方案详情
文
评估75个苹果品种种子的含油率、脂肪酸、类胡萝卜素和生育酚组成Assessing the Fatty Acid, Carotenoid, and Tocopherol Compositions of Seeds from Apple Cultivars (Malus domestica Borkh.) Grown in Norway.
方案详情

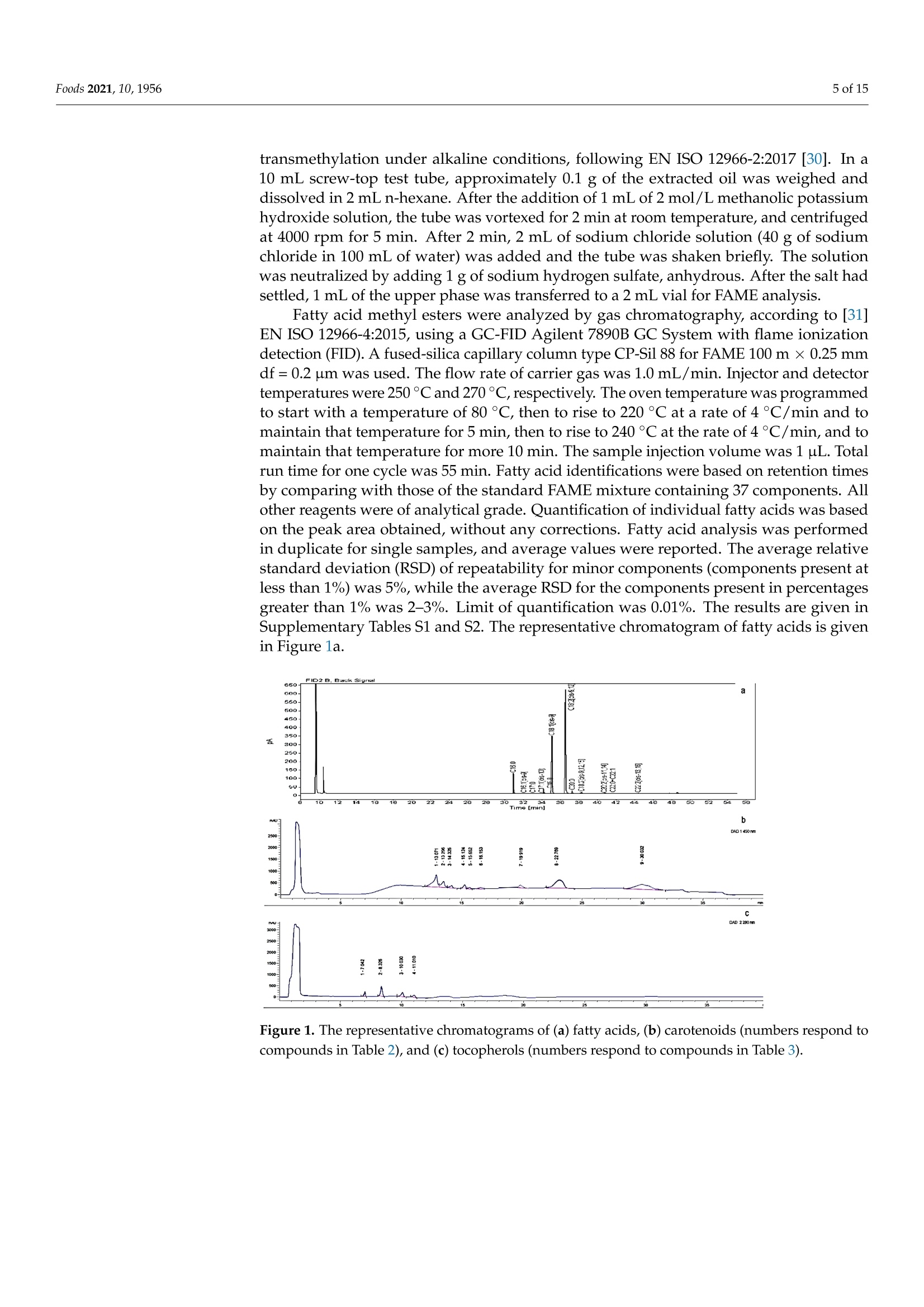
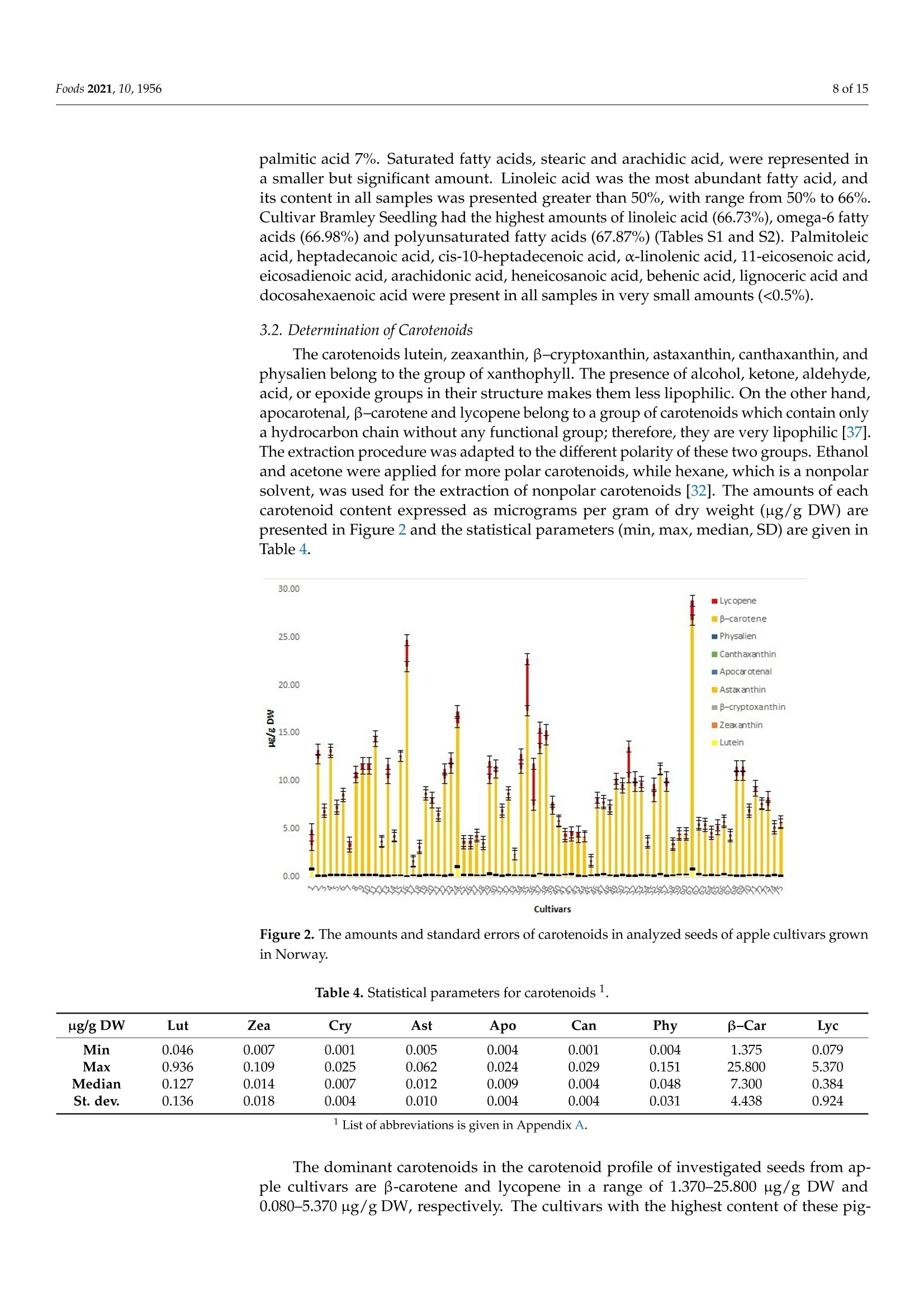
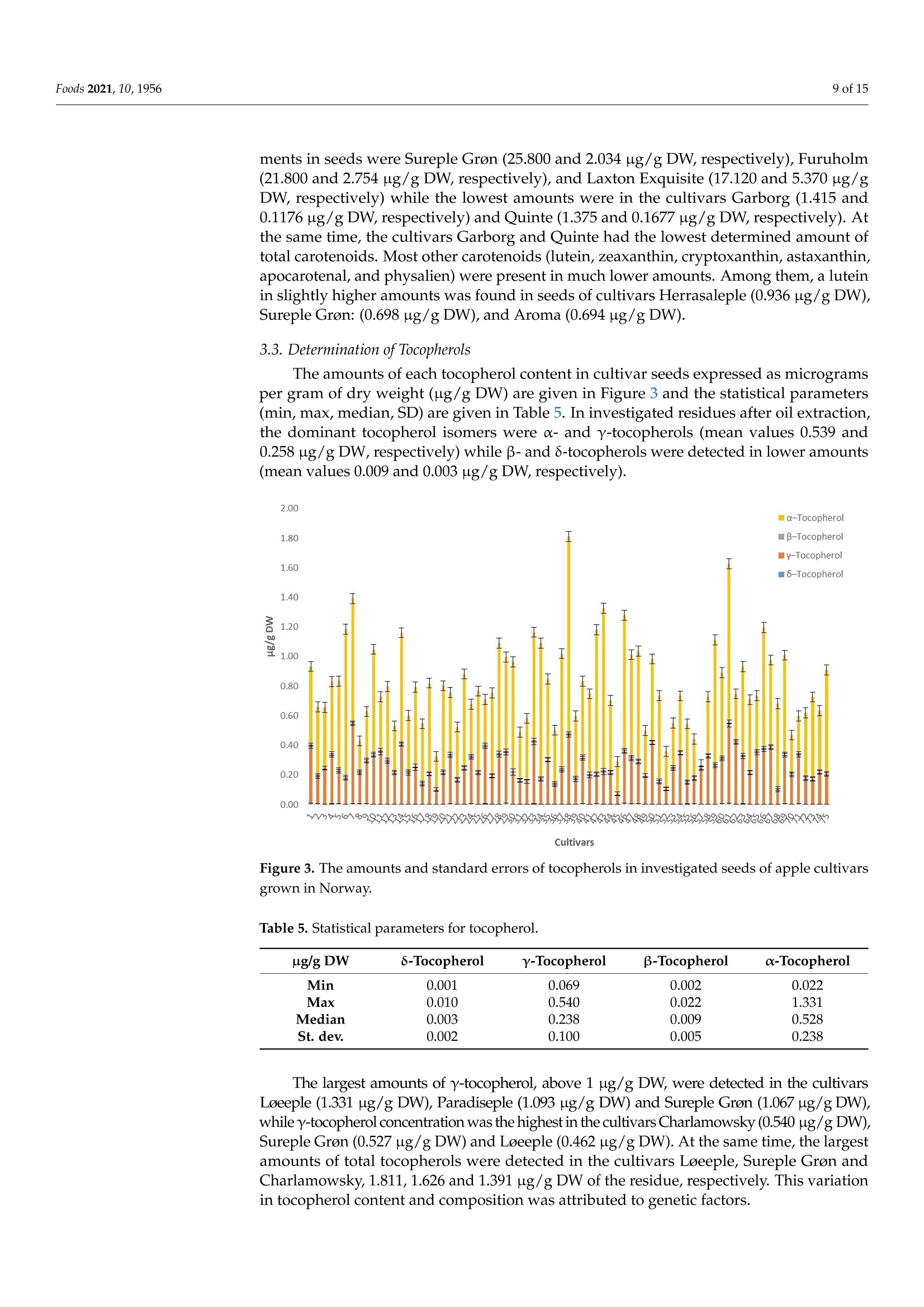
评估75个苹果品种种子的含油率、脂肪酸、类胡萝卜素和生育酚组成Assessing the Fatty Acid, Carotenoid, and Tocopherol Compositions of Seeds from Apple Cultivars (Malus domestica Borkh.) Grown in Norway.评估75个苹果品种种子的脂肪酸、类胡萝卜素和生育酚组成 Foods 2021, 10,19562 of 15 Article Assessing the Fatty Acid, Carotenoid, and Tocopherol Compositions of Seeds from Apple Cultivars (Malus domestica Borkh.) Grown in Norway Milica Fotiric Aksic 1, Kristina Lazarevic2, Sandra Segan D, Maja Natic 4D, Tomislav Tosti4D, Ivanka Ciric 5D and Mekjell Meland 6,* Faculty of Agriculture, University of Belgrade, 11080 Belgrade, Serbia; fotiric@agrif.bg.ac.rs Citation: Akic, M.F.; Lazarevic,K.;Segan, S.; Natic , M.; Tost i, T .; Ciric , I.;Meland , M. Assessing the Fatty Acid,Carotenoid,and Tocopherol Compositions of Seeds f rom Apple Cultivars (Malus domes t ica Borkh.)Grown in N o rway. F o ods 2021,10,1956. ht t ps://doi.org/10.3390/foods10081956 Academic Editors : Ana Blandino and Ana Belen Diaz Received: 19 July 2021 Accepted: 19 August 2021 Published: 22 Augus t 2021 Publisher's Note: MDPI stays neutra l with regard to jurisdictional claims in publ i shed maps and institutiona l affil -iations. Copyright: C 2021 by the authors.Licensee MDPI , Basel, Switzerland.This article is an open access article distributed u un n d d e e r r t t h he e terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/). 2Centre for Food Analysis, 11080 Belgrade, Serbia;kristina.lazarevic@cin.co.rs 3 Institute of Chemistry , Technology, and Metallurgy , University of Belgrade, 11000 Belgrade, Serbia;sgaica@chem.bg.ac.rs 4 Faculty of Chemistry , University of Belgrade, 11000 Belgrade, Serbia; mnatic@gmail .com(M.N.);tosti@chem.bg.ac.rs (T .T .) 5 Innovation Centre of Faculty of Chemistry Ltd., 11000 Belgrade, Serbia; ivankai@chem.bg.ac.rs 6 Norwegian Institute of Bioeconomy Research, NIBIO U l lensvang, Ullensvangvegen 1003,N-5781 Lofthus, Norway 准 Correspondence: mekjell.meland@nibio.no Abstract: Apple production generates large amounts of apple pomace including seeds, leading to high transportation costs, public health hazards and undesirable odor. A new reuse strategy of this kind of waste could solve environmental issues and/or create unconventional sources of health beneficial products. In total, seeds from 75 apple cultivars grown in Norway (both domestic and international ) have been analyzed for the f irst t ime for oil content and fatty acid profile together with tocopherols and carotenoids quantification in defatted seeds. Seeds from cultivar Hakonseple had the highest oi l content (22.10%), with linoleic, oleic acid, and palmitic acid as the most abundant fatty acids. The levels of B-carotene and lycopene carotenoids and -tocopherol were the highest in defatted seeds of the cultivar Sureple Gron. Principal component analysis separated cultivars