
方案详情
文
An important point with such a sample transition is thus the inclusion of a fast and precise autofocus system (such as the LabRAM Piezo 100), so that the sample surface will remain in the sampling position throughout an experiment and provide optimum spectroscopic results throughout.
So, but the use of a vapour pressure cell, it is possible through the application of Raman microscopy to follow the gain and loss of water molecules within a crystal structure.
In this instance for the morphine salt it was a repeatable and reversible transition.
方案详情

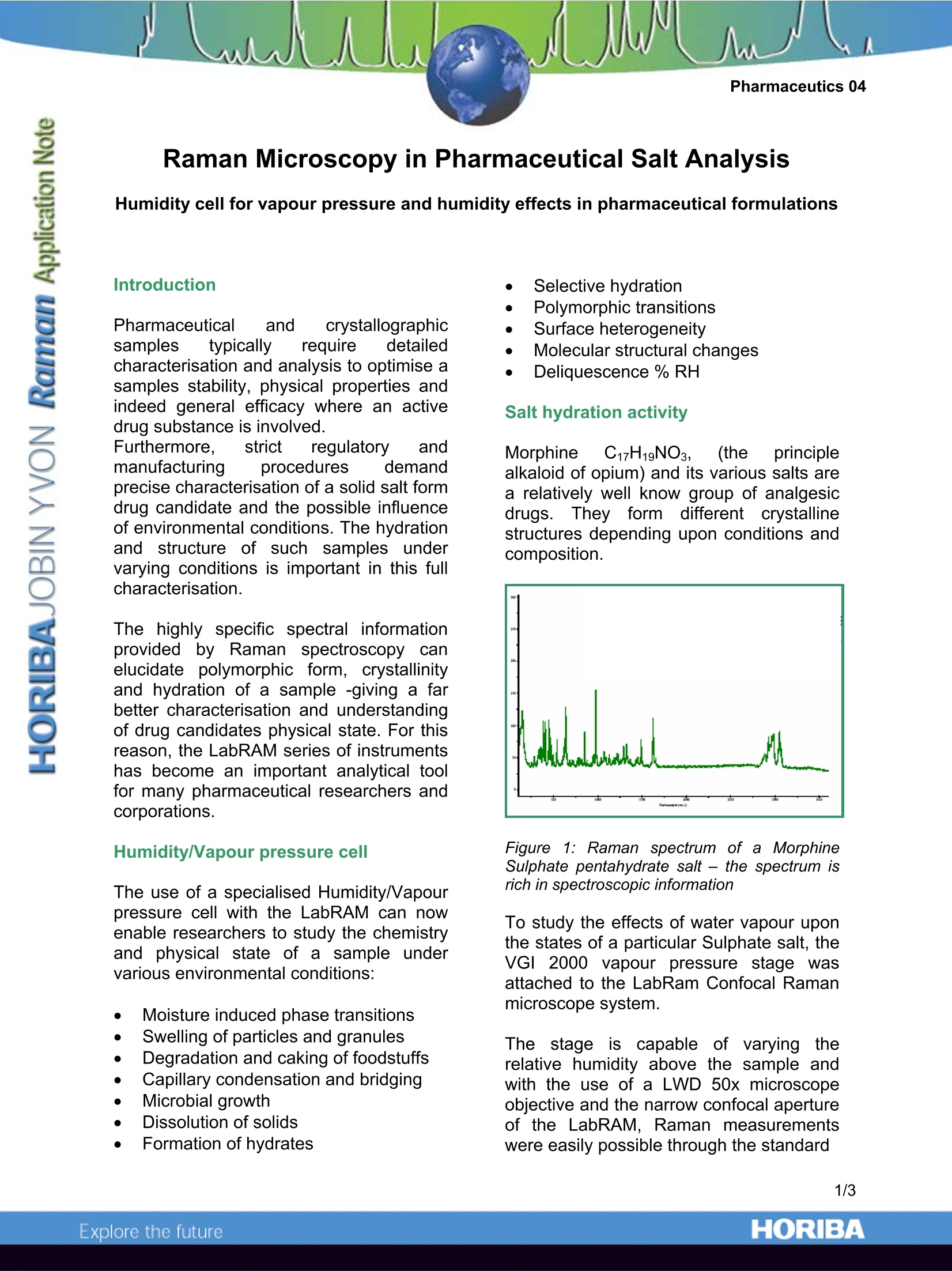
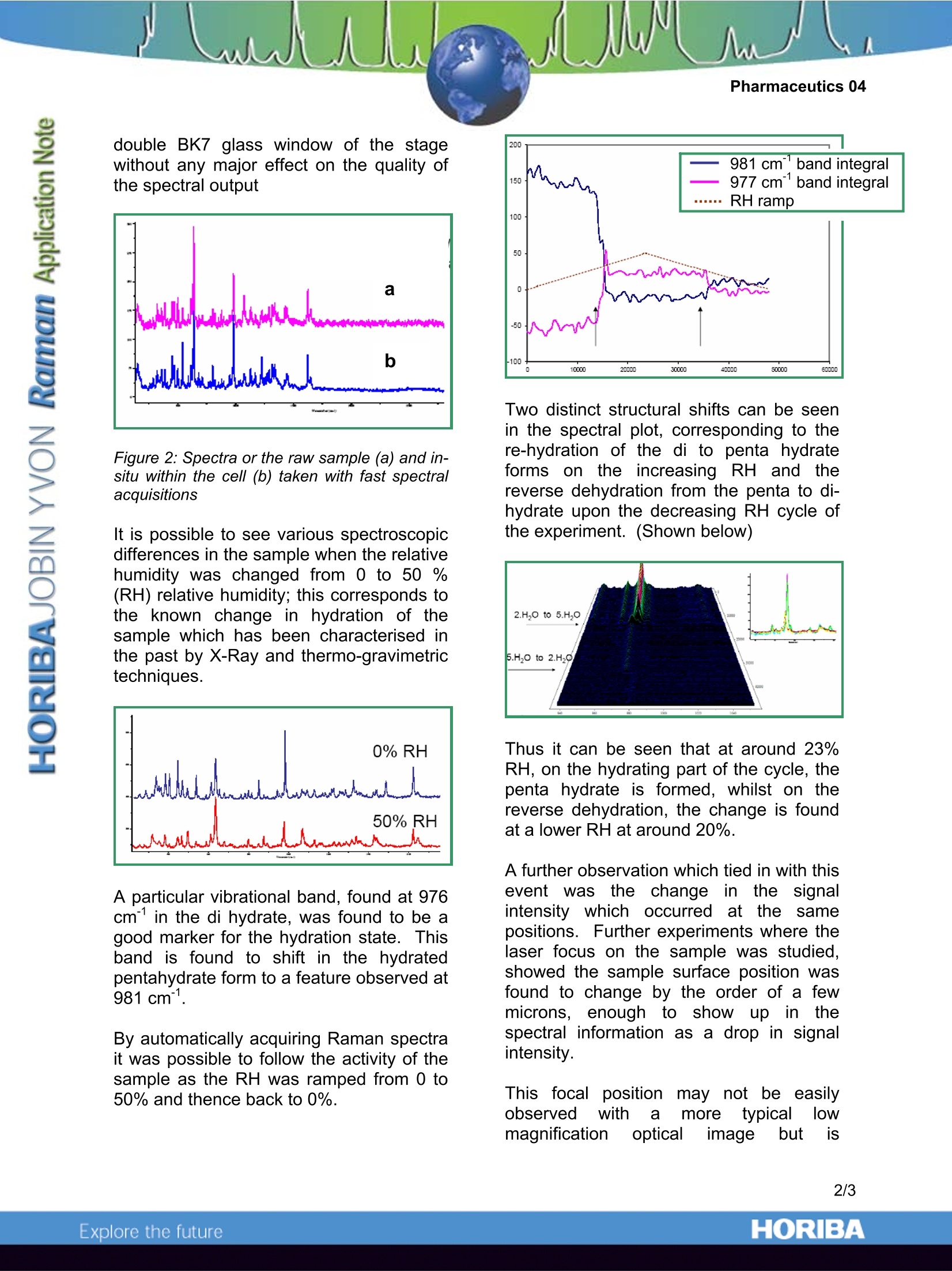
Pharmaceutics 04 Raman Microscopy in Pharmaceutical Salt Analysis Humidity cell for vapour pressure and humidity effects in pharmaceutical formulations Introduction Pharmaceutical and crystallographicsamples typically irequire dcetailedcharacterisation and analysis to optimise asamples stability, physical properties andindeed general efficacy where an activedrug substance is involved. Furthermore. strict regulatory andmanufacturing procedures cdemandprecise characterisation of a solid salt formdrug candidate and the possible influenceof environmental conditions. The hydrationand structure of such samples undervarying conditions is important in this fullcharacterisation. The highlyspecific spectral informationprovided by Raman spectroscopy carelucidate polymorphic form, crystallinityand hydration of a sample-giving a farbetter characterisation and understandingof drug candidates physical state. For thisreason, the LabRAM series of instrumentshas become an important analytical toolfor many pharmaceutical researchers andcorporations. Humidity/Vapour pressure cell The use of a specialised Humidity/Vapourpressure cell with the LabRAM can nowenable researchers to study the chemistryand physical state of a sample undervarious environmental conditions: Moisture induced phase transitions Swelling of particles and granules Degradation and caking of foodstuffs Capillary condensation and bridging