方案详情
文
南欧球花叶抗氧化、抗增殖、抗菌和抗生物膜作用的植物化学分析与评价Phytochemical Analysis and Evaluation of the Antioxidant, Antiproliferative, Antibacterial, and Antibiofilm Effects of Globularia alypum (L.) Leaves
方案详情

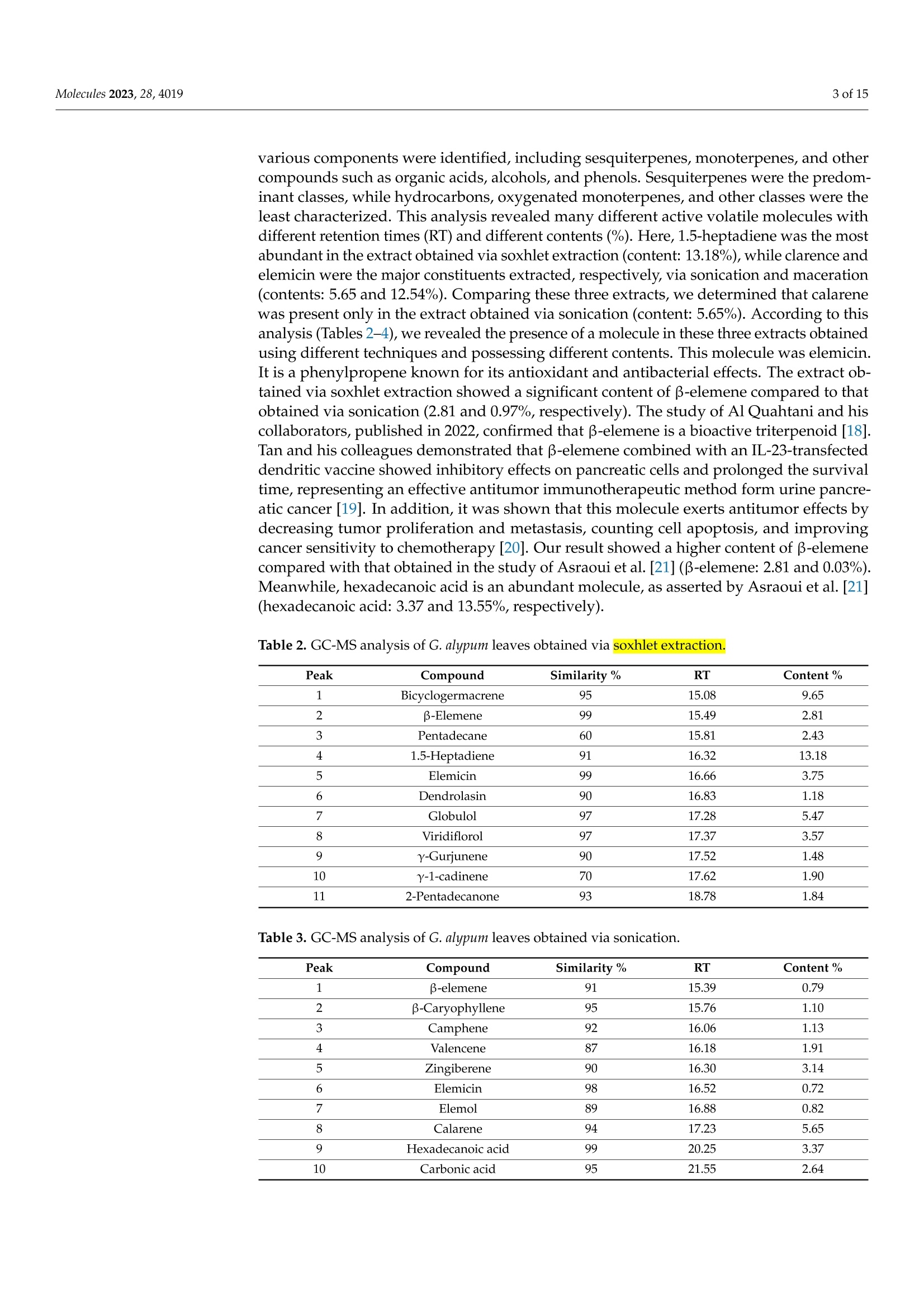
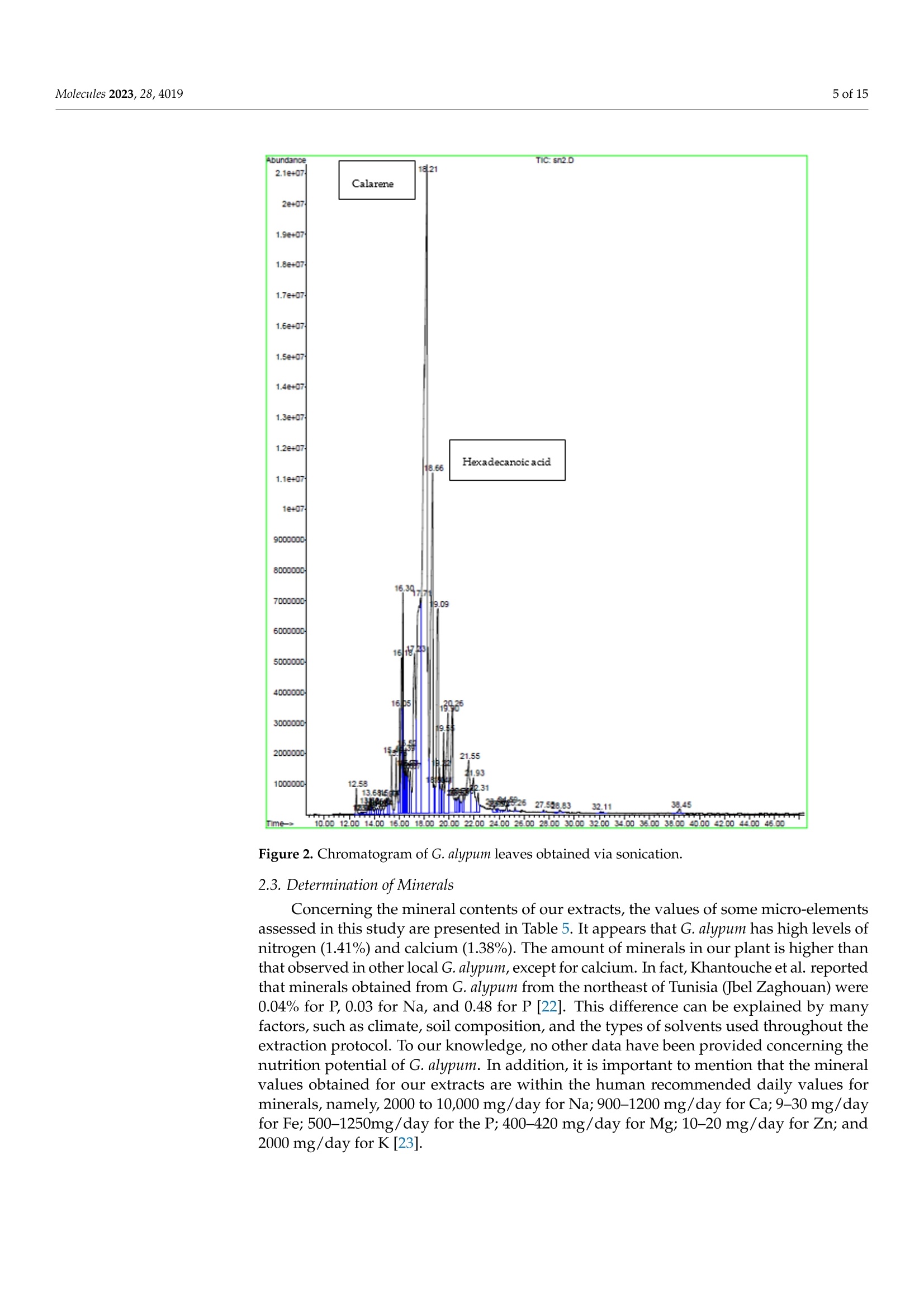
南欧球花叶抗氧化、抗增殖、抗菌和抗生物膜作用的植物化学分析与评价Phytochemical Analysis and Evaluation of the Antioxidant, Antiproliferative, Antibacterial, and Antibiofilm Effects of Globularia alypum (L.) Leaves2 of 15Molecules 2023,28,4019 南欧球花叶抗氧化、抗增殖、抗菌和抗生物膜作用的植物化学分析与评价 Citation: Nouir, S.; Dbeibia, A.; Bouhajeb, R.; Haddad ,H.;Khelifa, A.; Achour, L.; Ghardallou,M.; Zairi, A Phytochemical Analysis and Evaluation of the Antioxidant, Antiprol i ferative, Antibac t erial , and Antibiofilm Effects of Globularia alypum (L.) Leaves. Molecule s 2023, 28,4019. https://doi .org/10.3390/molecules28104019 Academic Editor: Riccardo Petrelli Received: 12 February 2023 Revised: 21 Apr il 2023 Accepted: 23 Apri l 2023 Published: 11 May 2023 Copyright: @ 2023 by the authors. L i censee MDPI, Basel, Switzerland. This article is an open access article distr i buted under the terms and conditions of the Creative Commons Attribution (CC BY ) l icense (https://creativecommons.org/licenses/by/4.0/) Abstract: Globularia alypum L. (GA) i s a Mediterranean plant of the Globulariaceae family which is widely used in traditional Tunisian medicine. The main goal of this study was to evaluate the phyto-chemical composition, antioxidant, antibacterial, and antibiofilm activities, and the antiproliferative potential of different extracts of this plant. The identification and the quantification of the different constituents of extracts were determined using gas chromatography-mass spectrometry (GC-MS). The antioxidant activities were evaluated using spec t rophotometric methods and chemical tests. The antiproliferative study was based on the use of colorectal cancer SW620 cells, including an antibacterial assessment with the microdilution method and analysis of the antibiofi l m effects via the crysta l violet assay. All extracts presented several components, mainly sesquiterpenes,hydro-carbon, and oxygenated monoterpenes. The results revealed that the maceration extract had the most important antioxidant effect (IC50 =0.04 and 0.15 mg/mL), followed by the sonication extract (IC50=0.18 and 0.28 mg/mL). However, the sonication extrac t demonstrated significant antiprolifer-ative (ICs0 =20 ug/mL), antibacterial (MIC = 6.25 mg/mLand MBC>25 mg/mL),and antibiofilm (35.78% at 25 mg/mL) proper t ies against S.aureus. The results achieved confirm the i mportant role of this plant as a source of therapeutic activities. Keywords: Globularia alypum (L.); phenolic compounds; antioxidant activity; antiproliferative activity; antibacterial activity; antibiofilm activity 1. Introduction Due to their curative properties, medicinal plants have been utilized as treatments for various diseases since ancient times [1]. Nowadays, many people are oriented to-wards "green t herapy" and the use of natural compounds from plants that are found to be free from side effects and less toxic than commercia l drugs [2]. The beneficia l effects of medicinal plants have mainly been attributed to several compounds, such as polyphenols, flavonoids, and phenolic acids [2]. These compounds have a variety of beneficial effects on human health [3]. Previous studies have reported that the consumption of plants and particularly phenolic compounds has been used for the treatment and prevention of a wide spec t rum of chronic diseases, including hypertension and hyperglycemia [4]. For this reason, several scientists have conducted extensive studies using different plant extracts to evaluate t he antioxidant, antibacterial, anti-inflammatory, and many other medicinal prop-erties of these extracts [5]. Globularia alypum L. Extracts may be candidates for such assays. G. alypum is a medicinal plant species belonging to the Globulariaceae family which grows in the Mediterranean basin. In Tunisia, it is known as "Zriga" and has been used in folk medicine for several diseases, such as kidney disease, cardiovascular disease, constipation, and diabetes [6]. I ts l eaves have long been used as hypoglycemic, laxative, and purgative agents [6]. According to biological activity studies, G. alypum has diverse biological activi-ties, such as antioxidant, antiobesity, antihyperglycemic, antihyperlipidemic, antipyretic, and analgesic properties [6,7]. Numerous studies have reported that G. alypum extracts are in secondary metabolites including flavonoids, t annins, and anthocyanins, which explains their numerous benefits and therapeutic properties [6,8]. However, several studies have reported that the extraction processes and techniques influence the nature and amount of secondary metabolites and have noticeable effects on the recovery of phytochemical contents. Thus, the choice of an adequate extraction method and solvent is necessary to obtain multiple secondary metabol i tes and for t he desired activities [9,10]. In this context, the aim of the present study was to conduc t a phytochemical analysis of extracts from the leaves of G. alypum and to evaluate their antimicrobial, antioxidant, and