according to the total oil content. The Norwegian apple cultivars Hakonseple, Kv i teple, Tolleivseple,Vinterrosenstrips, and Tokheimseple are recommended for obtaining vegetable oi l due to their high oil contents, while cultivar Sureple Gron can be separated due to i ts high levels of β-carotene,lycopene and total tocopherols. Keywords: seed oil; defatted seeds; l inoleic acid; oleic acid; B-carotene; l ycopene 1. Introduction Apple (Malus x domestica Borkh.) is economically and culturally the most important temperate fruit crop i n the world. It ranks second among the most widely produced fruits in t he world after banana. Due to climate adaptations and having a standing temperature between -30 °C and +40 C, apple trees are grown in 96 countries that produce apples for their domestic markets and export [1]. Apple contributes significantly to human daily consumption due to having less perishability than most other fruits, year-round availability,good transportability, comparatively low price, and nutritional qualities [2]. The global acreage is ~4.7 mi l ha, bearing ~87 mil tons of apples, where China produces ~42 mil tons,USA is in second place with ~5 mil tons and Turkey in the third place with ~3.6 mil tons [3]. Because of i ts precious characteristics (aroma, taste and its beneficial effects in coun-teracting obesity, cancer, cardiovascular disease, asthma, diabetes and others), out of the total production, about 70% of the fruit is consumed as fresh, while 25-30% of the fruit is processed [4,5]. Among numerous products, juice, concentrate, marmalade, jam, dried fruits and cider could be under l ined as the most important. Based on Statista (2021)[6] and AICV (2020) [7], more t han 2 million l iters of apple juice and more than 1.5 million l iters of cider, respectively, were produced in the EU in 2017. The total industrial production process is about 70-75% juice with 25-30% apple pomace and 5-11% sludge. Apple pomace consists mainly of apple skin/flesh (95%), seeds (2-4%) and stems (1%) [8]. According to Bhushan et al.[9], several million tons of apple pomace and several hundred thousand tons of apple seeds are generated worldwide. The bulky nature of apple pomace and its susceptibility to microbial decomposition are leading to public health hazards. Even dumping of such material is a big problem due to the high t ransportation costs and undesirable odor [9]. In the past, apple pomace was used as animal feed, fuel for boilers, added to soil as a conditioner, and as a substrate for microbial growth and later in the production of value-added products such as organic acids, enzymes, single cell proteins, low alcoholic drinks, ethanol , biogas, pigments and baker's yeast [10-12]. Recent studies have reported that the side-products of the Malus genus can be used in the pharmaceutica l and natura l cosmetic industries for the production of perfumes, toiletries and chemical addi t ives [13,14]. Still, there i s no systematic collection and utilization of this material; thus, a valuable product with a large industrial potential remains unexploited [15]. Apple seeds have been