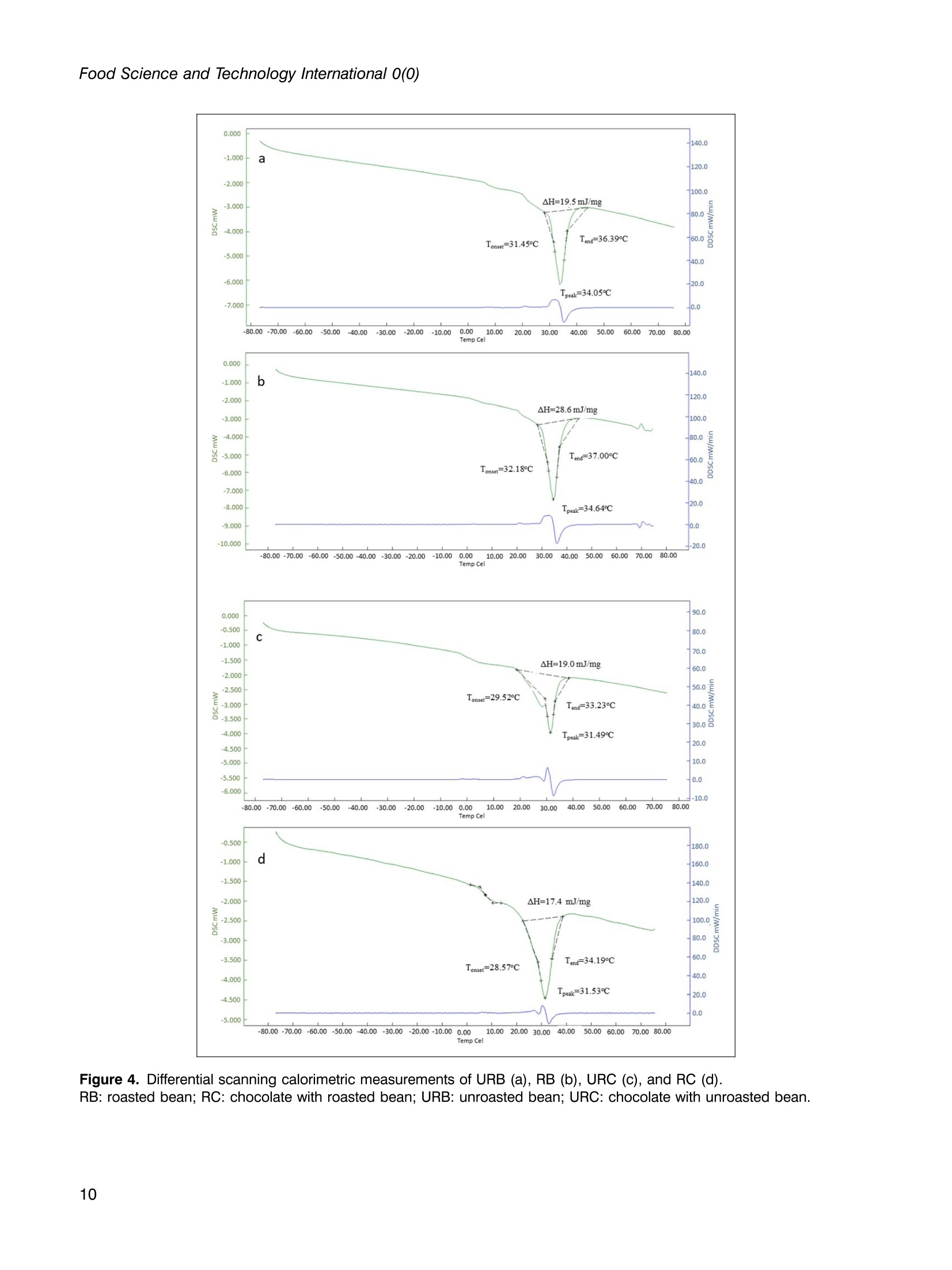
使用烤过和未烤过的可可豆生产的巧克力的氧化稳定性和酚类含量Phenolic content and oxidative stability of chocolates produced with roasted and unroasted cocoa beansTotal fat content:总脂肪含量:The total fat content of cocoa beans was determined using the Soxhlet procedure (AOAC, 1995). Fat was extracted with a fully automated extraction system (Gerhardt-Soxtherm, Germany) using petroleum ether as a solvent.可可豆总脂肪含量使用石油醚做为溶剂由格哈特全自动索氏提取系统-索克森Soxtherm提取。Check for updatesFOOD SCIENCE AND TECHNOLOGY INTERNATIONALOriginal Article Food Science and Technology International 0(0) Phenolic content and oxidative stability of chocolatesproduced with roasted and unroasted cocoa beans 使用烤过和未烤过的可可豆生产的巧克力的氧化稳定性和酚类含量 Inci CeritiDD, Omca Demirkol, Ayse Avci and Betul Sena Arkan Abstract The aim of this study was to produce chocolate using roasted (RB) and unroasted cocoa beans (URB). Theeffect of roasting on the total phenolic content (TPC), antioxidant activity [2,2-diphenyl-1-picrylhydrazyl (DPPH)scavenging activity, ferric reducing antioxidant power (FRAP), and cupric ion reducing antioxidant capacity(CUPRAC) values], phenolic compounds, caffeine, oxidative stability [free fatty acid, peroxide, conjugateddienes, conjugated trienes, and thiobarbituric acid reactive substances (TBARS)], Fourier transform infrared(FTIR), and differential scanning colorimetry (DSC) analysis of both cocoa beans and chocolate sampleswere analyzed. According to the results, the TPC of URB (24.96 mg gallic acid equivalent (GAE)/g sample)was higher than roasted beans (21.32 mg GAE/g sample). Similar results were also seen in the TPC of choc-olate samples. Although roasting did not affect the DPPH scavenging activity and caffeine content of cocoabeans, it decreased FRAP and CUPRAC values. (-)-Epicatechin and chlorogenic acid values were higher inunroasted bean and chocolate samples, but the amount of gallic acid increased with the roasting process.Free fatty acid, peroxide, conjugated dienes, conjugated trienes, and TBARS results of unroasted sampleswere lower than roasted ones, indicating better oxidative stability. The melting temperatures of cocoa beanschanged with roasting while it was similar between chocolate samples. Composition of the beans and thechocolate samples were qualitatively determined with FTIR. Keywords Chocolate, phenolics, antioxidants, roasting, oxidative stability INTRODUCTION Cocoa is the seed of the cocoa tree“Theobroma cacao L.”growing in tropical regions, especially in Africa and SouthAmerica (Campos-Vega et al., 2018). Cocoa is one of themost popular foods due to its unique properties, such as health-promoting effects and characteristic taste. It has been knownthat cocoa is rich in active secondary metabolites (polyphe-nols) and methylxanthines (theobromine and caffeine). Dueto high bioactive components, cocoa and chocolate havebeneficial effects on cholesterol levels, blood pressure, andpsychology (Acosta-Otalvaro et al., 2022; Rojas et al., 2022). Food Science and Technology International 0(0) 1-12 C The Author(s) 2023 Article reuse guidelines: sagepub.com/journals-permissions DOI: 10.1177/10820132231154429 journals.sagepub.com/home/fst QSAGE Cocoa beans undergo several processing steps until theyreach the consumer. After harvesting, the first stages are fer-mentation and drying of cocoa beans. Various microorgan-isms play a role to develop chocolate flavor precursorsduring the fermentation process, then the water content ofcocoa beans is decreased by drying (Rojas et al., 2022;Zyzelewicz et al., 2018). These preprocesses are necessaryto obtain good quality beans; however, roasting is one ofthe most important steps affecting the cocoa flavor.During roasting, complex chemical reactions, includingMaillard reactions, proteinndegradation,,and sugar Department of Food Engineering, Sakarya University, Esentepe,Sakarya, Turkey Corresponding author: Omca Demirkol, Department of Food Engineering, SakaryaUniversity, Faculty of Engineering, Esentepe, Sakarya 54187, Turkey. Email: omcad@sakarya.edu.tr caramelization occur, thus characteristic taste of cocoabeans is formed (Barisic et al., 2019; Rocha et al., 2017).In general, roasting temperature varies between 100℃and 150℃, depending on the processing type whichaffects the sensory properties and chemical compositionof the product (Pefia-Correa et al.,2022). It is known thathigh temperature causes the degradation of phenolic com-pounds and a substantial decrease in antioxidant activity.In addition to lipid oxidation during roasting, nonenzymaticbrowning reactions also lead to a loss in food quality due tothe formation of hydroxymethylfurfural and acrylamide,which are known to have harmful effects on health.Vitamin destruction and the reduction of protein digestibil-ity are the other results of the roasting process (Djikenget al., 2018; Garcia-Alamilla et al., 2017; Lambert et al.,2020). The chemical composition of fresh cocoa beans dependson the genotype, origin, and growth conditions, butunroasted cocoa beans (URB) contain about 32-39%water, 30-32% fat, 10-15% protein, and 5-6% polyphe-nols. The dominant flavonoids in cocoa beans are proantho-cyanins, catechins, flavan-3-ols, and anthocyanins. Otherbioactive compounds areePphenolicCacids,1flavones,phenols (clovamide and deoxyclovamide), flavonols (quer-cetin aglycone and its glycosides), alkaloids (theobromineandd caffeine),and hydroxylatedstilbene derivatives(Quiroz-Reyes and Fogliano,2018: Urbanska andKowalska, 2019). Besides their high antioxidant activity,bioactive compounds in cocoa beans possess antimicrobialeffects on bacteria