antiproliferative activities. Moreover, a comparative study of the extraction methods, including soxhlet, sonication, and maceration, was carried out. 2. Results and Discussion 2.1. Yield of Different Extraction Methods Various extraction techniques were used to obtain the phytochemicals from different plant parts. The selection of the proper extraction method is crucial for the qualitative and quantitative studies of bioactive compounds from plant materials. The extraction yield is dependent on the solvent, the method of extraction, and the extraction conditions, such as temperature and t ime. The extraction method must provide the recovery of the targeted compounds and must avoid their chemical modification or alteration [11,12]. Therefore, i t is necessary to selec t a suitable extraction method and solvent based on the sample matrix, chemistry of the bioac t ive compounds, and scientific expertise [13]. According to Table 1, the sonication method has the most significant yield (64.8%). This extrac t ion technique provides extracts in high yields with minimal changes in the functional properties of the extract [14]. However, the soxhlet technique uses a higher temperature for a longer period of time, which can alter the quality of the phenolic compounds. The study of Khlifi and his colleagues showed that methanol extract obtained using maceration had a lower yield compared to the one reported in our study (42.4 and 48.8%, respectively) [15]. Our results are in line with several studies which reported that sonication provides better yields in a short extraction time compared to the other methods. It has been reported that sonication preserves the nature of compounds that degrade at elevated temperatures [10]. Another study reported that sonication was found to lower the level of energy consumption, quicken the extraction process, and increase the amount of phytochemicals from annatto seeds [16]. Table 1. Yield percentage using different methods of extraction. Extraction Method Yield% Soxhlet 63.2±0.07D Sonication 64.8±0.45 Maceration 48.8±0.04C Results of the ANOVA t est are signi f icantly dif f erent at p<0.05, and each data point i s represented as the average of three r epetitions ±SD of one independent experiment. a: The highest value; b: T he medium value;: the lowest value. 2.2. GC-MS Analysis GC-MS plays an essential role in the phytochemica l analysis and chemotaxonomic studies of medicinal plants containing biologically active components [17]. According to the chromatographic analyses carried out via GC-MS (Tables 2-4) and (Figures 1-3), various components were identi f ied, including sesquiterpenes, monoterpenes, and other compounds such as organic acids, alcohols, and phenols. Sesquiterpenes were the predom-inant classes, while hydrocarbons, oxygenated monoterpenes, and other classes were the least characterized. This analysis revealed many different active volatile molecules with different retention t imes (RT) and different contents (%). Here, 1.5-heptadiene was the most abundant i n the extract obtained via soxhlet extraction (content: 13.18%), while clarence and elemicin were the major constituents extracted, respectively, via sonication and maceration (contents: 5.65 and 12.54%). Comparing these three extracts, we determined that calarene was present only in the extract obtained via sonication (content: 5.65%). According to this analysis (Tables 2-4), we revealed the presence of a molecule in t hese three extracts obtained using different techniques and possessing different contents. This molecule was elemic i n. It i s a phenylpropene known for its antioxidant and antibacterial effects. The extract ob-tained via soxhlet extraction showed a significant content of B-elemene compared to that obtained via sonication (2.81 and 0.97%, respectively). The study of Al Quahtani and his collaborators, published in 2022, confirmed that B-elemene is a bioactive triterpenoid [18]. Tan and his colleagues demonstrated that B-elemene combined with an IL-23-transfected dendritic vaccine showed inhibitory effects on pancreatic cells and prolonged the survival time, representing an effective antitumor immunotherapeutic method form urine pancre-atic cancer [19]. In addition, i t was shown that this molecule exerts antitumor effects by decreasing tumor proliferation and metastasis, counting cell apoptosis, and improving cancer sensitivity to chemotherapy [20]. Our result showed a higher content of B-elemene compared with that obtained in the study of Asraoui et al. [21] (B-elemene: 2.81 and 0.03%). Meanwhile, hexadecanoic acid is an abundant molecule, as asserted by Asraoui et al. [21](hexadecanoic acid: 3.37 and 13.55%, respectively). Table 2. GC-MS analysis of G. alypum leaves obtained vias soxhlet extraction. Peak Compound Similarity % RT Content% 1 Bicyclogermacrene 95 15.08 9.65 2 B-Elemene 99 15.49 2.81 3 Pentadecane 60 15.81 2.43 4 1.5-Heptadiene 91 16.32 13.18 5 Elemicin 99 16.66 3.75 6 Dendrolasin 90 16.83 1.18 7 Globulol 97 17.28 5.47 8 Viridiflorol 97 17.37 3.57 9 Y-Gurjunene 90 17.52 1.48 10 y-1-cadinene 70 17.62 1.90 11 2-Pentadecanone 93 18.78 1.84 Table 3. GC-MS analysis of G. alypum leaves obtained via sonication. Peak Compound Similarity % RT