shown to be rich in bioactive compounds such as proteins,carbohydrates, minerals, unsaturated (monounsaturated and polyunsaturated) fatty acids (mostly oleic acid and linoleic acid), tocopherols and tocotrienol homologues (tocochro-manols), carotenoids, dietary f iber and polyphenolics [16]. Apple seeds contain high amounts of fat that can reach up to 29% of oil but do not contribute to cholesterol formation in humans [17]. Thus, this kind of oil can be considered as a high-quality edible vegetable oil [18-21] proved to have antioxidant, antimicrobial and antiproliferative properties. Oil obtained from apple seeds is light yellow and aromatic. Nevertheless, seeds also store amygdalin (cyanogenic glycoside), which is potentially toxic in the presence of enzymes resulting in the releasing of hydrogen cyanide. The extraction of amygdalin can be car-ried out using a Soxhlet extractor, an ultrasonic bath, or the solid-phase extraction (SPE)method. Polar solvents are suitable for the extraction of amygdalin. Mostly these are ethanol , methanol, ethyl acetate, and water, but also 0.1% citric acid solution under reflux is used to increase the efficiency of extraction. To obtain a high-quality oil free of amygdalin,the extraction procedure should be optimized or cold-pressing can be carried out [22]. Fruit production in Norway is located in the southeastern part (around lakes) and in the southwestern part (around f jords), which are the most northerly fruit tree-producing areas in the world. Apples are the largest fruit crop produced in Norway. Consumption of fruit i s increasing and there i s a high demand for locally, sustainably produced apples of high quality. The acreage of Norwegian commercial apple production in 2020 was 1538 ha, where a tota l of 6783 tons were freshly consumed and 5674 tons were used for juice processing [28]. The by-product of the j uice industry—apple pomace-is a waste in Norway, currently has no commercial value, and is used for feeding animals. Recently, an interest in the usage of agro-industrial waste and unconventional oil sources has gained more attention. This kind of by-product from the apple industry can lower disposable problems, and obtained products can be a potential source of natural bioactive compounds with high biological importance and economically feasible produc-tion. The aim of this study was to analyze the seed oil from different traditional Norwegian apple cultivars, for the first time, and recommend the cultivars that can be used for oil production. Regarding the fact that the majority of scientists have dealt with tocopherol and carotenoid composition in seed oils [14,18,29], but not many have discussed tocopherols and carotenoids i n residues af t er oi l extraction, another goal was to quantify thei r presence in defatted seeds. 