Microbial growth Dissolution of solids Formation of hydrates Selective hydration FPolymorphic transitions Surface heterogeneity Molecular structural changes Deliquescence % RH Salt hydration activity Morphinee CC17H19NO3, (the principlealkaloid of opium) and its various salts area relatively well know qroup of analgesicdrugsThey form different crystallinestructures depending upon conditions andcomposition. Figure 11: Raman spectrum of a MorphineSulphate pentahydrate salt - the spectrum isrich in spectroscopic information To study the effects of water vapour uponthe states of a particular Sulphate salt, theVGI 2000 vapour pressure stage wasattached to the LabRam Confocal Ramanmicroscope system. The stage is capable oof varying therelative humidity above the sample andwith the use of a LWD 50x microscopeobjective and the narrow confocal apertureof the LabRAM, Raman measurementswere easily possible through the standard double BK7 glass window of the stagewithout any major effect on the quality ofthe spectral output Figure 2: Spectra or the raw sample (a) and in-situ within the cell (b) taken with fast spectralacquisitions It is possible to see various spectroscopicdifferences in the sample when the relativehumidity was changed ffram A trrom 0 to 50 %(RH) relative humidity; this corresponds tothe known change in hydration of thesample which has been characterised inthe past by X-Ray and thermo-gravimetrictechniques. A particular vibrational band, found at 976cm in the di hydrate, was found to be agood marker for the hydration state. Thisband is found to shift in the hydratedpentahydrate form to a feature observed at981cm. By automatically acquiring Raman spectrait was possible to follow the activity of thesample as the RH was ramped from 0 to50% and thence back to 0%. Two distinct structural shifts can be seenin the spectral plot, corresponding to there-hydration of the di to penta hydrateforms on the increasing RH and thereverse dehydration from the penta to di-hydrate upon the decreasing RH cycle ofthe experiment. (Shown