方案详情
文
Because it offers sensitive fluorescence measurements, full system control with our exclusive FluorEs-sence™ software, and modular construction, the Fluorolog spectrofluorometer is ideal for kinetic fluorescence determinations. The calibration procedure described herein can be applied readily to reaction-rate determinations® with your Fluorolog system.
方案详情

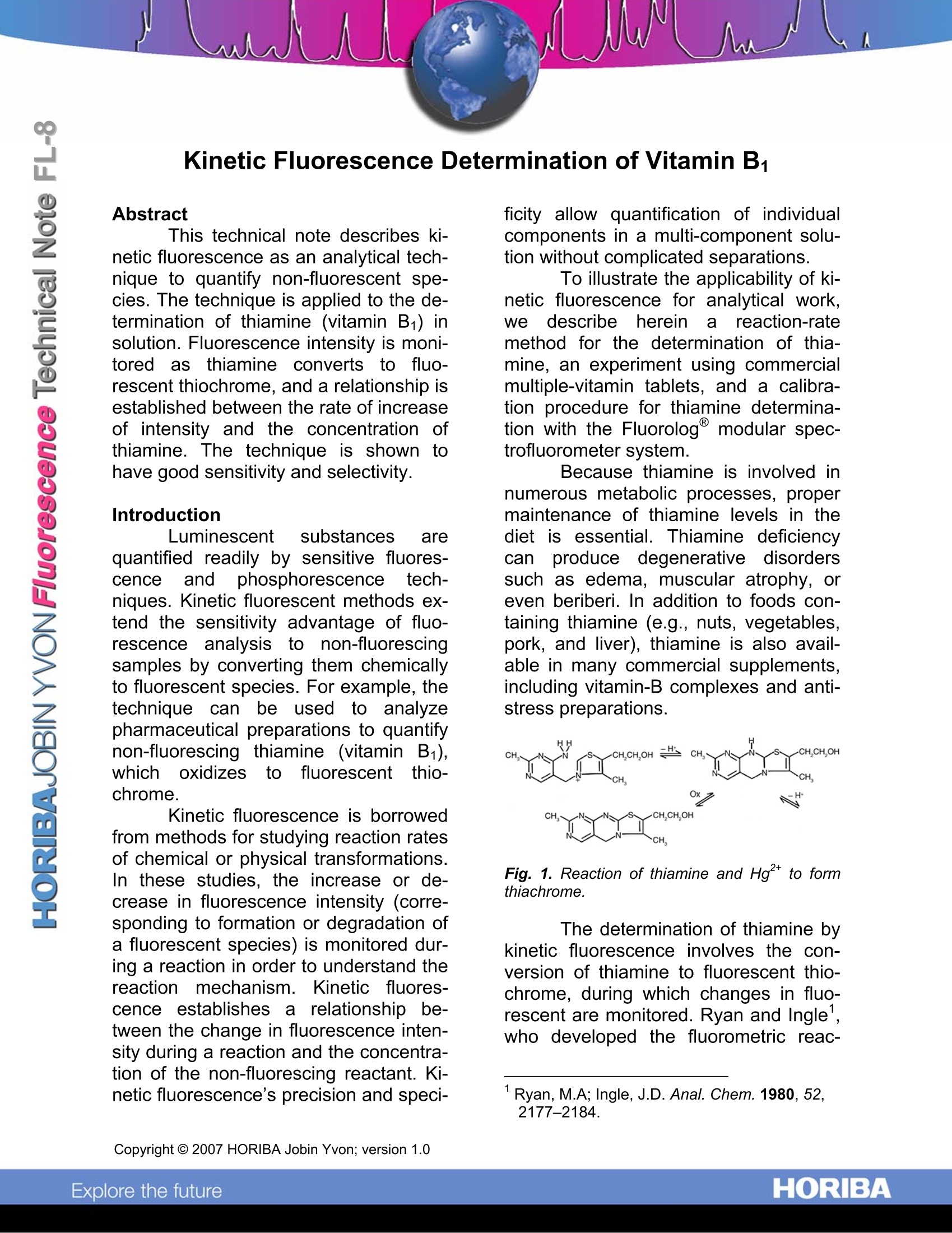
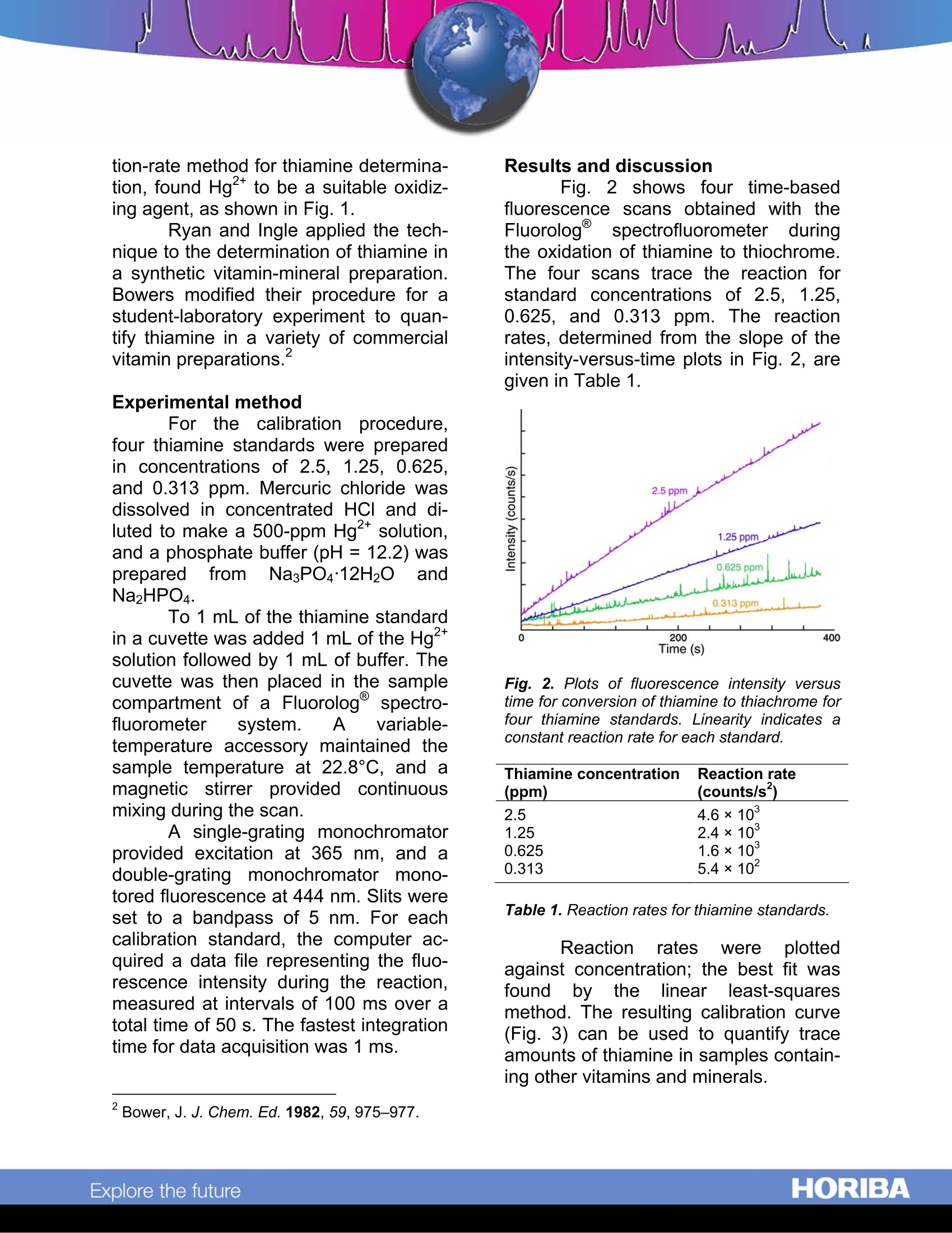
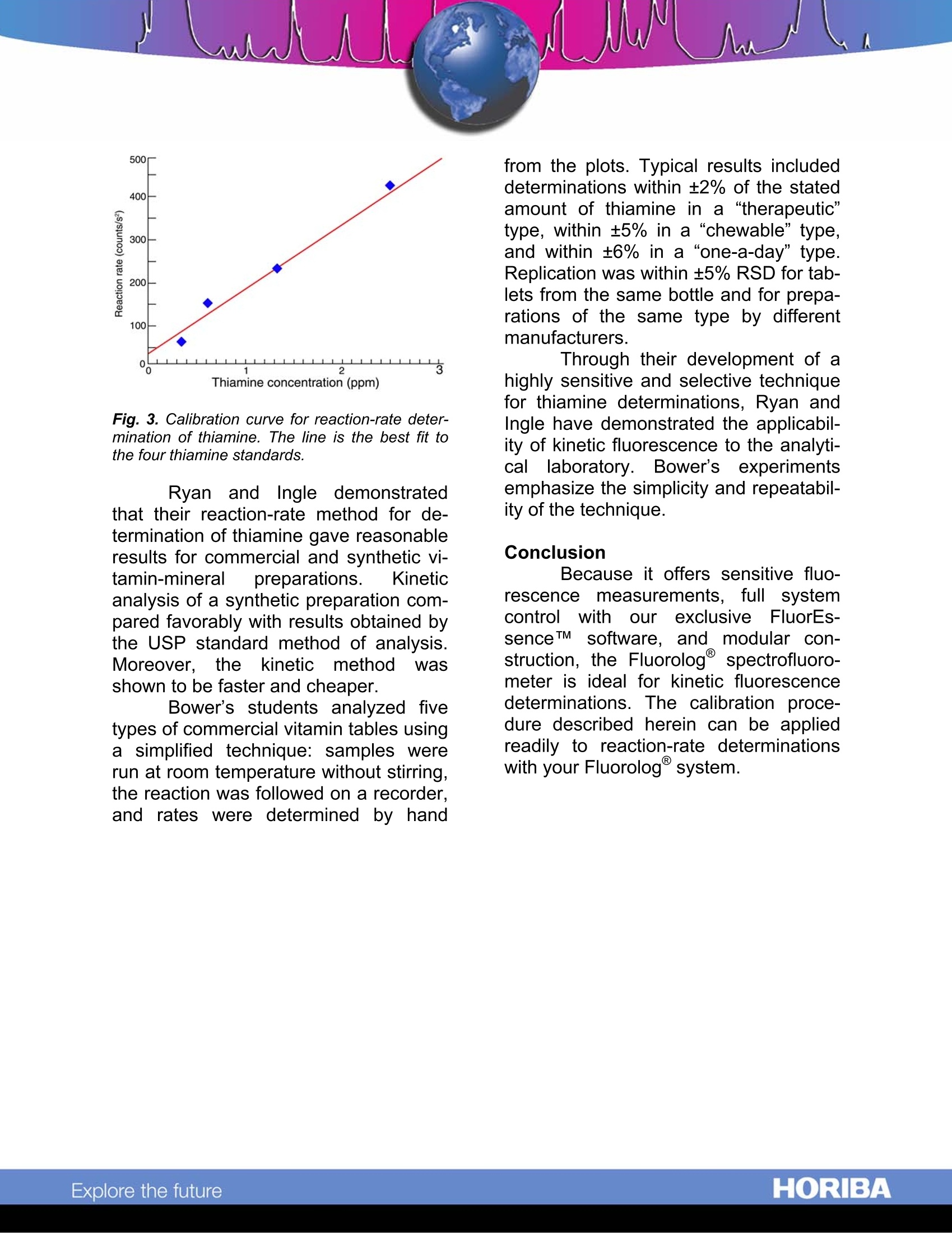
Kinetic Fluorescence Determination of Vitamin B. Abstract This technical note describes ki-netic fluorescence as an analytical tech-nique to quantify non-fluorescent spe-cies. The technique is applied to the de-termination of thiamine (vitamin B1) insolution. Fluorescence intensity is moni-tored asthiamine converts to fluo-rescent thiochrome, and a relationship isestablished between the rate of increaseof intensity and the concentration ofthiamine. The technique is shown tohave good sensitivity and selectivity. Introduction Luminescent substances arequantified readily by sensitive fluores-cenceand phosphorescence tech-niques. Kinetic fluorescent methods ex-tend the sensitivity advantage of fluo-rescence analysiss to non-fluorescingsamples by converting them chemicallyto fluorescent species. For example, thetechnique can be used to analyzepharmaceutical preparations to quantifynon-fluorescingthiamine (vitamin B1),whichoxidizestofluorescent thio-chrome. Kinetic fluorescence is borrowedfrom methods for studying reaction ratesof chemical or physical transformations.In these studies, the increase or de-crease in fluorescence intensity (corre-sponding to formation or degradation ofa fluorescent species) is