and yeasts, thus they may protect thecocoa products from spoilage microorganisms (Zyzelewiczet al., 2018). On the other hand, these compounds are sen-sitive to thermal processing such as roasting and drying. Inliterature, studies have reported that the roasting causes theloss or modification of flavanols resulting in the reductionof the total phenolic content (TPC) (Fernandez-Romeroet al., 2020; Spizzirri et al., 2019). Chocolate is a product that is consumed especially forenjoyment and pleasure. It is consumed in its pure formor used in other products such as ice cream or beverages.The primary raw material for the production of chocolateis cocoa liquor which is obtained by grinding the roastedcocoa beans (RB) into cocoa nibs. In addition to cocoaliquor, chocolate contains sugar, cocoa fat, emulsifiers,and other ingredients mixed first and milled to obtain asmooth texture(Fernandez-Romeroet al.,2020).Conching is also essential for final flavor development.The final step in the production is tempering, which pro-vides high-quality products (Toker et al., 2019). Recently, there has been a trend toward natural food by agrowing number of consumers as raw food preserves nutri-ents better. Raw cocoa beans are subjected to only fermen-tation and ddrying, so they contain more phenoliccompounds..Roastingalso causesStheformation ofthermal process contaminants although not as high as in bread or coffee. Moreover, roasting leads to a significantincrease in the aldehydes which affects cocoa butterquality (Penia-Correa et al., 2022). All of these factorschange the quality of chocolate because cocoa is the mostimportant component of chocolate. Therefore, it wasthought that using unprocessed beans in a chocolaterecipe could give a higher quality product. Taking all thisinto account, in this work, the phenolic and oxidative stabil-ity of URB were determined and compared with the roastedones. Then, chocolate was produced with these cocoa beansunder laboratory conditions. In literature, there have beenseveral researches about the effect of roasting on thequality of cocoa beans, but in this study, antioxidantactivities, oxidative stabilities, and melting propertieswere examined together. TPC, antioxidant activities,phenolic compounds, caffeine, free fatty acid, peroxidevalue, conjugated dienes, conjugated trienes, and thio-barbituric acid reactive substances (TBARS) values ofcocoa beans and chocolate samples were investigated.Furthermore, structural variations between the samplesand melting properties were determined with FTIR anddifferentialscanningcolorimetry ((DSC))analysis,respectively. MATERIALS AND METHODS Materials Methanol, iron (II) sulfate heptahydrate, sodium carbon-ate, Folin-Ciocalteu phenol reagent, and gallic acid(≥98.0%) wereesupplied fromMerck(Darmstadt,Germany). (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-car-boxylic acid (Trolox,≥97%), 2,2-diphenyl-1-picrylhydrazyl(DPPH), copper (II) chloride, TPTZ (24,6-tripyridyl-s-triazine),iron (ⅢII) chloride hexahydrate, neocuproine, (-)- epicatechin(≥98%), chlorogenic acid (≥95%), caffeine, 2-thiobarbituricacid were purchased from Sigma (St Louis, MO, USA). RBURB were supplied from Altinmarka Inc (Istanbul, Turkey).Cocoa butter, milk fat, vanillin, and lecithin were suppliedfrom Elvan Group (Istanbul, Turkey). Total fat content Chocolate production The ingredients used for chocolate production are listed inTable 1. First, RB and URB were ground in a mortar andthen sieved to facilitate the thinning. Cocoa nibs andcocoa butter were liquified by the bain-marie method, and Ingredients Percentage Cocoa nib 46.00% Granulated sugar 41.60% Cocoa butter 9.00% Milk fat 3.00% Vanillin 0.03% Lecithin 0.40% then ingredients in powder form were added and mixedwith a mixer (Fakir-Mezza plus, Germany). The mixturewas ground in melanger (Santha, USA). After 6 h of thin-ning and couching, lecithin and vanillin were added to themass and mixed for more 30 min. In the last step, the choc-olate mass was cooled to 28℃, then heated to 32℃ andtempering was completed (Cerit et al., 2016). The finalproduct was cooled and samples were stored at 4℃ untilanalyzed. Preparation of the extracts for the determination ofphenolic content, antioxidant activities, and HPLCanalysis RB,URB, chocolate with roasted bean (RC), and chocolatewith unroasted cocoa bean (URC) were ground and defattedwith hexane before extraction. For this, approximately 4grams of sample weighed, 20 mL of hexane was addedand samples were shaken in the water bath for 20 min atroom temperature. Then, the samples were centrifuged for5 min (13,130x g). The supernatant was poured and theabove steps were repeated three times. Finally, defattedsamples were dried at room temperature for 24 h. Theextraction was applied according to Yigit et al. (2021).One gram of chocolate or 0.5 g of cocoa bean wasweighed into the tubes. Three milliliters of methanol:water (75:25, v/v) solution was added and homogenized(Wiggens D-130, Germany). The extracts were then putinto an ultrasonic bath (Bandelin Sonorex, RK 100H,Germany) for 15 min to increase the efficiency of theextraction and centrifuged (13,130× g, 4℃) for 10 min.Supernatants were separated, another 3 mL of methanol:water solution was added, and the above procedure wasrepeated. All the supernatants were collected in a tube,and the final volume was adjusted to 10 mL. Determination of TPC After the appropriate dilutions of the extracts were pre-pared, 0.1 mL of the sample was transferred into a smalltest tube. Then, 0.2 mL of Folin-Ciocalteu reagent and2 mL of distilled water were added. It