
方案详情
文
在一篇ELSEVIER发表的题为“SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy”的文章中,首次提出了新冠病毒能通过物体表面及空气进行传播的理论,这对患者及医护人员的保护和公共卫生管理都具有深远意义。该研究结果进一步佐证了使用赛多利斯Airport MD8空气气溶胶采样器 有效采集监测新冠病毒的重要性,这对深入了解病毒传播途径及规避污染风险有重要作用。
方案详情

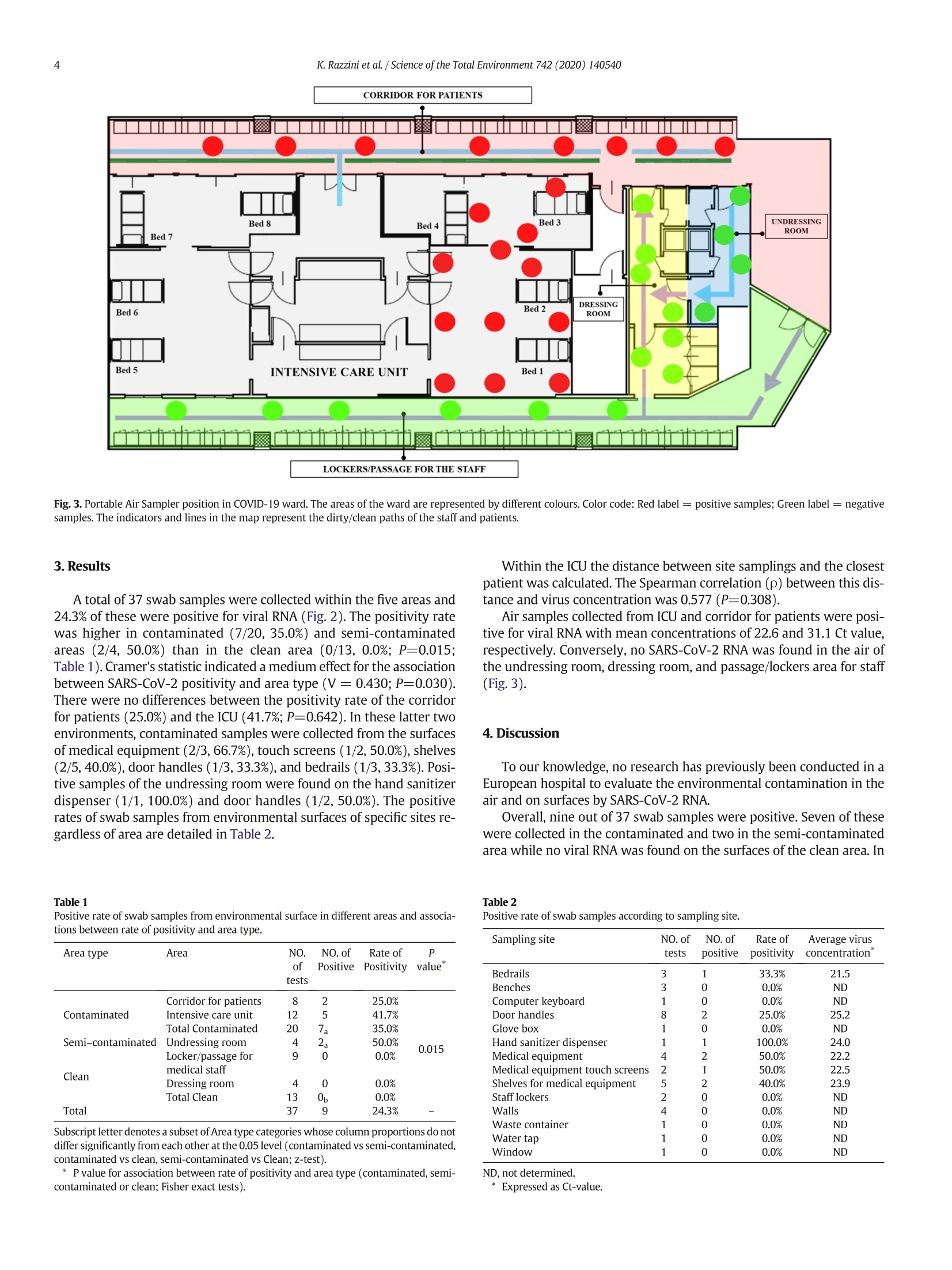
Science of the Total Environment 742(2020)140540 K. Razzini et al. /Science of the Total Environment 742 (2020) 1405402areas, but the results also support the need for strict disinfection, hand hygiene and protective measures forhealthcare workers as well as the need for airborne isolation precautions.◎ 2020 Elsevier B.V. All rights reserved. https://doi.org/10.1016/j.scitotenv.2020.140540 Contents lists available at ScienceDirectScience of the Total Environment journalhomepage: www.elsevier.com/locate/scitotenv SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19ward of a hospital in Milan, Italy Katia Razzini a·l, Marta Castrica b.*,l, Laura Menchetti C*,l, Lorenzo Maggi , Lucia Negroni , Nicola V. Orfeo,Alice Pizzoccheri, Matteo Stocco, Stefano Muttini, Claudia M.Balzarettib Saints Paolo and Carlo Hospital, Via Antonio di Rudini, 8,20142 Milan, Italy Department of Health, Animal Science and Food Safety “Carlo Cantoni",University of Milan, Via Celoria, 10, 20133 Milan, ItalyDepartment of Veterinary Medicine, University of Perugia, Via San Costanzo, 4, 06126 Perugia, Italy " Chemservice S.r.l.-Lab Analysis Group, Via F. Ili Beltrami, 15, 20026 Novate Milanese, Milan, Italy HIGHLIGHT S ·Air and surface samples were collectedin the COVID-19 ward of an Italian hos-pital. ·SARS-CoV-2 RNA detection was per-formed using RT-PCR. · Positive swab samples were found inthe semi-contaminated and contami-nated areas. ·Viral RNA was found in the air of inten-sive care unit and corridor for patients.·No positive samples were found in theclean areas of the ward. ARTIC L E INF O Article history: Received 27 May 2020 Received in revised form 24 June 2020 Accepted 24 June 2020 Available online 26 June 2020 Editor: Damia Barcelo Keywords:COVID-19CoronavirusOutbreakAirborne transmissionEnvironmental contaminationInfection control ABSTRACT The COVID-19 outbreak has rapidly progressed worldwide finding the health system, scientists and society un-prepared to face a little-known, fast spreading,and extremely deadly virus. Italy is one of the countries hardesthit by the pandemic, resulting in healthcare facilities bearing heavy burdens and severe restrictive measures.De-spite efforts to clarify the virus transmission, especially in indoor scenarios, several aspects of SARS-CoV-2 spreadare still rudimentary. This study evaluated the contamination of the air and surfaces by SARS-CoV-2 RNA in theCOVID-19 isolation ward of a hospital in Milan, Italy. A total of 42 air and surface samples were collected insidefive different zones of the ward including contaminated (COVID-19 patients' area), semi-contaminated(undressing room), and clean areas. SARS-CoV-2 RNA detection was performed using real time reverse transcrip-tion polymerase chain reaction. Overall, 24.3%of swab samples were positive, but none of these were collected inthe clean area. Thus, the positivity rate was higher in contaminated(35.0%) and semi-contaminated (50.0%) areasthan in clean areas (0.0%; P<0.05). The most contaminated surfaces were hand sanitizer dispensers (100.0%),medical equipment (50.0%), medical equipment touch screens (50.0%), shelves for medical equipment (40.0%),bedrails (33.3%), and door handles (25.0%). All the air samples collected from the contaminated area, namelythe intensive care unit and corridor,were positive while viral RNA was not detected in either semi-contaminated or clean areas. These results showed that environmental contamination did not involve clean 1. Introduction The novel coronavirus, named Severe Acute Respiratory SyndromeCoravirus 2(SARS-CoV-2),has rapidly progressed worldwide, and theimpact on health systems, science, and society is unprecedented (Torriand Nollo, 2020). The chronology of COVID-19 infections is as follows:On 31 December 2019 the coronavirus disease was first reported as acluster of pneumonia cases of unknown etiology by the Wuhan Munic-ipal Health Commission in Wuhan City, Hubei Province China (WHO,2020a).Subsequently, on 7 January 2020, Chinese authorities confirmedthat they had identified a novel virus belonging to the same family ofcoronaviruses as Severe Acute Respiratory Syndrome (SARS). Then on11 February 2020 the World Health Organization (WHO) announced aname for the new coronavirus disease: COVID-19. Due to the globalspread of COVID-19 various international concerns declared a State ofEmergency as COVID-19 was considered to be the third highest patho-genic human coronavirus that had emerged in the last two decades(WHO,2020a).On 11 March 2020 the WHO declared the COVID-19 out-break a global pandemic (WHO,2020a). Italy was the first European nation to be affected by COVID-19 with238,720 confirmed total cases and 34,657 deaths to date (Fig. 1; WHO,2020b). The pandemic broke out and was mainly located in northernItaly with the first Italian COVID-19 patient hospitalized on 21 February2020 at Codogno Hospital, Lodi (Lombardy-Italy) (Indolfi