Content% 1 B-elemene 91 15.39 0.79 2 β-Caryophyllene 95 15.76 1.10 3 Camphene 92 16.06 1.13 4 Valencene 87 16.18 1.91 5 Zingiberene 90 16.30 3.14 6 Elemicin 98 16.52 0.72 7 Elemol 89 16.88 0.82 8 Calarene 94 17.23 5.65 9 Hexadecanoic acid 99 20.25 3.37 10 Carbonic acid 95 21.55 2.64 Table 4. GC-MS analysis of G. alypum leaves obtained v i a maceration. Molecules 2023, 28, x FOR PEER REVIEW Peak Compound Similarity % RT Content% 1 α-Farnesene 76 16.42 0.92 2 Elemicin 99 16.70 12.54 3 Elemol 91 16.87 1.87 4 Spathulenol 99 17.24 3.28 5 Isospathulenol 94 17.66 1.20 6 Italicene 72 17.87 5.09 Figure 1. Chromatogram of G. alypum leaves obtained via soxhlet extraction. Molecules 2023, 28, x FOR PEER REVIEW Abundancs 2.12407 22+07 1.92407 1.82407 1.7e+07 1.62+071.50+071.42+071.324071.24021.1e+071240790000m80000m1700n0n6000001- 50000m1 Anonomm- 300n0E 2000000- 1n0n0m Calarene 16.30 16 161 12.5813.6 Figure 2.Chromatogram of G. alypum leaves obtained via sonication. 2.3. Determination of Minerals Figure 3. Chromatogram of G. alypum leaves obtained via maceration. Table 3. GC‐MS analysis of G. alypum leaves obtained viasonication. Peak N(%) Compound K(%) Similarity % RT Content % 1 1.41 β‐elemene 0.61 91 15.39 0.79 2 β‐Caryophyllene 95 15.76 1.10 2.4. 3Antioxidant Activitu Camphene 92 16.06 1.13 4 Valencene 87 16.18 1.91The antioxidant effect of G. alypum leaves was evaluated via ABTS and B-carotene assay 5s , a n d t h e r e s u l ts w Z e ingiberene 90 16.30 3.14re expressed as ICso values (Table 6). As presented i n Table 6, the extra 6c t s o f G . a l y p u m s h o w El e e d m i i m ci p n o r t a n t a n ti o x i d a n t a c ti v 9i 8ti e s , w i t h s i 1g 6n .5if 2ic a n t v a r 0i .a 7b 2ility betw 7e e n th e e x t r a ct s a n d th E e l e m m e o t l h o d s u s e d . T h e e x tr a c t o b 8t 9a i n e d vi a m 1a 6.c 8e 8ra t i o n p r 0e .s 8e 2nted the h 8i g h e s t a n t io x i d a n t p ot C e a n l t a i r a e l,n e with values of IC50=0.049 4m g / m L a n d 1I 7.C 2530 = 0 .1 5 m 5.g 6/5mL 9 Hexadecanoic acid 99 20.25 3.3710 Carbonic acid 95 21.55 2.64and 0.65 mg/mL, respectively). We also noted that the ABTS activity assay was the most sensitive in terms of IC5o when comparing the two methods used (Table 6). Phenolic compounds such as flavonoids, phenolic acid, and tannins contribute considerably to the Acid ABTS scavenging assay 0.06 ± 0.02 b 0.25 ± 0.01 d 0.18 ± 0.00 c ICs0(mg/mL) β‐carotene 0.59 ± 0.00 c 0.65 ± 0.05 d 0.28 ± 0.09 b Results of the ANOVA test are significantly different at p < 0.05, and each data point is represented by the average of three repetitions ± SD of one independent experiment. IC50: half maximal inhibi‐ tory concentration. Values with different superscripts within the same column are significantly B-carotene different (p < 0.05). of three repet i tions ± SD of one independent experiment. ICso: half maximal i nhibitory concentration. Values 2.5. Antiproliferative Effect In the present study, an evaluation of the antiproliferative effects of different ex‐ tracts from G . alypum leaves on SW620 was carried outat various concentrations. After 48h of incubation, the survival rates showed that all the extracts inhibited cell line pro‐liferationinaconcentration‐dependentmanner.Theextractobtainedvia presented the highest anti‐proliferative effect (Figure 4), with an IC50 = 20 μg/mL. This result can be explained by the richness of this extract of active molecules (Figure 2). Many studies have demonstrated that β‐caryophyllene has antioxidant [30], antimicro‐bial [31], anti‐inflammatory [32], and anticancer activities [33]. Comparing our results with those of the study of Friscic and his collaborators, we determined that our extract of G. alypum leaves has the most significant antiproliferative effect (IC50= 20 and 231.43μg/mL, respectively) [7]. antiproliferative effect (IC50=20 and 231.43 ug/mL, respectively) [7]. Concentration (ug/ml ) Figure 4. Antiprol i ferative effect of G. alypum leaves obtained via soxhlet extraction. The symbols *Figure 4. Antiproliferative effect of G. alypum leaves obtained via soxhlet extraction. The symbols *and ** indicate a significant (p ≤ 0.05) to highly significant **** (p ≤ 0.01) difference between different extracts ateach concentration. Viability rate (means ± standard deviations ) of SW620 cells treated with different types of extracts of G. alypum leaves. Soxhlet extract (IC50= 80μg/mL), sonication ex‐tract (IC50 = 20μg/mL), and maceration extract (IC50 = 100μg/mL). IC50: concentration required to reduce SW620 cell viability by 50%. 