was kept for 3 min,and then 1 mL of sodium carbonate (20%w/v) wasadded. The mixture was incubated for 1 hh at room temperature. Absorbance values were measured at 765nm wavelength using a UV-visible spectrophotometer(Shimadzu UV mini 1240, Japan) at the end of theperiod. Gallic acid was used as a standard, and a calibrationcurve was created with gallic acid solutions (0-1000 ppm;R²=0.9984). The amount of phenolic substance in thesample was given as mg gallic acid equivalent (GAE)/100g (Wojdylo et al.,2007). Determination of antioxidant activities The antioxidant activities of samples were given in threespectrophotometric methods: DPPH scavenging activity,ferric-reducing antioxidant power (FRAP), and cupric ionreducing antioxidant capacity (CUPRAC) assays. DPPHscavenging activity Was performedaccordingtBrand-Williams et al. (1995) with some modifications.Briefly, 200 uL of the prepared extract was mixed with3 mL of 0.051 mmol/L DPPH solution, followed by incuba-tion at room temperature for 30 min. The absorbance wasrecorded at 517 nm wavelength and the standard curvewas constructed using different concentration of standardTrolox solutions (0-50 ppm; R²=0.9983). The resultswere presented in mg Trolox equivalence per gram ofsample. FRAP values were determined using the method devel-oped by Benzie and Strain (1996). In a test tube, 0.1 mLof extract, 1.2 mL of distilled water, and 1.8 mL of FRAPreagent which was prepared by mixing 300 mmol/Lacetate buffer (pH 3.6), 10 mmol/L TPTZ, and 20 mmol/L FeCl3.6H2O at the ratio of 10:1:1 (v/v), were added andincubated at 37C for 15 min. The absorbance was deter-mined spectrophotometrically at 593 nm and the calibrationcurve was created with standard FeSO4 solutions (0-1000ppm;R’=0.9950). The results were given as mg FeSo4equivalent per gram of sample. CUPRAC assay was performed according to the methoddeveloped by Apak et al. (2004). One mL of each of 7.5×10-2 mol/L neocuproine (in 96% ethanol), 10-’mol/Lcopper(II) chloride (in distilled water), and ammoniumacetate buffer solution (pH 7.0))were mixed.Then.1.05 mL of distilled water and 0.5 mL of extract wereadded to give a final volume of 4.1 mL. Samples were incu-bated at room temperature for 1 h. The absorbance weredetermined spectrophotometrically at 450 nm, and the cali-bration curve was constructed with standard Trolox solu-tions (0-1000 ppm;R2=0.9921). The results were givenas mg Trolox equivalent per gram of sample. Determination of phenolic compounds andcaffeine content by HPLC Phenolic compounds, chlorogeniicc acid, anddccaffeinecontent of cocoa bean and chocolate samples were pre-dicted with the HPLC method described by Erol (2013). The extracts were filtered through a 0.45 um nylon filter andinjected into HPLC system including LC-20 AD Shimadzupumps, a CTO-10 ASVP column oven, and an SPD-M20Aphotodiode array (PDA) detector, a ShimadzuDGU-20A5R degasser and SLC-10 A VP system control-ler. The injection volume was 20 uL and the column usedwas a C18 reversed-phase Nova Select (250×4.6 mm ID,5pm). Orthophosphoric acid in water (A, 0.1%, w/v) andacetonitrile (B) were used for gradient elution and theelution profile was as follows: 8.0% B from 0 to 10 min,18.0% B to 57 min, and 24.0% B to 78 min and back to8.0% B until 88 min. The flow rate was adjusted to 1 mL/min. Phenolic compounds and caffeine in the sampleswere identified by comparing their retention times andUV spectra with those of their reference standards. Determination of oxidative changes Free fatty acid and peroxide values. The free fatty acidcontents and peroxide value of the oil samples were mea-sured according to the IUPAC (1987) and the officialmethod of AOCSOfficialMethod Cd8b-90(1989),respectively. Conjugated dienes and conjugated trienes values.The conjugated dienes and conjugated trienes values ofthe samples were measured according to Zyzelewicz et al.(2014). Approximately 0.1 g of butter was weighed and dis-solved in 10 mL isooctane. Then, the absorbance valueswere measured at 232 and 268 nm for conjugated dienesand conjugated trienes, respectively using a UV-visiblespectrophotometer (Shimadzu UV-1240, Tokyo, Japan). TBARS. To determine the effect of the roasting on the oxi-dative status of both chocolates and cocoa beans, TBARSassay was performed. Samples weighed (10 g) were homo-genized with 50 mL of distilled water for 2 min. The slurrywas poured into a Kjeldahl flask by washing it with47.5 mL of distilled water. Then, two to four drops of par-affin and 2.5 mL of 4M HCl were added. When 50 mL ofdistillate was collected, the distillation was ended. Fivemilliliters of the distillate and 5 mL of TBA solution (2.0mM, prepared in glacial acetic acid) were mixed in tightlyclosed glass tubes. The mixture was heated in a boilingwater bath for 35 min. After the tubes were cooled, absor-bances were measured at 538 nm. The calibration curvewas created with malondialdehyde solutions (0-1 mmol,R’=0.995). The TBARS values were calculated as mg ofmalondialdehyde (MDA) per kg of sample (Tarladgiset al., 1960; Yigit et al., 2021). Fourier transform infrared (FTIR) analysis FTIR spectroscopy analyses of the cocoa bean and thechocolate samples were performed on a Shimadzu IR spectrometer (Prestige 21, Nakagyo-Ku, Japan). Defattedsamples were crushed, and the spectra were obtained atroom temperature in the range of 4000 and 400 cm. Differential scanning calorimetry analysis Melting behavior of the cocoa and chocolate samples wasdetermined with differential scanning calorimetry analysis(Seiko, DSC 7020, Hitachi, Japan)) according to themethod reported by Toker et