andSpaccarotella, 2020). In the following days, in Lombardy,there was arapid increase in the number of cases. The mortality rate in this regionalone with a total of 16,579 deaths, is currently greater than that ofChina (4646 total deaths; Regione Lombardia,2020; WHO, 2020c). Inthis context the Italian National Healthcare Service was close tocollapse. Person-to-person transmission routes have previously been de-scribed for SARS-CoV-2 with incubation times between two and tendays (Chan et al., 2020). The spread is facilitated through direct personalcontact, droplets, hands, or contaminated surfaces (Chan et al., 2020).Laboratory experiments showed that SARS-CoV-2 can remain viably infectious in aerosols for hours and on surfaces for days (vanDoremalen et al., 2020). Unfortunately the diffusion of COVID-19 in hos-pital settings is facilitated by the presence of high viral loads in the re-spiratory tract of hospitalized infected persons, released in theenvironment through droplets spread via coughing or sneezing(Rothan and Byrareddy, 2020). In closed and stagnant environmentssuch as hospital wards, droplets can remain suspended for more than10 min and cover long distances, potentially maintaining their abilityto transmit disease (Bourouiba, 2020; Ong et al., 2020; Stadnytskyiet al.,2020). However, knowledge of several aspects of SARS-CoV-2spread in indoor scenarios is still rudimentary, and, as far as we know,inspections of the SARS-CoV-2 contamination in a European hospitalward have not yet been reported. The aim of this study was to evaluate the contamination of the airand surfaces by SARS-CoV-2 RNA in the COVID-19 ward of an Italianhospital in order to understand the extent of the viral shedding andthen to improve the management of healthcare settings and implementpublic safety measures. 2. Materials and methods 2.1. Sampling location and methods applied We conducted the present study on May 12,2020 inside the COVID-19 ward of the San Carlo Hospital in Milan, Lombardy (Italy). The areaunder control consisted of the COVID-19 ward, which included an oper-ating room converted into an intensive care unit (ICU) with eight beds(Fig.2). The air conditioning system in the COVID-19 ward consistedof a negative airflow system. Surfaces and objects were wiped downdaily with active chlorine (5-10%) disinfectant. On the day of the exper-imentation the sampling was carried out before thecleaning operations,precisely in the time interval from 8:00 am to 1:00 pm. On the day of theexperimentation the temperature and relative humidity (RH) of theCOVID-19 ward ranged from 20° to 22 °C and 40 to 60% respectively, Fig.1. WHO Italian situation report dated 24 June 2020.(Adapted from WHO, 2020b.) CORRIDOR FOR PATIENTS*25.0% LOCKERS/PASSAGE FOR THE STAFF *0.0% Fig. 2. Location of sampled surfaces and objects in COVID-19 ward. The areas of the ward are represented by different colours. Color code: Red label = positive samples; Green label =negative samples. Numeric code: 1.Bedrails; 2. Benches;3. Computer keyboard; 4. Door handles; 5. Gloves box; 6. Hand sanitizer dispenser; 7. Medical equipment; 8. Medicalequipment touch screen; 9. Shelf for medical equipment; 10. Staff lockers, 11. Walls; 12. Waste container; 13. Water tap, 14. Window. The indicators and lines in the map represent thedirty/clean paths of the staff and patients. while the average outdoor temperature and RH in Milan on that daywere 18 ℃ and 78% respectively. In this study, swab (n=37) and air (n=5) samples were collectedfrom three zones classified as contaminated, semi-contaminated, andclean areas. The contaminated area was a specifically designated areafor patients of COVID-19 (corridor for patients and ICU), the clean areawas a specifically designated area for non-contaminated items (lockersand passage for the medical staff and a dressing room),and a semi-contaminated area was set up between the contaminated area andthe clean area (undressing room). Patients were not allowed to enterthe semi-contaminated and clean areas. The different areas within theCOVID-19 ward were adjacent but separated by watertight doors withautomatic closing system in order to avoid possible contaminationsand transmissions. All watertight doors were marked with specific sign-age that highlighted the area type and the relative prescriptions to limitaccess to authorized medical staff. There were three patients confirmedwith COVID-19 within the ICU. Among these, two were intubated andsupported by a respirator (beds no.1 and2) while one patient (bedno. 