monitored dur-ing a reaction in order to understand thereactionmechanism.Kinetic fluores-cence establishes a relationship be-tween the change in fluorescence inten-sity during a reaction and the concentra-tion of the non-fluorescing reactant. Ki-netic fluorescence's precision and speci- ficity allow quantification of individualcomponents in a multi-component solu-tion without complicated separations. To illustrate the applicability of ki-netic fluorescence for analytical work,we: describeeihereinareaction-ratemethod for the determination of thia-mine, an experiment using commercialmultiple-vitamin tablets, and a calibra-tion procedure for thiamine determina-tion with the Fluorolog@ modular spec-trofluorometer system. Because thiamine is involved innumerous metabolic processes, propermaintenance of thiamine levels in thediet is essential. Thiamine deficiencycan produce degenerative disorderssuch as edema, muscular atrophy, oreven beriberi. In addition to foods con-taining thiamine (e.g., nuts, vegetables,pork, and liver), thiamine is also avail-able in many commercial supplements,including vitamin-B complexes and anti-stress preparations. Fig.1. Reaction of thiamine and Hg2+ to formthiachrome. The determination of thiamine bykinetic fluorescence involves the con-version of thiamine to fluorescent thio-chrome, during which changes in fluo-rescent are monitored. Ryan and Ingle',who developed the fluorometric reac- ( ' Ryan, M.A; Ingle, J.D. Anal. Chem. 1 980, 52, 2177-2184. ) tion-rate method for thiamine determina-tion, found Hg2* to be a suitable oxidiz-ing agent, as shown in Fig. 1. Ryan and Ingle applied the tech-nique to the determination of thiamine ina synthetic vitamin-mineral