2.6. Evaluation of Antibacterial Activity In the present research, initial screening was performed to evaluate the antibacterial effects of G. alypum leaves on some available bacterial strains (Table 7). All the presented bacteria showed resistance to the extracts, except for S. aureus. The extrac t obtained via sonication showed a significant antibacterial effect on S. aureus, with a zone of inhibition of 14.5 mm. The variation in the diameters of the zones of inhibition may be impacted by the microorganisms, the plant, and the antibacterial potential of the bioactive substances of the extract. Several studies have affirmed that the antimicrobial activity is strongly linked to the contents and composition of the extract. Phenolic compounds were also influenced by their diffusion capacity in agar medium [34]. All the tested extracts presented the same MIC and MBC (6.25 and >25 mg/mL, respectively) against S. aureus (Table 8). The study of Friscic et al. [7] revealed that t he methanolic extract of G. alypum l eaves obtained via soxhlet extraction had significant antibacterial activity against S. aureus i n comparison to our results, with MIC= 1.42 mg/mL. According to Lozienne et al. [35], the antibacterial activity depends on several factors, namely, the species of the plant, the preparation of the extract, the solvent used, and the sensitivity of the bacteria . In our study, the antibacterial profiles of the extracts against the tested strains indicated that S . aureus is the most sensitive bacterium of all the strains. In other words, the walls of Gram-negative bacteria are more complex than those of Gram-positive bacteria. They act as a diffusional barrier and render cells less sensitive to antimicrobial agents [36]. This difference in sensi t ivity may also be due to the inhibition of t he efflux pump of Gram-positive bacteria [37]. Several works have suggested that the antibacterial activity of flavonoids may be due to the following mechanisms [38]:damage to the cytoplasmic membrane caused by perforation and/or reduced membrane fluidity or the inhibition of nucleic acid synthesis caused by t he i nhibition of topoisomerase. P. aeruginosa has high internal resistance to almost all known antibiotics and antimicrobials, even synthetic drugs, owing to i t s very restrictive outer membrane barrier, which has been a serious problem worldwide [39]. Regarding our results, the antibacterial activity of the extract obtained via soxhlet extraction could be attributed to globulol , a sesquiterpenoid that was only present only in the extract obtained via soxhlet extraction, with a content equal to 5.47%. In addition, it was reported tha t globulol has antibacterial activity in the study of Tan and his collaborators in 2008 [40]. Table 7. Evaluation of antibacterial activ i ty of G. alypum leaves at 25 mg/mL Zone of Inhibition (mm) Strains Tetracycline Soxhlet Sonication Maceration E.coli 29.10±0.00 N N N P. aeruginosa 27.20±0.53 N N N S. typhimurium 25.01±1.02 N N N S. aureus 21.00±1.82a 11.2±0.30d 14.50±0.30D 12.00±0.06° Values are means of triplicate determination (n=3)± standard deviation. N, no zone of inhibi t ion was found. Values with different superscripts within the same column are significantly different (p<0.05). 2.7. Antibiofilm Activity Our results showed that the antibiofilm effect is dose-dependent (Table 9). After 24 h of culture, the antibiofilm inhibition percentage varied f rom 35.78% (25 mg/mL) to 16.01%(3.12 mg/mL) for the extract obtained via sonication. The elimination of the biofilm by the extracts may be due to their richness in active molecules such as phenolic compounds [41] and other volatile molecules. Resistance against the various disinfectants is more important in the case of older biofilms than younger ones [42]. This may be related to the fact that the majority of cells in young biofilms are still in the reversible phase of adhesion. In this phase, the bacteria are easily suppressed through the application of minimum forces [43]. Moreover, according to the study of Melo [44], several mechanisms influence bacterial attachment to surfaces, such as the characteristics of the microbial strains, the composition and roughness of the adhesion surface, the availability and concentration of nutrients, surface charge, pH, t emperature, electrolyte concentration, and f lux of materials, as wel l as the type of surface. Table 8. MIC and MBC determination of G. alypum l eaves. Strains E. Coli P. aeruginosa S. typhimurium S.aureus MIC (mg/mL) Soxhlet N N N 6.25 Sonication N N N 6.25 Maceration N N N 6.25 MBC (mg/mL) Soxhlet N N N >25 Sonication N N N >25 Maceration N N N >25 Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC). Table 9. Percentage of biofi l m inhibition after 24 h. Concentration (mg/mL) 25 12.5 6.25 3.12 Methods So S M So S M So S M So S M E.coli N N N N N N N N N N N N S. typhimurium N N N N N N N N N N N N S. aureus 20.49 35.78 27.19 15.06 28.15 20.01 10.59 20.3 17.10 8.50 16.01 10.24 P.aeruginosa N N N N N N N N N N N N N: no activity. So: Soxhlet. S: sonication . M: maceration. 3. Materials and Methods 3.1. Plant Material This study's use of plant material adhered to the appropriate institutional, national, and international rules and regulations. G. alypum was taken in the full blooming stage from Tunisia's Ouardanin area in January 2019 (Table 10). Prof. Fethia Harzallah Skhiri of Tunisia's High Institute of Biotechnology in Monastir completed the taxonomic iden-tification. The plant voucher specimen was cataloged as Ga 022 in the Herbarium of the Laboratory of Bioresources: I ntegrative Biology and Valorization (ISBM). Over 7 days, the leaves were washed and dried at room temperature. The material was dried until it reached a constant weight. To optimize the phenolic extraction, the leaves were milled . Three techniques of extraction were used (Table 11). The extracted ethanol