2. Materials and Methods 2.1. Plant Material In this study, fruits from a total of 75 apple accessions maintained in the ex situ collection located in western Norway (Hjeltnes college, a municipality of the Hardanger district-lat 60°33'N, long 6°55’E) were sampled during the falls of 2017 and 2018 (Table 1).Most sampled accessions are considered traditional Norwegian cultivars. However, some cultivars represent foreign cultivars with a longer or shorter tradition of commercial production in Norway. Each accession was represented with five trees. Cultivars were grafted on the M 26 rootstock and planted in the period 1995-2000. Planting distance was 3 × 5 m with an East-West row orientation and trained as spindle trees. Orchard floor management consisted of grass in the interrow and a 1 m-wide herbicide strip in the intrarow space. The trees were not irrigated. All trees received the same amount of fertilizers each spring based on soil analysis. The trees were managed according to the general agricultura l practices for the area inc l uding the plant protection program. Harvest time was according to the commercial standards for maturity based on fruit color, seed development and f irmness. Table 1. Investigated apple cultivars, country of origin, and ripening time. No. Cultivar Origin Ripening Time No. Cultivar Origin Ripening Time 1 Aroma Sweden October 39 Magelemer Denmark Sepember 2 Bananeple Norway September 40 Marta-Moster Norway Sepember 3 Beauty of Bath UK September 41 Norfolk Royal UK Sepember 4 Belle de Boskoop The Netherlands October 42 Oster Norway September 5 Bramley Seedling UK October 43 Paradiseple Norway August 6 Brureple Norway September 44 Prins Norway August 7 Charlamowsky Russia September 45 Quinte Canada August 8 Cox's Orange UK October 46 Raud Granat Norway October 9 Cox's Pomona UK October 47 Raud Gravenstein Denmark September 10 Early Red Bird Canada August 48 Raud Safstaholm Sweden August 11 Elraud Pigeon Denmark November 49 Raud Sommerkavil Norway August 12 Elstar Boerekamp The Netherlands October 50 Ribston UK October 13 Franskar Norway August 51 Rondestveit Norway August 14 Fristeren Norway August 52 Rossvolleple Norway August Table 1. Cont. No. Cultivar Origin Ripening Time No. Cultivar Origin Ripening Time 15 Fuhr Norway September 53 Rubinstep CzechRepublic October 16 Furuholm Norway August 54 Signe Tillish Denmark September 17 Garborg Norway August 55 Silkepele Germany Sepember 18 Geneva Early USA August 56 Sitroneple Norway August 19 Gront Laupsaeple Norway August 57 Stor Granat Norway Sepember 20 Gul Granat Norway September 58 Storesteinseple Norway Sepember 21 Gullspir UK August 59 Strutar Norway Sepember 22 Gyldenkoks Astrakan Sweden August 60 Summered Canada September 23 Haugeeple Norway September 61 Sureple Gron Norway Sepember 24 Herrasaleple Norway September 62 Sysekavil Norway August 25 Hjartneseple Norway September 63 Savstaholm Sweden August 26 Husmoreple Germany October 64 Tolleivseple Norway Sepember 27 Hoyneseple Norway October 65 Tokheimseple Norway August 28 Hakonseple Norway October 66 Tormodseple Norway Sepember 29 Ingrid Marie Denmark October 67 Transparente Blanche Baltic countries August 30 Kaupanger Norway October 68 Tveiteple Norway September 31 Kavill Norway August 69 Ulgenes Norway Sepember 32 Knuteple Norway August 70 Vanleg Torstein Norway October 33 Kviteple Norway August 71 Vintergul Norway Sepember 34 