al. (2018). Samples wereplaced into aluminum pans and were hermetically sealed.Then, the samples were heated from -80 to 80℃ at 10°C/min in N2 stream. Statistical analysis The results are expressed as mean ± SD. The statistical ana-lyses were performed using SPSS (version 11.5, SPSS Inc.,USA). T-test (α=0.05) was applied to show the effect ofthe roasting. RESULTS AND DISCUSSION Chocolate was defined as“it shall contain not less than 35%total cocoa solids (on a dry matter basis), of which not lessthan 18% shall be cocoa butter and not less than 14%fat-free cocoa solids” (Codex Alimentarius, 2003). Theaverage fat content of RB and URB used in this studywas 37.00%. In this way, all requirements for chocolate def-inition were provided. TPCs of RB, URB, RC, and URC are shown in Table 2.URB (24.96 mg GAE/g) had higher TPC than RB (21.32mg GAE/g), significantly. In addition, the TPC of URC(13.90 mg GAE/g) was considerably higher than RC(11.10 mg GAE/g) due to raw material properties.Salvador et al. (2019) analyzed the TPC of cocoa beansduring production. They determined that the TPC of rawbeans was 64.1 mg GAE/g, while RB from the same pro-duction cycle had only about 10.4 mg GAE /g of product.In another study, Giiltekin-Ozgiiven et al. (2016) investi-gated the effect of roasting and conching temperatures onthe chocolate manufacturing process. They observed areduction in TPC and flavonoid content depending on roast-ing temperature. In the study of Zyzelewicz et al. (2018),the chocolates were produced with roasted and raw cocoabeans in various proportions, and the TPC of productswas investigated. CH25, CH50, and CH75 chocolates(contain 25%, 50%, and 75% cocoa liquor from rawbeans, respectively) contained the highest concentrationof total polyphenols. Although the TPC of CHO (100%cocoa liquor from RB) chocolate was determined as841.17 mg/100 g, the value for CH100 (100% cocoaliquor from raw beans) chocolate was detected as 1074.38mg/100 g. The decrease in polyphenol contents during theroasting process was attributed to nonenzymatic oxidation Table 2. Antioxidant activities and total phenolic contents (TPCs) of samples. Samples DPPH (mg Trolox/g) FRAP (mg FeSO4/g) CUPRAC (mg Trolox/g) TPC (mg GAE/g) RB 59.33±0.50 104.58±1.54 561.92±3.20 21.32±1.03 URB 60.36±0.35 109.61±6.06 615.52±8.80* 24.96±1.00* RC 1.65±0.26 3.32±0.26 197.76±0.65 11.10±0.41 URC 2.85±0.06* 4.49±0.09* 208.16±3.46* 13.90±0.54* 厂 0.96 0.95 0.97 一 Data are presented as mean and ±SD for the three samples (n=3). r values represent the correlation between total phenolic content and antioxidant activities. Statistical analyses were performed for cocoa beans and chocolates separately.*Shows the significant difference between samples (p< 0.05).CUPRAC: cupric ion reducing antioxidant capacity; DPPH: 2,2-diphenyl-1-picrylhydrazyl; GAE: gallic acid equivalent; FRAP: ferric reducingantioxidant power; RB: roasted bean; RC: chocolate with roasted bean; TPC: total phenolic content; URB: unroasted bean; URC: chocolatewith unroasted bean. of these compounds to quinones and further condensationof oxidized compounds with other components like poly-phenols or proteins (Zyzelewicz et al., 2018). Roastingdecreased the TPCs of cocoa beans by 14.6% in this study. Cocoa has strong antioxidant activity due to its highpolyphenol content. It can scavenge superoxidee andhydroxyl radicals and inhibit lipid peroxidation. In thisstudy, three different antioxidant capacity assays wereapplied to evaluate thee antioxidantCcapacity of thesamples, and the obtained results are demonstrated inTable 2. The DPPH scavenging activities of RB, URB,RC, and URC samples were 59.33, 60.36, 1.65, and 2.85mg Trolox/g, respectively. Roasting did not affect theDPPH value of cocoa beans. However, the URC samplehad higher DPPH value than the RC sample. The FRAPvalues of RB and URB samples were calculated as104.58 and 109.61 mg FeSO4/g samples, respectively.When the FRAP values of chocolate samples were com-pared, the product with URB had significantly higherresults. Lin et al. (2021) applied DPPH assay on cocoabeans and calculated the results in terms of EC5o values.They reported that roasting caused a significant reductionin the antioxidant capacity. CUPRAC values were alsoinvestigated to determine the antioxidant activity perform-ance of the samples. RB, URB, RC, and URC sampleshad 561.92, 615.52, 197.76, and 208.16 mg Trolox/g ofCUPRAC values, respectively. While the direct comparisonof these antioxidant values with other researches is difficultdue to differences in assay and extraction methodology,Maldonado-Mateus et al. (2021) reported a significantdecrease in the antioxidant capacity of roasted cocoapowders. It can be seen from the results that the roastingprocess negatively affected the antioxidant powers of bothcocoa beans and chocolate samples. To examine the rela-tionship between TPC and antioxidant activity, linearregression was performed, and the results are shown inTable 2. A significant positive correlation was detectedbetween phenolic content and DPPH scavenging activity,FRAP, and CUPRAC assays (r=0.96, 0.95, and 0.97, respectively). These results showed that phenolic sub-stances constitute an important source of antioxidant cap-acity in cocoa beans and their products. Oracz and Nebesny(2016) demonstrated a good correlation between TPC andABTS antioxidant activity (R’=0.989), DPPH antioxidantcapacity (R’=0.968), and FRAP assay (R²=0.778) in rawand roasted seeds of different cocoa cultivar that is in agree-ment with the results of this study. In this study, using HPLC with a UV detector, quantifi-cation