was weighed and kept in an amber glass container in a refrigerator (4C) after being vaporized using a rotary evaporator. The extract solution was prepared using ethanol absolute for the measurement of active molecules. Table 10. Collec t ion site of G. alypum L. and i ts eco-geographical characteristics. Collection Site Geographical Location Longitude (E) Latitude (N) Altitude (m) Ouardanin 10°40'35” 35°42'35” 75 Table 11.Extraction methods of G. alypum leaves. Extraction Methods Soxhlet Sonication Maceration Solvent Ethanol Ethanol Ethanol Temperature (°C) 40 25 25 Time (min) 60 10 240 Plant: solvent proportion 5:100 5:100 5:100 Centrifugation Not applicable 5 min at 5000 rpm and 4°℃ Not applicable Equipment Not applicable Ultrasound bath (130 kHz) Not applicable 3.2. The GC-MS Analysis GC-MS was used to identify the volatile active compounds. The Varian Hp-5890 Gas Chromatograph was used, together with HP5 and Innovax (30 m, 0.25 mm, film thickness 0.25 m) bonded silica capi l lary columns and FID. The temperatures of the injector and detector were between 240 °C and 280 °C. The volume of nitrogen gas used was 1 mL/min. The oven temperature was set at 50 °C for 3 min and then to 280 °C for 3 min. A total of 0.1 uL of sample diluted in Hexane at a concentration of 1mg/mL was injected, and the integration of peak contents was used to assess the qual i ty and amount of the identified active molecules. To analyse the extracts, we used an HP 5972/A MS set to 70 eV and helium at 20 p.s.i. The identification of volatile molecules was based on a comparison of their retention indices with those of the Wiley Library search routines [45], which were founded on mass spectra that were qualified according to their fit and purity. Kovats index(I) was calculated as I = 100 [n +(Log (ti-to)- Log (tn - to)/Log(tn +1 - to) 一 Log(tn - t o)], where n is the carbon number of n-alkane. The parameters described above in this formula are: Peak heading: t is t he retention time of compound i in minutes; to is the air peak void time as the average velocity. 3.3. Determination of Minerals 3.3.1. Determination of Potassium and Calcium The powder materials of both the tested plant species were weighed in a numbered capsule, and then these samples were placed in a muffle furnace at a temperature T1equa l to 220 °℃ for 2 h and then at a temperature T2 equal to 550 °C for 6 h to ensure the destruction of the chemical bonds and thus obtain free atoms and ions. This step is called calcination. Following this step, concentrated hydrochloric acid was added to each capsule. After heating these samples on a hot plate until the total evaporation of the acid, 5 mL of N/10 hydrochloric acid was added. The next step was f il t ration. The solutions obtained were filtered into 50 mL volumetric flasks, and distilled water was added to the mark of the dipstick. Finally, the determination of potassium, sodium, and calcium was carried out via f lame photometry; therefore, i t was necessary to prepare stock solutions and calibration solutions of each element. 3.3.2.Determination of Nitrogen the mineralization operation was completed , the tubes were moved one by one into the Vapodest for potentia l distil l ation, and the pH was measured. 3.4. Antioxidant Activity 3.4.1. ABTS Scavenging Assay The spectrophotometric analysis of the activity of organic extracts from G. alypum, used to trap ABTS + cations, was determined according to the method of El Arem and his collaborators [47]. The ABTS cation radical was generated by mixing equal volumes of a solution of potassium persulfate K2S2Og and a stock solution of ABTS (39.2 mg), and the combined solution was stored away from light at room temperature for 16 h before use. The solution obtained was diluted with ethanol to obtain an absorbance between 0.7 and 0.8 at 734 nm. A volume of 975 pL of this freshly prepared solution was added to 25 uL of each extract, and the reading was carried out at 734 nm after 20 min for each analysis series. Ascorbic acid was used as a positive control. The antioxidant activity was determined according to the discoloration of the solution and expressed as the percentage inhibition (PI) of the absorbance at t he wavelength of 734 nm, at which point the ABTS+· radical presents a characteristic absorption band. Inhibition (%)=[(Abscontrol - Absextract)/Abscontrol]×100. 3.4.2. B-Carotene The oxidation of l inoleic acid generated the peroxide radicals which then oxidized the B-carotene, causing the disappearance of its red color. This test was based on an emulsion mixed with 0.5 mg B-carotene, 1mL chloroform, 25 uL linoleic acid, 200 mg Tween 20, and 100 mL distilled water. After that, 2.5 mL of this emulsion was added to 0.5 mL of extract or control BHT (butyl hydroxytoluene) and left to undergo incubation for 2 h at 50°C in a water bath. The emulsion was added to the sample and measured severa l times at intervals of 20 min [48] starting with the absorbance at To, which was measured at 490 nm. The percentage of inhibition of peroxidation was calculated according to t he following formula:%IP =B-carotene after 2 h/initial B-carotene ×100. 