Langballeeple Norway August 72 Vinterrosenstrips Norway August 35 Laxton Exquisite UK September 73 Worchester Pearmain UK September 36 Leiknes Norge September 74 Olands Kungseple Sweden August 37 Lord Lambourne UK September 75 Oskhaug Norway September 38 Loeeple Norway September Fruits from each cultivar were picked during full maturity (when fruits had typical color and taste and when seeds became brown). Seeds were extracted from 20 fruits (picked from five different trees and from all around the canopies), washed up with tap water and air dried for ten days. After, seeds were bagged and stored in a cool place until analysis. 2.2. Standards and Chemicals Lutein, zeaxanthin, B-cryptoxanthin, canthaxanthin, astaxanthin, apocarotenal , physalien,B-carotene, lycopene and tocopherols (, B, y and 8) were from Sigma-Aldrich, Inc. (St.Louis,MO,USA). Acetonitrile, methanol, ethanol, dichloromethane, acetone, n-hexane and ascorbic acid were of analytical-grade purity and purchased from Merck (Darmstadt , Ger-many). Water was prepared using a Millipore Simplicity 185 S.A., 67120, water purification system (Molshem, France). The standard FAME mixture of 37 components was purchased from Food Industry FAME Mix, RESTEK (Bellefonte, PA, USA) (lot 24676). 2.3. Oil Extraction and Fatty Acid Methyl Esters Determination The oil extraction and determination of fatty acid methyl esters of apple seeds were carried out according to standard method ISO 12966-2:2017 [30] and ISO 12966-4:2015 [31].Seed oil extraction was performed by Soxhlet extractor (Soxtherm-Gerhard) using n-hexane as a solvent. Oi l was extracted with 130 mL of n-hexane from 10 g of seed powder using a Soxhlet extraction system for 3 h. At the end of the extraction, the solvent was evaporated.The obtained oils were flushed with nitrogen to remove the residual traces of n-hexane and stored in the dark at 4C. Fatty acid methyl esters (FAME) were prepared using transmethylation under alkaline conditions, following EN ISO 12966-2:S 2 0W 17 [30]. In a 10 mL screw-top test tube, approximately 0.1 g of the extracted oil was weighed and dissolved in 2 mL n-hexane. After the addition of 1 mL of 2 mol/L methanolic potassium hydroxide solution, the tube was vortexed for 2 min at room temperature, and centrifuged at 4000 rpm for 5 min. After 2 min, 2 mL of sodium chloride solution (40 g of sodium chloride in 100 mL of water) was added and the tube was shaken briefly. The solution was neutralized by adding 1 g of sodium hydrogen sulfate, anhydrous. After the salt had sett l ed, 1 mL of the upper phase was transferred to a 2 mL via l for FAME analysis. Fatty acid methyl esters were analyzed by gas chromatography, according to [31]EN ISO 12966-4:2015, using a GC-FID Agilent 7890B GC System with f lame ionization detection (FID). A fused-silica capillary column type CP-Sil 88 for FAME 100 mx0.25mm df = 0.2 um was used. The flow rate of carrier gas was 1.0 mL/min. Injector and detector temperatures were 250 °C and 270°C, respectively. The oven temperature was programmed to start with a temperature of 80 °C, then to rise to 220 °C at a rate of 4 °C/min and to maintain that temperature for 5 min, then to rise to 240℃ at the rate of 4℃/min, and to maintain that temperature for more 10 min. The sample injection volume was 1 uL. Total run time for one cycle was 55 min. Fatty acid identifications were based on retention times by comparing with those of the standard FAME mixture containing 37 components. All other reagents were of analytical grade. Quantification of individual fatty acids was based on the peak area obtained, without any corrections. Fatty acid analysis was performed in duplicate for single samples, and average values were reported. The average relative standard deviation (RSD) of repeatability for minor components (components present at less than 1%) was 5%, while the average RSD for the components present in percentages greater than 1% was 2-3%. Limit of quantification was 0.01%. The results are given in Supplementary Tables S1 and S2. The representative chromatogram of fatty acids i s given in Figure 1a. Figure 1. The representative chromatograms of (a) fatty acids, (b) carotenoids (numbers respond to compounds in Table 2), and (c) tocopherols (numbers respond to compounds in T a ble 3). Table 2. Parameters of method validation for carotenoids No. Compound RT (min) Regression Equation R2 LOD LOQ Recovery (%) CV (%) 1 Lutein 13.071 y= 1.235x- 0.074 0.994 0.054 0.178 94.2-103.8 3.45 2 Zeaxanthin 13.296 y=1.012x+0.033 0.997 0.062 0.205 95.8-102.4 4.15 3 B-cryptoxanthin 14.325 y=1.007x-1.065 0.999 0.025 0.083 92.3-106.9 4.33 4 Canthaxanthin 15.124 y=1.154x-1.234 0.994 0.068 0.224 93.5-107.4 4.57 5 Astaxanthin 15.652 y=1.058x-0.863 0.992 0.076 0.251 95.8-107.6 4.18 6 Apocarotenal 16.153 y=1.023x+0.694 0.991 0.055 0.182 93.1-105.9 3.22 7 Physalien 19.919 y=1.195x -0.324 0.995 0.036 0.119 92.8-104.3 2.98 8 B-carotene 22.769 y=1.102x-0.753 0.997 0.029 0.096 91.7-109.3 3.74 9 Lycopene 30.032 y= 1.198x- 0.127 0.999 0.041 0.133 96.1-103.2 4.86 1 RT-retention t ime (min), Regression relationships, R2-coefficient of determination, LOD-limit of detection (ppm), LOQ -limit of quantification (ppm), Recovery (%), CV (%)-coefficient of variation, repeatability. Table 3. Parameters of method validation for tocopherols. No. Tocopherol RT1 (min) Regression Equation R2 LOD LOQ Recovery (%) CV (%) 1 8 27.042 y=1.114x+0.127 0.998 0.032 0.106 96.4-101.7 2.94 2 Y 83.326 y=1.076x +0.247 0.999 0.025 0.083 97.7-103.3 3.23 3 104.03 y=1.012x +0.541 0.997 0.021 0.069 98.5-101.9 3.54 4 C 11.01 y=1.337x +0.287 0.998 0.026 0.086 97.5-104.2 2.38 1 RT—retention t ime (min), Regression relationships, R2-coefficient of determination, LOD-limit of detection (ppm), LOQ -limit of quantification (ppm), Recovery (%)-, CV (%)-coefficient of variation, repeatabi l ity. 2.4. Carotenoids and Tocopherol Analysis 2.4.1. Extraction Procedure Extraction was performed by ultrasonication of 5 g of defatted seeds of each apple variety separately with solvents of different polarity (hexane, acetone, and ethanol) and the mixtures were left in an ice-cold VF ultrasonic bath (frequency 40 kHz, volume 4 L)for 15 min under a stream of nitrogen. I n order to i