preparation.Bowers modified their procedure for astudent-laboratory experiment to quan-tify thiamine in a variety of commercialvitamin preparations. Experimental method For the calibration procedure,four thiamine standards were preparedin concentrationnss ooff 22..55., 1.25, 0.625,and 0.313 ppm. Mercuric chloride wasdissolved in concentrated HCl and di-luted to make a 500-ppm Hg2+ solution,and a phosphate buffer (pH= 12.2) wasprepared tfrom Na3PO412H2OandNa2HPO4. To 1 mL of the thiamine standardin a cuvette was added 1 mL of the Hgsolution followed by 1 mL of buffer. Thecuvette was then placed in the samplecompartment of a Fluorolog spectro-fluorometer system. A variable-temperature accessory maintained thesample temperature at 22.8℃, and amagnetic stirrer pprovided continuousmixing during the scan. A single-grating monochromatorprovided excitation at 365 nm, and adouble-grating monochromator 0-tored fluorescence at 444 nm. Slits wereset to a bandpass of 5 nm. For eachcalibration standard, the computer ac-quired a data file representing the fluo-rescence intensity during the reaction,measured at intervals of 100 ms over atotal time of 50 s. The fastest integrationtime for data acquisition was 1 ms. Results and discussion Fig. 2 shows four time-basedfluorescence scans obtained with theFluorologspectrofluorometer duringthe oxidation of thiamine to thiochrome.The four scans trace the reaction forstandard concentrations of 2.5.1.25,0.625, and 0.313 ppm. The reactionrates, determined from the slope of theintensity-versus-time plots in Fig. 2, areqiven in Table 1. Fig. 2. Plots of fluorescence intensity versustime for conversion of thiamine to thiachrome forfour thiamine standards. Linearity