below) Thus it can be seen that at around 23%RH, on the hydrating part of the cycle, thepenta hydrate is formed, whilst on thereverse dehydration, the change is foundat a lower RH at around 20%. A further observation which tied in with thisevent wasthe change iinnthe signalintensity which occurred atthe samepositions. Further experiments where thelaser focus on the sample was studied,showed the sample surface position wasfound to change by the order of a fewmicrons, enoughhto showup in thespectral information as a drop in signalintensity. This focalpositionmay not be easilyobserved with amoretypical lowmagnification optical image but is reproducibly seenonthelaser focusimage and in the spectral data. It providesyet further evidence of a physical transitionin the sample. An important point with such a sampletransition is thus the inclusion of a fast andprecise autofocus system (such as theLabRAM Piezo 100), so that the samplesurface will remainin the samplingposition throughout an experiment andprovide optimumspectroscopic resultsthroughout. So, but the use of a vapour pressure cell,it is possible through the application ofRaman microscopy to follow the gain andloss of water molecules within a crystalstructure. In this instance for the morphine salt it wasa repeatable and reversible transition. ORIBAExplore the future ORIBAExplore the future An important point with such a sample transition is thus the inclusion of a fast and precise autofocus system (such as the LabRAM Piezo 100), so that the sample surface will remain in the sampling position throughout an experiment and provide optimum spectroscopic results throughout.So, but the use of a vapour pressure cell, it is possible through the application of Raman microscopy to follow the gain and loss of water molecules within a crystal structure.In this instance for the morphine salt it was a repeatable and reversible transition.
确定

还剩1页未读,是否继续阅读?
HORIBA(中国)为您提供《盐类药物中主要成分含量检测方案(激光拉曼光谱)》,该方案主要用于原料药中含量测定检测,参考标准--,《盐类药物中主要成分含量检测方案(激光拉曼光谱)》用到的仪器有
相关方案
更多
该厂商其他方案
更多