of flavan-3-ol compounds, phenolic acids and alka-loids were performed in cocoa and chocolate samples.The contents of phenolic compoundss areshown inFigure 1. From the class of flavan-3-ols and phenolicacids, (-)-epicatechin, gallic acid, and chlorogenic acidwere identified and the sample of HPLC chromatogramsare shown in Figure 2. (-)-epicatechin content of URBwas found as 0.16 mg/g of defatted extract while it was0.13 mg/g in RB. In addition, 0.27 mg/g of chlorogenicacid and 0.08 mg/g of gallic acid were detected in URB. (--)-epicatechin and chlorogenic acid values were significantlyhigher, on the contrary, the amount of gallic acid was lowerin URB and URC samples (p<0.05). A number of studieshave shown that technological process conditions causechanges in phenolic compounds. Payne et al. (2010)showed that a distinct loss was detected in the content of (--)-epicatechin and (+)-catechin with drying, fermentation,and roasting of cocoa beans. However, when the beanswere roasted at higher than 70℃, catechin level in beansincreased significantly, which shows the importance of pro-cessing conditions on phenolic compounds. In the study ofSomporn et al. (2011), they found that the concentration ofchlorogenic acid reduced, but the values of syringic acid,p-coumaric acid, gallic acid, and sinapic acid in coffeeincreased under light roasted. They also reported that phen-olic compounds may vary according to their binding statusdue to the difference in their chemical structures. Therefore,the applied treatments may not affect the release of phenoliccompounds from a plant tissue in the same way. It can beseen from the results that the amount of gallic acid Figure 1. (-)-Epicatechin (a), gallic acid (b), chlorogenic acid (c), and caffeine (d) values of samples.Data are presented as mean and ±SD for the three samples (n=3). Statistical analyses were performed for cocoa beansand chocolates separately. *Shows the significant difference between samples (p<0.05). RB: roasted bean; RC: chocolate with roasted bean; URB: unroasted bean; URC: chocolate with unroasted bean increased with roasting while the TPC decreased. Thereason may be due to the results of TPC assay were givenas GAE, but it reflects the sum of all individual phenoliccompounds. In this study, epicatechin and chlorogenicacid decreased while gallic acid increased after the roastingprocess. Therefore, this event may cause a decrease in theTPC. In the course of the research, the content of caffeinewas also carried out. The concentration of this compoundin RB, URB, RC, and URC samples were 0.93, 0.89,0.44, and 0.39 mg/g, respectively. Alanon et al. (2016)examinedd commercialcchocolates1from theUnitedKingdom market and they reported caffeine content is inthe range of 0.05-0.31 mg/g, which is similar to theresults of this article. Roasting did not affect the caffeinecontent significantly (p>0.05). Cocoa beans have high lipid content that was reported as32-53% by weight in the literature (it was determined as37% in this study). Lipids react with oxygen ketones, alde-hydes, alcohols, acids, and hydrocarbons are produced atthe end of the reaction. Lipid oxidation occurs quickly during roasting because heat treatment causes more poros-ity in the cocoa beans (Rojas et al.,2022). Therefore, a sig-nificant increase in the primary and secondary oxidationstates can be seen as a result of roasting. Free fatty acid, per-oxide, conjugated dienes, conjugated trienes, and TBARSanalysesSVwereperformed11to evaluateethe oxidativechanges of cocoa and chocolate products. The results ofthese tests are given in Table 3. There was a significant dif-ference in the free fatty acid values of roasted and unroastedsamples (p<0.05). This might be attributed to the thermalhydrolysis of triglycerides in roasted samples. The peroxidevalue gives information on the primary oxidation state andno significant difference was observed between the perox-ide values of the samples. Conjugated dienes and trienesvalues are also an important indicator of the oxidativestatus of oils (Celebi et al., 2021). The statistical analysisdemonstrated that the effect of roasting on the conjugateddienes and trienes level of cocoa bean and chocolatesamples was significant. Secondary metabolites of lipid oxi-dation can assess with the thiobarbituric acid test. This Figure 2. Sample HPLC chromatograms of chocolate with unroasted bean (a) and chocolate with roasted bean (b).Gallic acid, caffeine, and epicatechin were detected at 270 nm. Chlorogenic acid was detected at 325 nm. 1 =gallic acid,2=chlorogenic acid, 3=caffeine, 4=epicatechin. Samples Free fatty acid (%) Peroxide (meq 02/kg) Conjugated dienes Conjugated trienes TBARS (mg MDA/kg) RB 0.82±0.00* 1.05±0.05 1.26±0.00* 0.23±0.00* 1.32±0.19* URB 0.63±0.03 0.98±0.01 1.10±0.01 0.16±0.01 1.01±0.03 RC 1.01±0.00* 1.08±0.00 1.65±0.02* 0.43±0.01* 1.19±0.16 URC 0.66±0.03 1.12±0.08 1.39±0.01 0.35±0.02 1.10±0.17 Data are presented as mean and ±SD for the three samples (n=3). Statistical analyses were performed for cocoa beans and chocolatesseparately. *Shows the significant difference between [samples] samples (p<0.05).RB: roasted bean; RC: chocolate with roasted bean; TBARS: thiobarbituric acid reactive substances; URB: unroasted bean; URC: chocolatewith unroasted bean. procedure determines MDA concentration, an abundantsecondary product of oxidation. TBARS analysis is asimple and quick technique in which a reaction occursbetween MDA and TBA. As a result of the reaction, theabsorbance of the red chromogen was detected (Yigitet al., 2021; Tarladgis et al., 1960). The results of theTBARS