indicates aconstant reaction rate for each standard. Thiamine concentration Reaction rate (ppm) (counts/s) 2.5 4.6×10° 1.25 2.4×10° 0.625 1.6×10° 0.313 5.4×10 Table 1. Reaction rates for thiamine standards. Reaction rates were plottedagainst concentration; the best fit wasfound bythe linear least-squaresmethod. The resulting calibration curve(Fig. 3) can be used to quantify traceamounts of thiamine in samples contain-ing other vitamins and minerals. Fig. 3. Calibration curve for reaction-rate deter-mination of thiamine. The line is the best fit tothe four thiamine standards. Ryan and Ingle demonstratedthat their reaction-rate method for de-termination of thiamine gave reasonableresults for commercial and synthetic vi-tamin-mineral preparations. Kineticanalysis of a synthetic preparation com-pared favorably with results obtained bythe USP standard method of analysis.Moreover.,1the kinetic method Wasshown to be faster and cheaper. Bower’s students analyzed fivetypes of commercial vitamin tables usinga simplified technique: samples were......run at room temperature without stirring,the reaction was followed on a recorder.and rates were determined by hand from the plots. Typical results includeddeterminations within ±2% of the statedamount of thiamine in a “therapeutic”type, within ±5% in a “chewable”type,and within ±6% in a “one-a-day”type.Replication was within ±5% RSD for tab-lets from the same bottle and for prepa-rations of the same type by differentmanufacturers. Through their development of ahighly sensitive and selective techniquefor thiamine determinations, Ryan andIngle have demonstrated the applicabil-ity of kinetic fluorescence to the analyti-cal laboratory. Bower’s experimentsemphasize