mprove the efficacy of total carotenoids extraction, solvents of different polarity were used. Hexane and acetone were used for extraction of less polar carotenoids (lycopene, B-carotene, B-cryptoxanthin, canthaxanthin),while ethanol was used for extraction of other more polar carotenoids (lutein, zeaxanthin,apocarotenal, physalien). The mixtures were centrifuged at 8000× g for 10 min and supernatants, which contained extracted carotenoids and tocopherols, were removed. The solid residues were re-extracted with fresh extraction solvent, applying the same procedure.The obtained extracts for each solvent were filtered, combined, and evaporated under N2.The residues were re-dissolved in 1 mL mixture of dichloromethane/acetone/ethanol (6:2:2,v/v/v), whereby the ethanol contained 0.5 g of ascorbic acid per 100 mL. This solvents mixture enables simultaneous extraction of less and more polar carotenoids [32]. The obtained extracts were filtered through 0.22 um and a volume of 100 uL was injected into the HPLC column. 2.4.2. HPLC Analysis An Agilent 1200 HPLC system (Agilent, Santa Clara, CA, USA) equipped with a Quat Pump (G1311B), Injector (G1329B) 1260 ALS, TCC 1260 (G1316A), Detector 1260DAD VL+ (G1315C), and t hermostated column compartment was used. Chromatographic separation was performed on octadecyl silica as a stationary phase (Nucleosil C18 analytical column, 4 mm x 150 mm, 5 um) using a mobile phase consisting of deionized water (A),acetonitrile (B), and methanol (C) [33]. The optimized HPLC method was developed to allow appropriate separation of individual carotenoids and tocopherols. The gradient protocol was: 0-4 min 15% A, 60% B, 25% C, 4-6 min 15%A →0%A, 60%B→70%B,6-34 min 0% A, 70% B, and 30%C, 34-35 min 0% A → 15%A,70%B→60%B,30%C→ 25%C, 35-40 min 15% A, 60% B,25% C. The mobile phase temperature was set at 30 °C,the flow rate was 1.0 mL/min. UV-vis diode array detection was set at 450 nm for carotenoids and at 280 nm for tocopherols. Carotenoids and tocopherols were identified by comparing their retention times and spectral data with those of the authentic standards. Quantification was performed by comparing peak area with standard reference curves. 2.4.3. Method Validation for Carotenoids and Tocopherols The carotenoid standards lutein, zeaxanthin, B-cryptoxanthin, and physalien were prepared a t a concentration of 1.00 mg/mL i n acetone-ethanol(1:1). The beta carotene and ly-copene were prepared at a concentration of 1.00 mg/mL in acetone [34] and dichloromethane,respectively. The standard solutions of tocopherols were prepared in an extraction mixture (dichloromethane/acetone/ethanol (6:2:2, v/v/v with ascorbic acid)) and a volume of 20 pL was injected. The parameters of method validation for carotenoids are given in Table 2 and the parameters of method validation for tocopherols are given in Table 3. The representative chromatograms of carotenoids and tocopherols are given in Figure 1b,c, respectively. 