analysis are given in Table 3. The TBARSvalues of RB and URB were determined as1.32 mgMDA/kg and1.011 mg MDA/kg, respectively, whichshows that roasting significantly increased the amount ofmalondialdehyde (p<0.05). Moreover, URC had a lowerTBARS value than RC, but the difference was insignificant.The reason may be that the decomposition of primary oxi-dation products enhances and hydroperoxides are brokendown into malondialdehyde with heat (Womeni et al.,2016). These results are in accordance with those ofDjikeng et al. (2018), who investigated the effect of roast-ing on the physicochemical properties of fermented cocoabeans. They found that while the TBARS values of freshand dried cocoa beans were 0.8 ppm and 1.4 ppm, thevalues after roasting for 5 min and 10 min were 3.8 ppmand 4.1 ppm, respectively. They reported that roastingtime and temperature significantly increased the numberof secondary oxidation products. FTIR spectroscopy is a rapid, cost-effective, and non-destructive technique that can be used for identifying bio-logical samples by determining the presence of mainfunctional groups and chemical bonds (Palacio et al.,2014; Hospodarova et al., 2018). FTIR spectra of the defat-ted bean and the chocolate samples were determined inorder to detect the structural variations between thesamples. The spectra of URB and RB were almost identical;however, the transmittanceintensitiesweredifferent(Figure 3(a)). The URB sample had lower transmittancevalues than the RB samples which meant higher absorbancevalues (Indiarto et al., 2019). Thus this indicated that thenumber of functional groups decreased during the roastingprocess as a result of chemical changes. A wide peakaround 3000-3644 cm-was observed in all the samples,which were attributed to the stretching vibration of O-Hgroups in phenols, alcohols, polysaccharides, etc. (Batistaet al., 2016; Hospodarova et al., 2018). The peaks at 2923and 2851 cm corresponded to C-H stretching in the aromatic rings (Batista et al., 2016). The C=0 stretchingpeak was observed at around 1705cm-which was attributedto carboxylic acids, phenolic acids, flavonoids, aldehydes,and ketones (Grillo et al., 2019; Oracz and Zyzelewicz2019). The peaks around 1630 and 1549 cm-may be asso-ciated with C=0 stretch (amid I) and NH2 vibration (amidII) in proteins, respectively (Grillo et al., 2019). The regionfrom 1500 to 500 cm is the fingerprint region wherestrong absorption peaks were observed (Patle et al. 2020).The intense peak at 1023 cm- was due to the stretching ofC-O-C and C-O and it was attributed to the polysaccharidessuch as cellulose and hemicellulose (Wijaya, 2016; Oraczand Zyzelewicz, 2019). FTIR spectra of the defatted choc-olate samples produced from RB and URB are shown inFigure 3(b). In addition to the peaks obtained with beansamples, the characteristic peaks corresponding to sucrose(according to NIST standard reference database)wereobserved. DSC gives information about the thermal properties ofsolid materials such as glass transition temperature, enthalpyvalues, and melting temperature. It does not require extensivesample preparation and provides fast results (Leyva-Porraset al., 2019). In this study, the melting characteristics ofcocoa beans and chocolate samples were investigated andDSC curves are given in Figure 4. Onset temperature(Tonset), peak temperature (Tpeak), end temperature (Tend),and energy required for the complete melting of thesamples (AH) were calculated using thermograms. Tonsetand Tend values were found as 31.45℃, 32.18℃, and36.19℃, 37.00℃ for URB and RB, respectively Tonsetparameter representIsStthetemperatureatwhichthemelting begins. Tend refers to the temperature at whichthe complete liquefaction occurs. (Toker et al., 2018).Tonset and Tend values of RB were higher than those ofthe unroasted sample. In addition, Tpeak and AH valuesof URBand RBwere 34.05°℃ and 34.64°C and19.5 mJ/mg and 28.6 mJ/mg, respectively. It can be seenthat melting temperature (Tpeak) and AH values (amountof energy for full melting of fat) also increased by roast-ing. Ostrowska-Ligeza et al. (2021)) compared thethermal characteristics of raw and RB from two differentorigins. They found that while melting characteristicswere similar in one type of cocoa beans, they changed Figure 3. Fourier transform infrared (#FTIR) spectra of beans (a) and chocolate samples (b).RB: roasted bean; RC: chocolate with roasted bean; URB: unroasted bean; URC: chocolate with unroasted bean. with roasting in the other. They reported that long-chainsaturated fatty acids may cause higher melting points.Regarding the melting properties of chocolates, there were no great differences between Tpeak values of URCin comparison with RC samples. One of the reasonscould be that the multi-component nature of chocolate Q 名 8 名 E E Figure 4. Differential scanning calorimetric measurements of URB (a), RB (b), URC (C), and RC (d).RB: roasted bean; RC: chocolate with roasted bean; URB: unroasted bean; URC: chocolate with unroasted bean may depress the peak temperature and enthalpy values(Ioannidi et al.,2021). CONCLUSION In this study, RB and URB were used in chocolate produc-tion and the effect of roasting on TPC, antioxidant capaci-ties, bioactive compounds, and oxidative changes wereexamined. The results indicated that roasting decreasedthe TPCs of both cocoa beans and chocolate samples.Moreover, the antioxidant activities of cocoa beans werenegatively affected by the roasting process, and a high posi-tive correlation was detected between TPC and all antioxi-dant activity assays. It can be noted that roasting influencedthe