3) was not intubated and without C-PAP nasal mask support(Figs. 2, 3). The ICU patients in beds no. 4,5,6,7, and 8 were dismissedthe same day of experimentation. For this reason, no sampling was car-ried out in the area surrounding these beds as the air and surface sam-ples were taken at the locations where the highest risk ofcontamination was assumed. The medical and paramedical staff consisted of eight people obli-gated to wear personal protective equipment (PPE), specifically gloves,aprons,long sleeved gowns, goggles, fluid-repellant surgical masks, facevisors,and respirator masks. Sterile premoistened swabs with a specific virus preservation solu-tion (Biocomma Limited, ShenZhen, PR, China) were used to sample po-tentially contaminated surfaces and objects such as the bedrails,benches, computer keyboards,door handles, glove boxes,hand sanitizerdispensers, medical equipment, medical equipment touch screens,shelves for medical equipment, staff lockers, walls, waste containers,water taps, and windows (Fig.2). The air samples were collected using an MD8 Airport Portable AirSampler with Gelatine Membrane Filters (Sartorius, Varedo, MB, Italy). One gelatin membrane filter was used for each monitored area. The du-ration of each aspiration cycle was 40 min with a flow of 50 l/min, for atotal volume sampled of 2 m. The detector was positioned 1.5 m abovethe floor. The long duration required by the aspiration cycle did notallow repeated sampling in the same area due to operational limita-tions, but the detector was moved at predefined times to different loca-tions of each area randomly identified (Fig. 3). 2.2. Laboratory analysis: SARS-C0V-2 detection After air samples and surface swabs were collected, all samples weretransferred to the Chemservice -Labanalysis Group (Milan, Italy) labo-ratory under cool conditions (temperature range between 2 and 8C).Laboratory confirmation of the virus was performed using real time re-verse transcription polymerase chain reaction (RT-PCR) using theVETfinder“Detection of CoV-19 and SARS and Recovery control in envi-ronmental sample”detection kit (Generon s.r.l., San Prospero, Modena,Italy), which is able to detect both SARS-CoV-2 and SARS virus group. Each reaction contained 20 pl of Ready-to use Mastermixes and 5 plof RNA sample (final volume 25 ul). Each reaction was run with initialconditions of 55 C for 10 min (one cycle), 95℃ for 3 min (one cycle),followed by 45 cycles of 95℃ for 15 s, and 58 ℃ for 30 s, as instructedby the kit supplier. A sample was considered positive when the qRT-PCR Ct value was ≤40. 2.3. Statistical analysis Descriptive statistics were used to present data as numbers and per-centages. The differences in the positive rates between the areas werecompared by Fisher exact tests while a z-test was used to compare col-umn proportions. Cramer’s V was reported as measure of effect size.Correlation between virus concentration and distance from patientswas examined using Spearman rank correlations (p). The number ofsample locations was calculated based on the area of each room in ac-cordance with the ISO 14644-1.Statistical analyses were performedwith SPSS Statistics version 25 (IBM, SPSS Inc., Chicago, IL, USA). Statis-tical significance occurred when P<0.05. Fig. 3. Portable Air Sampler position in COVID-19 ward. The areas of the ward are represented by different colours. Color code: Red label = positive samples; Green label= negativesamples. The indicators and lines in the map represent the dirty/clean paths of the staff and patients. 3.Results A total of 37 swab samples were collected within the five areas and24.3% of these were positive for viral RNA (Fig. 2). The positivity ratewas higher in contaminated (7/20, 35.0%) and semi-contaminatedareas (2/4, 50.0%) than in the clean area (0/13, 0.0%; P=0.015;Table 1). Cramer’s statistic indicated a medium effect for the associationbetween SARS-CoV-2 positivity and area type (V= 0.430; P=0.030).There were no differences between the positivity rate of the corridorfor patients (25.0%) and the ICU (41.7%; P=0.642). In these latter twoenvironments, contaminated samples were collected from the surfacesof medical equipment (2/3,66.7%), touch screens (1/2, 50.0%), shelves(2/5, 40.0%), door handles (1/3,33.3%), and bedrails (1/3, 33.3%). Posi-tive samples of the undressing room were found on the hand sanitizerdispenser (1/1, 100.0%) and door handles (1/2,50.0%). The positiverates of swab samples from environmental surfaces of specific sites re-gardless of area are detailed in Table 2. Table 1 Positive rate of swab samples from environmental surface in different areas and associa-tions between rate of positivity and area type. Area type Area NO. NO.of Rate of P of Positive Positivity value" tests Corridor for patients 8 2 25.0% Contaminated Intensive care unit 12 5 41.7% Total Contaminated 20 7a 35.0% 0.015 Semi-contaminated Undressing room 4 2 50.0% Clean Locker/passage for 9 0 0.0% medical staff Dressing room 4 0 0.0% Total Clean 13 0 0.0% Total 37 9 24.3% 一 Subscript letter denotes a subset of Area type categories whose column proportions do notdiffer significantly from each other at the 0.05 level (contaminated vs semi-contaminated,contaminated vs clean, semi-contaminated vs Clean; z-test). * P value for association between rate of positivity and area type (contaminated, semi-contaminated or clean; Fisher exact tests). Within the ICU the distance between site samplings and the closestpatient was calculated. The Spearman correlation (p) between this dis-tance and virus concentration was 0.577 (P=0.308). Air samples collected from ICU and corridor for patients were posi-tive for viral RNA with mean concentrations of 22.6 and31.1 Ct value,respectively. Conversely, no SARS-CoV-2 RNA was found in the air ofthe undressing room, dressing room, and passage/lockers area for staff(Fig.3). 