3.5. Antiproliferative Effect An evaluation of the antiproliferative effect was performed using the MTT test for the determination of the cellular metabolic activity with a measure of the SW620 cells extracted from colorectal human cells. These cells were cemented on a plate (MultiScreen@filtration plates , 96-well plates) at a density of5×10 in an i ncubator at 37℃ with 5% CO2. The cells were extracted from the medium culture and treated with the extracts (diluted in DMSO) at different concentrations (100; 50; 40; 20;10 ug/mL). This assay was repeated three times. The treatment with MTT solution (1 mg/mL in culture medium named RPMI 1640supplemented with 10% fetal bovine serum and 10 ug/mL antibiotic: 5 ug/mL penicillin and 5 ug/mL streptomycin) began after 48h of i ncubation. The appearance of purple formazancrystals from the yellow tetrazolium sal t was an indicator of metabolically active cel l s. To calculate the percentage of survival of these cells, we used the ratio (OD in the test group/OD in the control group x100), which was measured using an ELISA reader at 570 nm after 2 h of incubation in an incubator at 37 °C with 5% CO2. Three independent experiments were performed. 3.6. Antibacterial Activitu 37 °C for 24 h, the antibacterial activity was evaluated by measuring the inhibition zone formed around the disc. Each assay was performed in triplicate. The MIC was evalu-ated [50]. Serial dilutions of the extracts (0.05-25 mg/mL) were applied to plates with 96U-bottomedwells (Nunc, Roskilde, Denmark) together with MH broth and the target bacteria. After incubation a t 37 °C for 24 h, the MBC was evaluated by transferring 10 uL showing no bacterial growth after the MIC assay on MH agar f rom the well. After 24 h of incubation at 37°C, the bacterial growth was examined, and the MBC was determined as the lowest concentration of the sample having bactericidal activity. All assays were performed in triplicate. Three i ndependent experiments were performed. 3.7. Antibiofilm Assay The antibiofilm assay was determined via the crystal violet assay [50]. The extracts di l uted in sterile disti l led water at different concentrations (0.05-25mg/mL) were mixed with pathogenic bacteria suspension (grown in BHI, for 24 h at 37°℃; 10 5 CFU/mL)on plates with 96 U-bottomed wells (Nunc, Roskilde, Denmark) containing brain heart infusion (BHI) (Oxoid) with 2% glucose (w/v).Wells containing only BHI with 2% glucose and BHI with 2% glucose inoculated with the pathogenic strain served as the negative and positive controls, respec t ively. After incubation at 37 °C for 24 h, the plates were rinsed 3 t imes with PBS. Cells in the biofilm were fixed with methanol for 15 min, air-dried, and stained with 1% crysta l violet. Biofilm formation was quantified by measuring the absorbance at 595 nm using a microplate reader (GIO. DE VITA E C, Roma, Italy). All assays were performed in triplicate. Three independent experiments were carried out. Inhibition (%)=(ODcontrol-ODExtract)/ODcontrol×100. 3.8. Statistical Analysis The results are represented as mean values ± standard deviation. Statistical analyses were based on SPSS version 22. ANOVA and the Student-Newman-Keuls test were used in these analyses. Data were considered statistically different at a p-value of 0.05 or less. 4. Conclusions Based on t he results of this study, the chemical analysis of G. alypum leaves showed the plant's richness i n polyphenols extracted with different methods. In addition, GC-MS analysis revealed t he presence of other active molecules. The ethanol extract obtained via maceration had a powerful abil i ty to inhibi t oxidation. In addition, the extracts obtained via soxhlet extraction and maceration had important anti-inflammatory effects. However, the extract obtained via sonication demonstrated a significant antiproli f erative property against SW620. Because of the richness of G. alypum leaves in active molecules, this medicinal plant could be exploited not only for the treatment of diseases related to oxidative stress and colorecta l cancer but also for use against bacteria l infec t ions. Globally, the sonication method enables the bursting of plant cells by releasing the active molecules through vibration. Comparing these three extraction methods, our results revealed that the extract obtained via sonication presented the highest potential, while the soxhlet method altered the quality of the active molecules due to the long extraction time and the high temperature required. However, the maceration extract, obtained at room temperature, is not the best for evaluating antimicrobial activity. Author Contributions: S.N. wrote the main manuscript. A.D., R.B., H.H., A.K., L.A. and M.G. contributed to the preparation of figures. A.Z. revised the work. All authors have read and agreed to the published version of the manuscript. Funding: This research received no external funding Institutional Review Board Statement: Not applicable Informed Consent Statement: Not applicable. Data Availability Statement: Materials, data, and associated protocols are available to readers without undue qualifications regarding material transfer agreements. For data retrieval, please contact (email: zairi _amira@yahoo.fr). Acknowledgments: We thank Kamel Maaloul, Scientific Translation Expert, Sfax