content of phenolic compounds both positively andnegatively. DSC analysis showed that the melting tempera-ture of cocoa beans was affected by the roasting process.Specific peaks related to proteins, polysaccharides, and phe-nolics were determined with FTIR. It can be concluded thatroasting decreases the antioxidant capacities and increasesthe oxidation of metabolites. Therefore, chocolate produc-tion with URB may be increased on an industrial scaletaking into account the desire of people to reach raw andhealthy food. However, roasting may also affect otherquality parameters such as production of thermal processcontaminants or formation of volatile compounds that canbe further examined in future studies. DECLARATION OF CONFLICTING INTERESTS The author(s) declared no potential conflicts of interest with respect tothe research, authorship, and/or publication of this article. FUNDING The author(s) received no financial support for the research,authorship, and/or publication of this article. DATA AVAILABILITY STATEMENT All data generated or analyzed for this study are included in thispublished article. ETHICAL APPROVAL Ethical approval was not required for this research. ORCID iDInci Cerit https://orcid.org/0000-0002-3106-8951 REFERENCES Acosta-Otalvaro E, Dominguez-Perles R, Mazo-Rivas JC, et al.(2022) Bioavailability and radical scavenging power of phen-olic compounds of cocoa and coffee mixtures. Food Scienceand Technology International 28: 514-523. Alanion ME, Castle SM, Siswanto PJ, et al. (2016) Assessment offlavanol stereoisomers and caffeine and theobromine content incommercial chocolates. Food Chemistry 208: 177-184. AOAC (1995) Official methods of analytical chemist, 5th ed.Washington, DC: Association of Official Analytical Chemist. AOCS Official Method Cd 8b-90 (1989) Peroxide value aceticacid-isooctane method. In: Official methods and recommendedpractices of the American Oil Chemists’ Society. Champaign,Il: American Oil Chemists’Society. Apak R, Guelu K, Ozyiirek M, et al. (2004) Novel total antioxi-dant capacity index for dietary polyphenols and vitamins Cand E, using their cupric ion reducing capability in the presenceof neocuproine: CUPRAC method. Journal of Agriculturaland Food Chemistry 52(26): 7970-7981. Barisic V, Kopjar M, Jozinovic A, et al. (2019) The chemistrybehind chocolate production. Molecules 24: 17. Batista NN, de Andrade DP, Ramos CL, et al. (2016) Antioxidantcapacity of cocoa beans and chocolate assessed by FTIR. FoodResearch International 90:313-319. Benzie IFF and Strain JJ (1996) The Ferric Reducing Ability ofPlasma (FRAP) as a measure of “antioxidant power": TheFRAP assay. Analytical Biochemistry 239(1): 70-76. Brand-Williams W, Cuvelier ME and Berset C (1995) Use of afree radical method to evaluate antioxidant activity. LWT -Food Science and Technology 28(1):25-30. Campos-Vega R, Nieto-Figueroa KH and Oomah BD (2018)Cocoa (Theobroma cacao L.) pod husk: Renewable source ofbioactiveecompounds. Trends in Food ScienceeandTechnology 81: 172-184. Celebi K, Cerit I and Demirkol O (2021) Effect of hops oil on sun-flower oil thermal Stability. Progress In Nutrition 23(4):1-10.DOI: 10.23751/pn.v23i4.12087. Cerit I, Senkaya S, Tulukoglu B, et al. (2016) Enrichment of functionalproperties of white chocolates with cornelian cherry, spinach andpollen powders. Gida / the Journal of Food 41:311-316. Codex Alimentarius (2003) Standard for Chocolate and ChocolateProducts. International Food Standards 11: 87-1981. Djikeng FT, Teyomnou WT, Tenyang N, et al. (2018) Effect oftraditional and oven roasting on the physicochemical propertiesof fermented cocoa beans. Heliyon 4(2): e00533. DOI: 10.1016/J.HELIYON.2018.E00533. Erol NT (2013) Determination of phenolic compounds fromvarious extracts of green tea by HPLC. Asian Journal ofChemistry 25(7):3860-3862. Fernandez-Romero E, Chavez-Quintana SG, Siche R, et al. (2020)The kinetics of total phenolic content and monomericFlavan-3-ols during the roasting process of Criollo Cocoa.Antioxidants 9(2): 7-10. Garcia-Alamilla P, Lagunes-Galvez LM, Barajas-Fernandez J,et al. (2017) Physicochemical changes of cocoa beans duringroasting process. Journal of Food Quality 2017: 2969324.DOI: 10.1155/2017/2969324. Grillo G, Boffa L, Binello A, et al. (2019) Analytical dataset ofEcuadorian cocoa shells and beans. Data in Brief 22: 56-64. Gultekin-Ozgiiven M, Berktas I and Ozcelik B (2016) Influence ofprocessing conditions on procyanidin profiles and antioxidantcapacity of chocolates: Optimization of dark chocolate manu-facturing by response surface methodology. LWT ·FoodScience and Technology 66: 252-259. HospodarovaV, Singovszka E and Stevulova N((2018)Characterization of cellulosic fibers by FTIR spectroscopyfortheir further implementationto buildingmaterials.American Journal of Analytical Chemistry 9: 303-310. Indiarto R, Pranoto Y, Santoso U, et al. (2019) In vitro antioxidantactivity and profile of polyphenol compounds extracts and theirfractions on cacao beans. Pakistan Journal of BiologicalSciences 22: 34-44. Ioannidi E, Risbo J, Aarpe E, et al. (2021) Thermal analysis ofdark chocolate with differential scanning calorimetry—limita-tions in the quantitative evaluation of the crystalline state. FoodAnalytical Methods 14(12): 2556-2568. IUPAC (1987) Standard methods for the analysis of oils, fats andderivatives. Lambert J, Seymore T, Dai Q, et al. (2020) Effect of roasting andalkalization on the chemical composition and in vitro anti-inflammatory effects of cocoa. Current Developments inNutrition 4(Supplement_2): 421-421. Leyva-Porras C, Cruz-Alcantar P, Espinosa-Solis V, et al. (2019)Application of differential scanning calorimetry (DSC) andmodulated differential scanning calorimetry (MDSC) in foodand drug industries. Polymers 12(1):5. Lin YC, Choong YM and Chu HL (2021) Effects of processingtime and temperature on flavanol and procyanidin, proantho-cyanidin and antioxidant activity of cocoa bean in Taiwan.Journal of Food and Nutrition Research 9(1):10-17. Maldonado-Mateus LY, Perez-Burillo S, Lerma-Aguilera A, et al.