4. Discussion To our knowledge, no research has previously been conducted in aEuropean hospital to evaluate the environmental contamination in theair and on surfaces by SARS-CoV-2RNA. Overall, nine out of 37 swab samples were positive. Seven of thesewere collected in the contaminated and two in the semi-contaminatedarea while no viral RNA was found on the surfaces of the clean area. In Table 2Positive rate of swab samples according to sampling site. Sampling site NO. of NO. of Rate of Average virus testsS positive positivity concentration Bedrails 3 1 33.3% 21.5 Benches 3 0 0.0% ND Computer keyboard 1 0 0.0% ND Door handles 8 2 25.0% 25.2 Glove box 1 0 0.0% ND Hand sanitizer dispenser 1 1 100.0% 24.0 Medical equipment 4 2 50.0% 22.2 Medical equipment touch screens 2 1 50.0% 22.5 Shelves for medical equipment 5 2 40.0% 23.9 Staff lockers 2 0 0.0% ND Walls 4 0 0.0% ND Waste container 1 0 0.0% ND Water tap 1 0 0.0% ND Window 1 0 0.0% ND ND, not determined. particular the positivity rate of the ICU was 41.7%, which is in closeagreement with those obtained in the ICUs of Wuhan, China, by Guoet al. (2020)(43.5%) and by Wu et al.(2020)(37.5%). In the present study the correlation between the virus concentrationand the distance from patients was also evaluated. The Spearman coef-ficient suggests that there may be a moderate correlation and that theviral load of the surfaces increases with the patient proximity.However,there was not sufficient evidence to confirm these data as they did notquite achieve significance. The correlation analysis was only carriedout on the five positive samples collected within the ICU. As the p-value for a correlation coefficient is affected by the sample size, analyseson a greater number of positive samples could elucidate the relationshipbetween virus concentration and distance from patients. Other aspectsshould be further investigated. For example,Santarpia et al. (2020)evaluated the association between patients' body temperature andshedding of virus in the environment even if they did not find a statisti-cally significant correlation. The positivity rate of the corridor for patients was lower but not sta-tistically different than that of the ICU. Moreover, two positive swabsamples were obtained from the surfaces of the undressing room. Thisfinding emphasized the criticality of the undressing procedures. Con-versely, as mentioned above, no contaminated surfaces by SARS-CoV-2 were found in the area for passage and lockers of medical staff, andin the dressing room. Thus, our findings showed contamination of sur-faces in both contaminated and semi-contaminated areas but not inthe clean area. This result suggests that the pathway separating semi-contaminated and clean areas as well as the definition of separate dress-ing and undressing zones are essential. In particular the PCR-positive samples were obtained from the sur-face of hand sanitizer dispensers, medical equipment, medical equip-ment touch screens, shelves for medical equipment, bedrails, and doorhandles. On the contrary no samples collected from the walls were pos-itive. These results confirmed previous studies in the Wuhan, Nebraska,and South Korea hospitals that reported relatively high positivity ratesfor the surface of the objects that are frequently touched by medicalstaff or patients such as computer mice, bedrails, water machine but-tons, door handles, telephones, oxygen cylinder valves, personal com-puters, iPads, cellular phones, reading glasses, and remote controls fortelevisions (Guo et al., 2020; Ryu et al., 2020; Santarpia et al., 2020;Wu et al., 2020). Ong et al. (2020), analysing some toilet sites in theSingapore hospital, also showed viral RNA contamination of toiletbowl and sink samples. Although the RT-PCR does not determine the infectivity, the identi-fication of viral RNA in these items indicates the shedding of the virus,and it can be a marker of ineffective cleaning and disinfection (Otteret al., 2016). As previously demonstrated (Wojgani et al., 2012), thedoor handles are critical points for infection control, but our findingsconfirm that a special emphasis should be placed on the disinfectionof all these surfaces including staff and patients'personal items. Medicaland, in particular,electronic equipment needs particular attention as itis possible that their cleaning is intentionally neglected to avoid inter-ference with medical procedures. Fixed use of equipment for each pa-tient and frequent disinfection of all reusable medical equipmentcould limit the spread of the virus (Wuet al., 2020). Our findings alsosupport the importance of hand hygiene as it could break the cycle de-riving from touching contaminated surfaces (Lai et al., 2020). As claimedby the WHO (WHO,2020d),hand hygiene is an important measure toprotect patients, health-care workers, and the environment from con-tamination. There is still no direct evidence that hand hygiene reducestransmission of SARSCoV-2 (Yang, 2020). However, the fact that SARS-CoV-1 and MERS-CoV virus can survive on surfaces for extended periods(Kampf et al., 2020; Otter et al., 2016) indicates that hand hygiene is ahighly