the simplicity and repeatabil-ity of the technique. Conclusion Because it offers sensitive fluo-rescence measurements.full systemcontroll with our exclusive FluorEs-senceTM software, and modular con-struction, the Fluorologspectrofluoro-meter is ideal for kinetic fluorescencedeterminations. The calibration proce-dure described herein can be appliedreadily to reaction-rate determinationswith your Fluorolog@system. ( USA: H ORIBA Jobin Yvon In c ., 3 8 80 Park Avenue, Edison, NJ 08 8 20-3012, Toll-Free:+1 - 866-jobinyvon Tel: +1-732-494-8660, F ax: + 1 - 732-549-5125, E - mail: info@jobinyvon.com,www.jobinyvon.com France: H ORIBA Jobin Yvon S . A . S., 1 6-18, rue du Canal , 9116 5 Longjumeau Cedex, ) ( T el: +33 ( 0) 1 6 4 54 1 3 0 0 , Fax: + 33 (0) 1 69 09 9 3 1 9 , w w w.jobinyvon.fr Japan: H ORIBA Ltd., J Y Optical Sales Dept, Hig a s h i-Kanda, Daij i Building, 1-7-8 Higashi-Kanda ) ( Chiyoda-ku, T okyo 1 0 1-0031, T e l: +81 (0) 3 3861 8231, www.jyhoriba.jp Germany: +49(0)89462317-0 I taly:+39 0 2 57603050 U K:+44 (0) 20 8204 8142 China: ) Copyright C HORIBA Jobin Yvon; version .HORIBAExplore the future HORIBAExplore the future Because it offers sensitive fluorescence measurements, full system control with our exclusive FluorEs-sence™ software, and modular construction, the Fluorolog spectrofluorometer is ideal for kinetic fluorescence determinations. The calibration procedure described herein can be applied readily to reaction-rate determinations® with your Fluorolog system.
确定

还剩2页未读,是否继续阅读?
HORIBA(中国)为您提供《合成维生素矿物质制剂,商业维生素制剂中维他命B1检测方案(分子荧光光谱)》,该方案主要用于化药制剂中特殊物质和基团检测,参考标准--,《合成维生素矿物质制剂,商业维生素制剂中维他命B1检测方案(分子荧光光谱)》用到的仪器有HORIBA Fluorolog®-3科研级荧光光谱仪
相关方案
更多
该厂商其他方案
更多