University, Tunisia. We also thank Fethia Harzallah Skhiri of the for the botanic identification of the plant: Globularia alypum (L.). Conflicts of Interest: The authors declare no conflict of interest. References 1. Siddiqui , A.J.; Danciu, C.; Ashraf, S.A.; Moin, A.; Singh, R.; Alreshidi, M.; Patel, M.; Jahan, S.; Kumar, S.; Alkhinjar, M.I.M.;et al. Plants-derived Biomolecules as potent antiviral phytomedicines: New insights on ethnobotanica l evidences against coronaviruses. Plants 2020, 9, 1244. [CrossRef ] [PubMed] 2. Bartekova, M.; Adameova, A.; Gorbe, A.; Ferenczyova, K.; Pechanova, O.; Lazou, A.; Dhalla, N.S.; Ferdinandy, P.; Giricz, Z. Natural and synthetic antioxidants targeting cardiac oxidative stress and redox signaling in cardiometabolic diseases. Free Radical Biol. Med. 2021, 169,446-477.[CrossRef] [PubMed ] 3. Ciampi,F.;Sordillo, L .M.; Gandy, J.C.; Caroprese, M.; Sevi , A.; Albenzio, M.; Santi l lo, A. Evaluation of natural plant extracts as antioxidants in a bovine i n vitro model of oxidative stress. J. Dairy Sci. 2020, 103,8938-8947.[CrossRef ] [PubMed ] 4. El Hachlafi, N.; Chebat, A.; Bencheikh, R.S.; Fikri, B.K. Ethnopharmacological study of medicinal plants used for chronic diseases treatment in Rabat-Sale-Kenitra region (Morocco). Ethnobot . Res. Appl . 2020,20, e02191. [CrossRef ] 5. Zekeya, N.; Ibrahim, M.; Mamiro, B.; Ndossi , H.; Kilonzo, M.; Mkangara, M.; Chacha, M.; Chilongola,J.; Kideghesho, J. Potential of natural phenolic antioxidant compounds from Bersamaabyssinica (Meliathacea) for treatment of chronic diseases. Saudi J. Biol. Sci. 2022,29,103273. [CrossRef ] 6. T i ss, M.; Souiy, Z.; Achour, L.; Hamden, K. Anti-obesity, anti-hyperglycaemic, anti-antipyretic and analgesic activities of Globulariaalypum extracts. Arch. Physiol . Biochem. 2020, 128,1453-1460. 7. Friscic, M.; Petlevski, R.; Kosalec, I.; Madunic, J.; Matulic, M.; Bucar, F; Hazler Pilepic, K.; Males, z. Globulariaalypum L. and Related Species: LC-MS Profiles and Antidiabetic, Antioxidant, Anti-Inflammatory, Antibacterial and Anticancer Potential. Pharmaceuticals 2022, 15, 506.[CrossRef ] 8. Rahman,M.;Rahaman, S.;Islam, R.; Rahman, F .;Mithi , F.M.; Alqahtani,T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwai l i, A.S.; Hossain, S.; et al . Role o f Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022,27,233. [CrossRef ] 9. Abubakr, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied. Sci. 2020, 12, 1-10. [CrossRef ] 10. Chibuye, B.; Singh, S.I.; Chimuka, L.; Maseka, K.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci . Afr. 2023,19, e01585.[CrossRef ] 11. Hayouni, E.A.; Abedrabba, M.; Bouix, M.; Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological act i vi t ies i n vitro of Tunisian Quercuscocc i fera L. and Juniperusphoenica L. frui t extracts. Food Chem. 2007, 105, 1126-1134.[CrossRef | 12. Lameirao, F.; Pinto, D.F.; Vieira, E.F.; Peixoto, A.; Freire, C .; Sut , S.; Rodrigues, F. Green-sustainable recovery of phenolic and antioxidant compounds from industrial chestnut she l ls using ultrasound-assisted extraction: Optimization and evaluation of biologica l activities in vitro. Antioxidant s 2020, 9,267. [CrossRef] [PubMed ] 13. Alara,O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sc i . 2021,4,200-214.[CrossRef ] 14. Quispe Candori, S.; Foglio, M.A.; Rosa, P.T.V.; Meireles, M.A.A. Obtaining b-caryophyllene from Cordiaverbenacea de Candolle by super crtitical fluid extraction. J. Supercrit. Fluids. 2008, 46,27-32. [CrossRef] 18. Al-Qahtani, W.H.; Dinakarkumar, Y.; Arokiyaraj , S.; Saravanakumar, V.;Rajabathar, J.R.; Arjun, K.; Gayathri, P.K.; Appaturi, J.N. Phyto-chemical and biological activ i ty of Myristicafragrans, an ayurvedic medicina l plant in Southern India and its ingredient analysis. Saudi J . Biol. Sci . 2022,5,3815-3821. [CrossRef ] 19. Tan, G.; Wang, Z.;Che, L.; Yin, S. Immunotherapeutic effects on murine pancreatic carc i noma by beta-elemene combined with dendritic cells modified with genes encoding interleukin-23. Front . Med. China 2007,1, 41-45. [CrossRef ] [PubMed ] 20. Li-fei , M.; Wei-Min, H.; Jun, L.; Jing, S. Study on the antioxidant effects of B-elemene. Chin. J. Clin. Pharmacol. Therap. 2012,7,727-731. 21. Asraoui, F;Kounnoun, A.; El Cadi, H.; Cacciola,F; El Majdoub,Y.O.; Alibrando,F.;Mandolfino, F; Dugo, P; Luigi Mondello, L.; Louajri, A.A. Phytochemical investigation and antioxidant activity of Globulariaalypum L. Molecules 2021, 26, 759.[CrossRef ] 22. Khantouche, L.;Guesmi, F;Motri, S.; Mejri, M.; Abderabba,M. Nutritional Composition, Analysis of Secondary Metabolites and Antioxidative Effects of the Leaves of Globulariaalypum L . Indian J. Pharm. Sci . 2018,80,274-281.[CrossRef ] 23. Ndomou, S.C.H.;Djikeng, F.T.; Teboukeu, G.B.; Doungue, H.T. Hermann Arantes Kohole Foffe, Cristelle Tsapla Tiwo,Hilaire Macaire Womeni . J . Agric. Food Res. 2021, 3, 100105. 24. Jrah Harzallah, H.;Neffati, A.; Skandrani , I.; Maaloul, E.; Chekir Ghedira, L.; Mahjoub, T. Antioxidant and antigenotoxic activities of Globulariaalypum l eaves extracts. J. Med. Plant Res. 2010, 4, 2048-2053. 