defensible measure also in the SARSCoV-2 scenario (Yang,2020).Moreover, van Doremalen et al. (2020) have recently showedthat, under laboratory conditions, SARS-CoV-2 can remain stable on sur-faces such as plastic and stainless steel for up to 72 h with a stability similar to that of SARS-CoV-1.Since in the experiment of van Doremalenet al. the virus was artificially nebulized, studies conducted under clini-cal conditions would be necessary to confirm these data (Peters et al.,2020). Other recent experimental data demonstrated that, in additionto the surface type, the stability of the virus is affected by temperatureas the time for inactivation was reduced at 70℃ (Chin et al., 2020).The same authors also proved the effectiveness of some disinfectantssuch as ethanol, povidone-iodine, chlorhexidine, and benzalkoniumchloride (Chin et al.,2020). Likewise, Ong et al. (2020) showed thatthe samples collected from COVID-19 patients'rooms after routinecleaning were negative. In this regard detailed guidelines have beenprovided for cleaning and disinfection procedures in both healthcareand non-healthcare settings from the Centers for Disease Preventionand Control (CDS, 2020; European Centre for Disease Prevention,2020) and from the WHO (WHO, 2020d). Repeated sampling at severaltimes after the routine cleaning would have provided information onthe effectiveness of the disinfection measures and on the persistenceof RNA in the environment. Our results also brought attention to the undressing procedures and,more generally, to the behavioural measures within the undressingroom, which is one of the areas affected by surface contamination. Indi-cations on the dressing and undressing procedures are provided by theItalian Ministry of Health (2020). All the air samples collected from contaminated areas were positivefor viral RNA. Our results are consistent with those of Guo et al. (2020)and Liu et al. (2020) referring to hospitals in Wuhan. Santarpia et al.(2020) demonstrated the positivity of air samples for SARS-CoV-2 inthe Nebraska Medical Center as well. Moreover, under laboratory condi-tions, van Doremalen et al. (2020) showed that SARS-CoV-2 remainedviable in aerosols at least for three hours. These findings suggest thatthe virus could be transported by aerosol processes in the surroundingenvironment, potentially even in the absence of aerosol-generating pro-cedures (Santarpia et al., 2020). They support the idea that the airborneroute has to be considered an important pathway for SARS-CoV-2 con-tamination as already suggested for SARS-CoV-1 (Booth et al., 2005;Yu et al., 2014). Indeed, several retrospective studies and fluid dynamicssimulations concluded that airborne transmission was the main route ofSARS-CoV-1 in indoor environments including hospital wards (Li et al.,2005; Yu et al.,2005). Conversely, Wu et al. (2020) and Cheng et al. (2020b) found no pos-itivity for SARS-CoV-2 in air samples collected in Wuhan No.7 and HongKong Hospitals, respectively. Similar results were obtained in Iran(Faridi et al., 2020) and Singapore (Ong et al., 2020) hospitals.Differ-ences in the sampling and analysis methodology could explain theseinconsistencies. Regardless, a precautionary approach is desirable. The WHO recom-mends a ventilation rate of at least 288 m’per hour per person for con-trol of opportunistic airborne transmission (such as SARS and influenza)in health care settings, highlighting the fact that some aerosol-generating procedures (i.e. tracheal intubation, non-invasive ventila-tion, tracheotomy, cardiopulmonary resuscitation, manual ventilationbefore intubation, and bronchoscopy) are associated with a significantincrease in the risk of disease transmission (WHO, 2009). Moreover,supplementary and precautionary measures should be used including:indoor air purifiers; frequent disinfection of the room; ventilation;avoidance of air recirculation; medical staff training; and appropriateseparation between all patients (WHO, 2020d; Zhao et al., 2020). Isola-tion rooms at negative pressure in segregated areas would be desirable(Fathizadeh et al., 2020; Liu et al., 2020) and, where it is not possible,keep infected patients at distance (at least 1 m according to WHO,2020b). Moreover, although studies on COVID-19 are not yet fully con-clusive (Cheng et al., 2020a), the use of physical barriers such as facemasks and FFP or N95 respirators must be recommended for healthcareworkers, even when performing aerosol-generating procedures in pa-tients without clinical features (Fathizadeh et al., 2020; Guo et al.,2020; Romano-Bertrand et al., 2020). Several evidences have indeed proven their effectiveness in preventing the spread of respiratory virusinfections, including SARS epidemic (Jefferson et al., 2009). Future epi-demiological studies will have to confirm the effectiveness of hospitalinfection control measures also for SARS-CoV-2. In the present study, all air samples collected within the semi-contaminated and clean areas were negative. The variability in the envi-ronmental contamination between areas is of interest as it suggests theeffectiveness of the nosocomial airborne isolation precautions. Ventila-tion systems, physical