(2021) Effect of roasting conditions on cocoa bioactivity andgut microbiota modulation. Food & Function 12(20):9680-9692. NIST standard reference database. 2007. Fructose. Available at:https://www.nist.gov/srd (accessed 10 December 2022). Oracz J and Nebesny E (2016) Antioxidant properties of cocoa beans(Theobroma cacao L.): Influence of cultivar and roasting conditions.International Journal of Food Properties 19(6): 1242-1258. Oracz J and Zyzelewicz D (2019) In vitro antioxidant activity andFTIR characterization of high-molecular weight melanoidinfractions from different types of cocoa beans. Antioxidants(Basel) 8(11): 60. Ostrowska-Ligeza E, Dolatowska-Zebrowska K, Wirkowska-WojdylaM, et al. (2021) Comparison of thermal characteristics and fattyacids composition in raw and roasted cocoa beans from Peru(Criollo) and Ecuador (Forastero). Applied Sciences 11(6): 2698. Palacio S, Aitkenhead M, Escudero A, et al. (2014) Gypsophilechemistry unveiled: Fourier transform infrared (FTIR) spec-troscopy provides new insight into plant adaptations togypsum soils. PLoS ONE 9(9): e107285. Patle TK, Shrivas K, Kurrey R, et al. (2020) Phytochemicalscreening and determination of phenolics and flavonoids inDillenia pentagyna using UV-vis and FTIR spectroscopy.Spectrochimica Acta Part A: Molecular and BiomolecularSpectroscopy 242: 118717.DOI:10.1016/j.saa.2020.118717. Payne MJ, Hurst WJ, Miller KB, et al. (2010) Impact of fermentation,drying, roasting, and Dutch processing on epicatechin and cat-echin content of cacao beans and cocoa ingredients. Journal ofAgricultural and Food Chemistry 58(19): 10518-10527. Pefia-Correa RF, Atac Mogol B and Fogliano V (2022) The impact ofroasting on cocoa quality parameters. Critical Reviews in FoodScience and Nutrition Early access:1-14. DOI: 10.1080/10408398.2022.2141191. Quiroz-Reyes CN and Fogliano V (2018) Design cocoa process-ing towards healthy cocoa products: The role of phenolicsand melanoidins. Journal of Functional Foods 45: 480-490. Rocha IS, De Santana LRR, Soares SE, et al. (2017) Effect of theroasting temperature and time of cocoa beans on the sensorycharacteristics and acceptability of chocolate. Food Scienceand Technology 37(4): 522-530. Rojas M, Hommes A, Heeres HJ, et al. (2022) PhysicochemicalPhenomena in the Roasting of Cocoa (Theobroma cacaoL.). Food Engineering Reviews 14(3): 509-533. DOI: 10.1007/s12393-021-09301-z. Salvador I, Massarioli AP, Silva APS, et al. (2019) Can we con-serve trans-resveratrol content and antioxidant activity duringindustrial production of chocolate? Journal of the Science ofFood and Agriculture 99(1): 83-89. Somporn C, Kamtuo A, Theerakulpisut P, et al. (2011) Effects ofroasting degree on radical scavenging activity, phenolics andvolatile compounds of Arabica coffee beans (coffea arabicaL. cv. Catimor). International Journal of Food Science &Technology 46(11):2287-2296. Spizzirri UG, Ieri F, Campo M, et al. (2019) Biogenic amines,phenolic, and aroma-related compounds of unroasted androasted cocoa beans with different origin. Foods (Basel,Switzerland) 8(8):06. Tarladgis BG, Watts BM, Younathan MT, et al. (1960) A distilla-tion method for the quantitative determination of malonalde-hyde in rancid foods. Journal of the American Oil ChemistsSociety 37(1): 44-48. Toker OS, Konar N, Palabiyik I, et al. (2018) Formulation of darkchocolate as a carrier to deliver eicosapentaenoic and docosa-hexaenoic acids: Effects on product quality. Food Chemistry254:224-231. Toker OS, Palabiyik I and Konar N (2019) Chocolate quality andconching. Trends in Food Science and Technology 91(April):446-453. Urbanska B and Kowalska J (2019) Comparison of the total poly-phenol content and antioxidant activity of chocolate obtainedfrom roasted and unroasted cocoa beans from differentregions of the world. Antioxidants 8(8):83. Wijaya WM (2016) The isolation of compound polyphenol fromwajo district cacao bean and cacao waste through fermentationprocess. Jurnal Sains Dasar 5(2):81-85. Wojdylo A, Oszmianski J and Czemerys R (2007) Antioxidantactivity and phenolic compounds in 32 selected herbs. FoodChemistry 105(3):940-949. Womeni HM, Djikeng FT, Anjaneyulu B, et al. (2016) Oxidativestabilization of RBD palm olein under forced storage condi-tions by old Cameroonian green tea leaves methanolicextract. NFS Journal 3: 33-40. Yigit GG, Cerit I and Demirkol O (2021) Oxidative stability ofcocoa hazelnut cream enriched with inactive yeast cells.Journal of Food Processing and Preservation 45(6):-9. Zyzelewicz D, Budryn G, Krysiak W, et al. (2014) Influence ofroasting conditions on fatty acid composition and oxidativechanges of cocoa butter extracted from cocoa bean ofForasterovariety cultivated in Togo. FoodResearchInternational 63: 328-343. Zyzelewicz D, Budryn G, Oracz J, et al. (2018) The effect on bio-active components and characteristics of chocolate by functio-nalization with raw cocoa beans. Food Research International113:234-244.
确定






还剩10页未读,是否继续阅读?
产品配置单
中国格哈特为您提供《巧克力总脂肪含量的检测》,该方案主要用于巧克力及制品中营养成分检测,参考标准--,《巧克力总脂肪含量的检测》用到的仪器有格哈特全自动超级总脂肪测定系统、格哈特快速干燥仪STL56、格哈特强力高重现振荡器LS500/RO500、德国加液器MM、玻璃滤筒、滤纸筒
相关方案
更多
该厂商其他方案
更多