25. Rossi,P.; Bao, L .; Luc i ani, A.; Panighi,J.;Desjobert, J .M.; Costa, J.; Casanova,J;J ean-Bolla, M.; Berti , L . (E)-Methylisoeugenol and Elemicin: Antibacterial Components of Daucuscarota L. Essential Oil against Campylobacter jejuni . J. Agric . Food Chem. 2007,55,7332-7336.[CrossRef ][PubMed ] 26. Surveswaran,S.; Cai, Y.Z.;Corke, H.;Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants . Food Chem. 2007, 102,938-953. [CrossRef ] 27. Chen, J; Wang, R.; Wang, T .;Ding, Q.; Khalil, A.; Xu, S.; Lin, A.; Yao, H.; Xie, W.; Zhu, Z.; et al . Antioxidant Properties of Novel Dimers Derived from Natural β-Elemene through I nhibiting H2O2-Induced Apoptosis. ACS Med. Chem. Lett . 2017, 8,443-448.[CrossRef ][PubMed] 28. Trevizan, L.N.F .; Nascimento, K.F.; Santos, J.A.; Kassuya, C.A.L.; Cardoso, C.A.L.; Vieira, M.D.C.; Moreira, F.M.F .; Croda, J.; Formagio, A.S.N. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylusedulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016,4,510-515.[CrossRef ] 29. Tiwari , M.; Kakkar, P. Plant derived antioxidants-Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitr . 2009,23,295-301. [CrossRef ][PubMed ] 30. Huang, M.S.; Moreiras, A.M.S.; Abel , C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-B-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193,997-1008.[CrossRef ] 31. Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.P.; Calixto, B.J. B-caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARy pathway. Am. J. Pathol. 2011, 178,1153-1166.[CrossRef | 32. Amiel , E .; Of ir , R.; Duda i , N.; Soloway, E.; Rabinsky, T.;Rachmilevitch, S. B-caryophyllene, a compound i solated f rom the biblical balm of gilead (Commiphoragileadensis), is a selective apoptosis inducer for tumor cell l ines. Evid.-Based Complement. Altern. Med.2012,2012,872394. [CrossRef] [PubMed ] 33. Legault, J.; Pichette, A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007,59,1643-1647.[CrossRef] [PubMed] 34. Carneiro, A.I.B.; Teixeira, M.F.S.; De Oliveira, V.M.A. Screening of Amazonian plants from the Adolpho Ducke forest reserve, Manaus, state o f Amazonas, Brazi l , for antimicrobia l activity. Mem. Inst . Oswaldo Cruz 2008, 103, 31-38. [CrossRef ] Lozienne, K.; Ausra, S.; Juozas, L. Scavenging and antimi 38crobial properties of extracts. Food Chem. 2007,103,546-559.[CrossRef ] Hailu, T.; Endris, M.; Kaleab, A.; Tsige, G.M. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the t reatment of skin disorders. J. Ethnopharmacol. 2005, 100,168-175.[CrossRef ] 37. Tegos, G.; Stermitz, F.R.; Lomovskaya,O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicro-bials. Antimicrob. Agents Chemother. 2002,46,3133-3141. [CrossRef ] 38. T im Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99-107.[CrossRef ] 39. Hayakawa, S.; Kawamura, M.; Sato, T.; Hirano, T.; Fujimura, S. An c-lipoic acid derivative, and anti-ros agent, prevents the acquisition of multi-drug resistance in clinical i solates of Pseudomonas aeruginosa.J. Infect. 2018,25,28-33.[CrossRef ] [Pu b Med ] 40. Tan, M.; Zhou, L.;Huang, Y.; Wang, Y.;Hao, X.; Wang, J . Antimicrobia l activity of globulol isolated from the fruits of Eucalyptus globulus Labill. Nat. Prod. Res. 2008, 22,569-575. [CrossRef] [PubMed ] 41. De Oliveira, M.M.M.; Brugnera, D.F. Disinfectant action of Cymbopogon sp. essential oils in different phases of biofilm formation by Listeria monocytogenes on stainless steel surface. Food Control. 2010,21,549-553.[CrossRef ] 42 Anwar, H.;Dasgupta, M. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob. Agents Chemother.1990, 34, 2043-2046. [CrossRef] [PubMed ] Watnick,P .; Kolter, R. Minireview: Biofilm, city of microbes. J. Bacteriol. 2000,182,2675-2679. [CrossRef] [P 43.ubMed] Melo, L . Biofilm f ormation and its role in f ixed film processes. In The Handbook of Water and Wastewater Microbiology; Academic Press: London, UK, 2003; pp. 337-349. 45. Adams,R.P. Identification of Essential Oi l Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. 46. Martin-Prevel, P.; Gagnard, J.; Gautier, P. L'analyse Vegetale dans le Controle de L'alimentation des Plantes Temperees et Tropicales; Lavoisier Tec e t Doc: Paris, France, 1984; p. 810. 47. El Arem, A.; Saafi , A.; Mechri, B.; Lahouar, L.; Issaoui, M.; Hammami, M.; Achour, L . Effects of the Ripening Stage on Phenolic Profile, Phytochemical Composition and Antioxidant Activity of Date Palm Fruit. J. Agric. Food Chem. 2012, 60,10896-10902. I CrossRe f 48. Barros, L.; Ferreira, M.J.; Queiros, B.; Ferreira, I.C.; Baptista, P. Total phenols, ascorbic acid, p-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007,2,413-419. [CrossRef | 50. Chaieb, K.; Kouidhi, B.; Jrah, H.; Mahdouani , K.; Bakhrouf, A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biof i lm formation. BMC Complement. Altern. Med. 2011, 11,29. [CrossRef ] Disclaimer/Publisher's Note: The statements, opinions and data contained i n all publications are solely those of the i ndividual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, i nstructions or products referred to in the content.
确定












还剩13页未读,是否继续阅读?
中国格哈特为您提供《南欧球花叶有效成分的提取以及氮含量的检测》,该方案主要用于中药材和饮片中萃取检测,参考标准--,《南欧球花叶有效成分的提取以及氮含量的检测》用到的仪器有格哈特全自动快速溶剂萃取仪Sox416、格哈特传统经典索氏提取/萃取仪EV6 AII16、格哈特快速凯氏定氮烧瓶红外消化炉KI 11/26、格哈特全自动凯氏定氮仪VAP500、棕色避光防紫外线萃取杯、德国移液器MM、凯氏定氮催化剂5.0g K2SO4+0.5g CuSO4 x 5H2O
相关方案
更多
该厂商其他方案
更多
