barriers as well as behavioural managementmay have contributed to the prevention of the spread of SARS-CoV-2from contaminated to clean areas. In this context the home isolation of patients with COVID-19 maynot be a good control strategy as the control of airborne transmissionis more difficult. Moreover, family members usually do not have ade-quate protective measures or training to limit viral shedding. Unfortu-nately, in an emergency context with overly-saturated hospitalfacilities, as it occurred in Lombardy, it is difficult to find otheralternatives. This research has some limitations. Although RT-PCR is routinelyused to detect causative viruses from respiratory secretions (Cormanet al., 2020), it is worthwhile to stress that it is a marker of virus shed-ding, but it does not necessarily indicate the presence of viable virus(Otter et al., 2016). Thus, viral culture should be done to demonstrate vi-ability. Another drawback of our study is that, due to operational limita-tions during an outbreak, it was only possible to investigate a limitednumber of rooms. Moreover, repeated sampling would increase knowl-edge of viral RNA persistence and effectiveness of the cleaning proce-dures. Indeed, all samples were collected before the disinfectionoperations. Finally, routine and extended investigations would be indi-cated to confirm these preliminary results, evaluate the effectiveness ofthe protective equipment, and monitor the hospital environment. 5. Conclusion This study provides the first report on the SARS-CoV-2 shedding inthe air and on object surfaces in a hospital in northern Italy with impor-tant implications for the patients and medical staff protection as well asfor the management of hospitals and public health. Indeed, it demon-strated that both air and surfaces within areas designated for patientswere contaminated by SARS-CoV-2 RNA. This finding suggests thatstrict structural and personal protection measures as well as systematicdisinfections should be implemented to reduce the risk of infection forhealthcare professionals working in these areas. Moreover, the contam-ination of surfaces within the undressing room highlighted not only therisk of virus spread from the ICUs but also the criticality of theundressing procedures. However, the airborne spread of the viral RNAdid not involve the areas where patients do not have access indicatingthe effectiveness of the physical barriers and staff behavioural precau-tions. Routine and extensive investigations in health-care settingswould be desirable. CRediT authorship contribution statement Katia Razzini: Conceptualization, Funding acquisition, Methodol-ogy,Supervision, Writing-review & editing. Marta Castrica: Conceptu-alization, Formal analysis, Software, Visualization, Writing-originaldraft, Writing-review & editing. Laura Menchetti: Conceptualization,Formal analysis, Software, Visualization, Writing-original draft, Writing-review & editing. Lorenzo Maggi:Conceptualization, Funding acquisi-tion, Investigation, Methodology, Writing -review & editing. LuciaNegroni: Data curation, Validation, Writing-review & editing. NicolaV. Orfeo: Data curation, Validation, Writing- review & editing. AlicePizzoccheri: Data curation, Validation, Writing-review & editing.Matteo Stocco: Data curation, Validation, Writing-review & editing.Stefano Muttini: Data curation, Validation, Writing-review & editing.Claudia M. Balzaretti: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing-original draft, Writing-review & editing. Declaration of competing interest The authors declare that they have no known competing financialinterests or personal relationships that could have appeared to influ-ence the work reported in this paper. Acknowledgments The authors wish to thank the staff of San Carlo, Hospital (Milan,Italy) and the staff of Chemservice,(Novate Milanese,Milan, Italy) andLabAnalysis, Casanova Lonati, (Pavia, Italy) laboratories. The authors wish to thank Ed Weisman for English revision. ( References ) ( Booth, T.F. , Kournikakis, B., B a stien, N., Ho, J., K obasa, D. , Stadnyk, L., Li, Y., Spence, M., P aton, S ., Henry, B ., Mederski, B. W hite, D . , Low, D . E., McGeer, A., S i mor, A., Vearncombe,M., Dow n ey,J.,Jamieson, F.B., Tang, P., Plummer, F., 2005. Dete c tion of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental c ontamination in SARS outbreak units. J. In f ect. D i s. 1 9 1, 1472-1477.https:/ /d o i. or g/10.1 0 86/429634 . ) ( Bourouiba, L., 2020. Turbulent gas clouds and respiratory pathogen emissions: potential i mplications for redu c in g transmissio n o f COVID-19.JAMA-J . Am. Med. A ssoc. 323, 1837-1838. h t tps :// do i .or g /1 0 .1 0 0 1/ j a ma.20 20.47 56. ) ( CDS, 2020. Guidance fo r cleaning and disinfecting public spaces, workplaces, businesses, schools, and homes.[WWW document].URL. http s : / / www. c d c.g o v / co ro n av ir us/ 20 19- nc ov /c o mmu n i t y/ p d f /R e op e ni ng _Ameri ca _Gu i danc e. p d f . ) ( C h an , J.F.W., Yuan, S., Ko k , K.H., To, K.K . W., Chu, H., Yang, J ., X ing, F., Liu, J., Yip, C.C. Y ., Poon , R .W.S., Tsoi, H.W., Lo, S.K.F., Chan, K. H ., P o o n, V.K . M., Chan , W.M., Ip, J.D., Cai, J.P., C heng, V . C.C., Chen, H . , Hui, C.K.M., Yuen, K . Y., 2020. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person t ransmis- s ion: a study of a fami l y cluster . Lance t 395 , 514-523. h t tps: / / doi . org /1 0. 1 0 1 6 / S 0 14 0 - 67 3 6 ( 20)30 15 4- 9 . ) ( Cheng, V . C.C., Chen, J.H.K., Yip, C .C.Y., Wong, S.-C., Chuang,V.W . M., Tsa n g, O.T.Y., Sridhar, S ., Chan, J .F.W., Ho, P.-L. , Yuen, K.-Y . , 2020a. Es calat i ng infe ct i o n c o ntr o l r e s pon s e t o t he r a p i d ly e v o l vi n g e pide m i olog y of t h e c o ro na v i r us d i seas e 2 019 ( C O VID- 19) du e t o S AR S -C o V - 2 in H o ng Ko n g. Infect. C ontro l Ho sp. E p i de m iol. 4 1 , 493- 4 98. ) ( Cheng, V.C.C., Wong, S.-C., Chan, V.W.-M., So,S.Y.-C., Chen, J.H.-K., Yip, C.C.-Y., Chan, K.-H., C hu, H ., Chung, T. W .-H., Sr i dhar, S., To, K . K.-W., Chan, J. F .-W., H u ng, I. F .-N., Ho, P.-L., Y uen, K.-Y., 2020b. Air and environmental sampling for SARS-CoV-2 around hospital- ized patients with coronavirus disease 2 0 19 (C O VID-19). Infect. Co n trol Ho s p. E pidemiol., 1 -32 h t tps://d o i. org /1 0 . 10 1 7/ i c e.2020 .28 2. ) ( C hin, A.W.H., Chu, J.T.S., Perera, M.R.A., Hui, K . P.Y., Yen, H . -L., Chan, M.C.W., Peiris, M . , P oon, L.L.M. , 2020. Stability of SARS-CoV-2 in different e nvironmental conditions. The L ancet Microbe h ttps:/ /d oi.o r g / 1 0 .10 1 6 /s2666 -5 24 7 ( 2 0)30 00 3-3. ) ( Corman,V.M., La n dt, O., K a iser, M., Molenkamp,R., Meijer, A., Chu, D.K.W., Bl e icker, T . , B runink, S., Schneider,J., S chmidt, M.L., Mulders, D . G.J.C., Haagmans, B. L ., Van De r V eer, B., Van Den Brink, S. , Wijsman, L., Goderski, G., Romette, J.L.,Ell i s, J. , Z ambon, M ., P eiris, M., Goossens, H.,R e usken, C., Koopmans, M.P. G ., Dros t en, C., 2020 . Detec- t ion o f 2019 novel coronavirus (2019 - nCoV) by real-time RT- P CR. Eurosurveillance 2 5. h t t ps : / /d o i. org / 1 0.2807 / 1 5 6 0-7 917.E S . 20 2 0 . 25 . 3 . 200 0 045. ) ( van D oremalen, N., Bushmaker, T., M o rris, D., Holbrook, M ., Gamble, A., Williamson, B ., Tamin, A . , Harcourt, J., Thornburg,N. , Gerber, S., Lloyd-Smith, J. , d e Wit, E. , M u nster, V ., 2020. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV- 1 . N . Engl.J. Med. 382,1564-1567. h t t ps : / /d o i . or g / 10.1 056/N EJ Mc 200 497 3 . ) European Centre for Disease Prevention, 2020. Disinfection of Environments in ( H e al thc a re and N o n-he a l thc are S ettin g s P o te n tia l ly C ont a mi n at e d With SA R S-CoV - 2. Faridi, S., Niazi, S.,Sadeghi,K., Naddafi, K., Yavarian, J. , Shamsipour, M ., J andaghi, N.Z.S., S adeghniiat, K ., Nabizadeh, R ., Y unesian, M., M omeniha, F. , Mokamel, A., H assanvand, M.S., MokhtariAzad, T., 2 020.A f i e ld i nd o or a i r mea s ur e ment of SARS - CoV - 2 in t h e p a tient r o o ms of th e l a rge s t hosp i ta l i n I ran. S ci . Total En vi ro n . 72 5 , 1 38401 . ) ( Fathizadeh,H., Maroufi, P. , Momen-Heravi, M., Da o ,S., Kose, S., G a nbarov,K., Pa g liano, P., E sposito, S., Kafil, H.S., 2020 . Prot e ct i on a nd di s in fection p o l i c i es agai n s t SA RS - C o V-2 ( C O V ID- 19). Le Infez. M e d. 28 , 1 8 5 -191. ) ( G uo,Z.-D.,Wang,Z.-Y.,Zhang, S .-F., Li , X ., L i , L., L i, C . ,Cui, Y., Fu, R . -B., Dong , Y.-Z., Chi,X . -Y., Z hang, M.-Y., Liu, K., C a o, C., Liu, B., Zhang, K . , Gao, Y.-W., Lu, B., C h en, W. , 2020. Aero- sol and s urface distribution of severe acute r e spiratory syndrome coronavirus 2 in h ospital w ards, Wuhan, China, 2020. Eme r g. Infect. Dis. 26. ht t ps:/ / d o i. o r g / 10.3 201/ e i d 2 6 0 7. 2 0 0 885. ) ( I ndolfi, C., Spaccarotella, C., 2020 . The outbreak of COVID-19 i n I t aly: fighting the pan- d emic. JACC Case R e ports htt ps: // d o i.o rg / 1 0 .10 16 / j.j a c cas .20 2 0.0 3 . 01 2 . ) ( I talian Ministry of Health, 2020. Disposition o f the Italian M inister of Health n . 5443. N e w i ndications and c l arifications [WWW document]. URL. http://2. flc g i l . s tgy .it /f i l es / p df / 2 0 2 0 0328/ci rc ol a r e- m i n i s t eriale - 5443- d el- 22 - f ebbr ai o-2 020- in d ic az ioni- c hi ar ime nt i-m i nis tero -della - s a lut e -c ov id - 19.pdf. ) Jefferson, T., Del Mar, C., Dooley, L., Ferroni,E., Al-Ansary, L.A., Bawazeer,G.A., Van Driel,M.L., Foxlee, R., Rivetti, A., 2009. Physical interventions to interrupt or reduce thespread of respiratory viruses: systematic review. BMJ 339, 792. https://doi.org/10.1136/bmj.b3675. Kampf, G., Todt, D., Pfaender, S., Steinmann, E., 2020. Persistence of coronaviruses on in-animate surfaces and their inactivation with biocidal agents. J. Hosp. Infect.104,246-251.https://doi.org/10.1016/j.jhin.2020.01.022. Lai, T.H.T., Tang, E.W.H., Fung, K.S.C., Li, K.K.W., 2020.Reply to"does hand hygiene reduceSARS-CoV-2 transmission?". Graefes Arch. Clin. Exp.Ophthalmol. 258, 1135. https://doi.org/10.1007/s00417-020-04653-4. Li, Y., Huang, X., Yu, I.T.S., Wong, T.W., Qian, H., 2005. Role of air distribution in SARStransmission during the largest nosocomial outbreak in Hong Kong. Indoor Air 15,83-95.https://doi.org/10.1111/j.1600-0668.2004.00317.x. Liu, Y.Y.,Ning, Z., Chen, Y., Guo, M., Liu, Y.Y., Gali, N.K., Sun, L., Duan, Y., Cai, J., Westerdahl,D.,Liu, X., Ho, K., Kan, H., Fu, Q., Lan, K., 2020. Aerodynamic Characteristics and RNAConcentration of SARS-CoV-2 Aerosol in Wuhan Hospitals During COVID-19 Out-break. bioRxiv 2020.03.08.982637. https://doi.org/10.1101/2020.03.08.982637. Ong, S., Tan, Y., Chia, P., Lee,T., Ng, O., Wong, M., Marimuthu, K., 2020. Air,surface envi-ronmental, and personal protective equipment contamination by severe acute respi-ratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAM 323.https://doi.org/10.1001/jama.2020.3227. Otter, J.A.,Donskey, C., Yezli, S.,Douthwaite, S., Goldenberg,S.D., Weber, D.J.,2016. Trans-mission of SARS and MERS coronaviruses and influenza virus in healthcare settings:the possible role of dry surface contamination. J. Hosp. Infect. 92, 235-250. https://doi.org/10.1016/j.jhin.2015.08.027. Peters, A., Parneix, P., Otter,J., Pittet, D., 2020. Putting some context to the aerosolizationdebatee around SARS-CoV-2. J.Hosp.Infect.https://doi.org/10.1016/j.ihin.2020.04.040. Regione Lombardia, 2020. Covid-19[WWW document].URL. https://experience.arcgis.com/experience/0a5dfcc103d0468bbb6b14e713ec1e30/. Romano-Bertrand, S., Aho-Glele, L.-S., Grandbastien, B., Gehanno, J.-F., Lepelletier, D.,2020. Sustainability of SARS-CoV-2 in aerosols: should we worry about airbornetransmission? J. Hosp. Infect. https://doi.org/10.1016/j.jhin.2020.06.018. Rothan, H.A., Byrareddy, S.N., 2020. The epidemiology and pathogenesis of coronavirusdisease (COVID-19) outbreak. J. Autoimmun. 109. https://doi.org/10.1016/j.jaut.2020.102433. Ryu, B.-H., Cho, Y., Cho,O.-H., Hong, S.I., Kim,S., Lee,S., 2020. Environmental contamina-tion of SARS-CoV-2 during the COVID-19 outbreak in South Korea. Am. J. Infect. Con-trol https://doi.org/10.1016/j.ajic.2020.05.027. Santarpia, J.L., Rivera, D.N., Herrera, V., Morwitzer, M.J., Creager, H., Santarpia, G.W.,Crown, K.K., Brett-Major, D., Schnaubelt, E., Broadhurst, M.J., Lawler, J.V., Reid, S.P., Lowe,JJ., 2020. Transmission Potential of SARS-CoV-2 in Viral Shedding Observedat the University of Nebraska Medical Center. medRxiv 2020.03.23.20039446.https://doi.org/10.1101/2020.03.23.20039446. Stadnytskyi, V., Bax, C.E., Bax, A., Anfinrud, P., 2020.The airborne lifetime of small speechdroplets and their potential importance in SARS-CoV-2 transmission. Proc.Natl.Acad.Sci. U.S.A. 117, 11875-11877.https://doi.org/10.1073/pnas.2006874117. Torri, E., Nollo, G., 2020. Public health decision-making in the real world: four points toreshape it after COVID-19. Disaster Med. Public Health Prep. https://doi.org/10.1017/dmp.2020.108. WHO, 2009. Natural Ventilation for Infection Control in Health-care Settings. WHO Press,Geneva, Switzerland. WHO, 2020a. WHO timeline -COVID-19 [WWW document]. URL. https://www.who.int/ news-room/detail/27-04-2020-who-timeline-covid-19 (accessed 5.18.20). WHO, 2020b. covid19.who.italy [WWWdocument]. URL. https://covid19.who.int/region/euro/country/it (accessed 6.23.20). WHO,2020c.cov19.who.china [WWW Document]. URL https://covid19.who.int/region/wpro/country/cn (accessed 6.23.20). WHO, 2020d. Coronavirus Disease (COVID-19) Technical Guidance: Infection Preventionand Control during Health Care When COVID-19 Is Suspected: Interim Guidance. _LF Wojgani, H., Kehsa, C.,Cloutman-Green, E., Gray, C., Gant, V., Klein,N., 2012. Hospital door handle design and their contamination with Bacteria: a real life observational study.Are we pulling against closed doors? PLoS One 7. https://doi.org/10.1371/journal.pone.0040171. Wu, S., Wang, Y., jin, X., Tian, J., Liu, J., Mao, Y., 2020. Environmental contamination bySARS-CoV-2 in a designated hospital for coronavirus disease 2019. Am. J. Infect. Con-trol https://doi.org/10.1016/j.ajic.2020.05.003. Yang, C., 2020. Does hand hygiene reduce SARS-CoV-2 transmission? Graefes Arch. Clin.Exp.Ophthalmol. https://doi.org/10.1007/s00417-020-04652-5. Yu, I.T.S., Wong, T.W., Chiu, Y.L., Lee, N., Li, Y., 2005. Temporal-spatial analysis of severeacute respiratory syndrome among hospital inpatients. Clin. Infect. Dis. 40,1237-1243. https://doi.org/10.1086/428735. Yu, I.T.S., Qiu, H., Tse, L.A.,Wong, T.W., 2014. Severe acute respiratory syndrome beyondamoy gardens: completing the incomplete legacy. Clin. Infect. Dis. 58, 683-686.https://doi.org/10.1093/cid/cit797. Zhao, B., Liu, Y., Chen, C., 2020. Air purifiers: a supplementary measure to remove air-borne SARS-CoV-2. Build. Environ. 177. https://doi.org/10.1016/j.buildenv.2020.106918. 在一篇ELSEVIER发表的题为“SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy”的文章中,首次提出了新冠病毒能通过物体表面及空气进行传播的理论,这对患者及医护人员的保护和公共卫生管理都具有深远意义。为了了解病毒空气传播途径,研究人员深入意大利疫情一线,对米兰一家医院隔离病房中空气及物品表面的病毒进行了监测,共采集了污染区(患者病房区)、半污染区(换衣间)和洁净区等五个区域的42个样本,并在处理后进行了qPCR检测。结果表明,有24.3%的表面拭子呈阳性;ICU病房及走廊空气样本呈阳性,半污染区和洁净区空气中未检测到病毒。这个结果说明,处在疫情一线的医院不仅需要对物品表面进行严格消毒,注意手部卫生清洁及医护人员防护;同时,更要加强空气气溶胶的监测及防护。该研究结果进一步佐证了使用赛多利斯Airport MD8空气气溶胶采样器 有效采集监测新冠病毒的重要性,这对深入了解病毒传播途径及规避污染风险有重要作用。—— 了解详细监测方法 ——↓ 点击下载阅读全文 ↓
确定






还剩5页未读,是否继续阅读?
德国赛多利斯集团为您提供《气溶胶中新冠病毒检测方案(微生物采样器)》,该方案主要用于其他中生化检验检测,参考标准--,《气溶胶中新冠病毒检测方案(微生物采样器)》用到的仪器有赛多利斯便携式浮游菌采样仪 Airport MD8 、赛多利斯台式浮游菌采样仪 MD8 Airscan
相关方案
更多
该厂商其他方案
更多