2.5. Statistical Analysis Basic statistics: minimum (min), maximum (max), median and standard deviation (SD) of the obtained amounts of fatty acids, carotenoids and tocopherols in apple cultivar seeds were calculated with Excel software ((Microsoft Office Professional Plus 2013, Santa Rosa,CA, USA). Principal component analysis (PCA) is one of the most widely used multivariate methods that reduces the dimensionality of the original dataset to a new set of non-correlated variables, called principal components (PCs), without losing much information.The results of a PCA are usually interpreted in terms of components (the transformed variable values corresponding to a data point), scores which correspond to the original samples, and loadings which correspond to the original variables. Graphical presentation of PCs as 2D or 3D patterns gives an overview of the relations between variables and between samples looking for groups and trends, sorting out out l iers [35,36]. Principal component analysis (PCA) was performed with the PLS Toolbox statistical package (version 5.7, Eigenvectors Inc.) from MATLAB version 7.4.0.287 (R2007a) (MathWorks INC, Natick,MA,USA) on a data matrix containing amounts of carotenoids, tocopherols and fatty acids in apple cultivar seeds. The data overview is obtained by using a singular value decomposition algorithm (SVD) and a 0.95 confidence level for Q and T2 Hotelling limits for outliers. In order to obtain variables on the same scale, the data pretreatment method called autoscaling (centering and rescaling to unit standard deviation) was performed. 3. Results 3.1. Oil and Fatty Acids Determination Results showed a high variability in total oil content among the apple seeds from the different cultivars (Table S1). The oil content ranged from 7.73 up to 22.10%. The average oil content in investigated apple seeds was 16.00 ± 3.39 g/100 g seeds. The lowest oil content was determined in seeds from the cultivar Bramley Seedling (7.73%),and it was also found in low amounts (less than 10 g/100 g) in the cul t ivar Haugeeple (9.69%), Knuteple (9.96%), Leiknes (9.71%),Oster (8.50%), Raud Gravenstein (9.69%), and Rossvol l eple (8.67%). The sample with the highest oil content was sample Hakonseple,with oil content of 22.10%. Oi l content higher than 20% was also determined in the cultivars Kviteple, Rubinstep, Tolleivseple, Vinterrosenstrips, and Tokheimseple, and the range was from 21.21% to 20.22%. In total, sixteen fatty acids were identified and quantified, where the most abundant were linoleic acid , oleic acid and palmitic acid, in decreasing order (Table S1). These three unsaturated fatty acids represented more than 95% of the total fatty acid contents. Of the total fatty acids content, l inoleic acid averaged 59%, oleic acid averaged 29%, and palmitic acid 7%. Saturated fatty acids, stearic and arachidic acid , were represented in a smaller but significant amount. Linoleic acid was the most abundant fatty acid, and its content in all samples was presented greater than 50%, with range from 50% to 66%.Cultivar Bramley Seedling had the highest amounts of linoleic acid (66.73%), omega-6 fatty acids (66.98%) and polyunsaturated fatty acids (67.87%) (Tables S1 and S2). Palmitoleic acid,heptadecanoic acid, cis-10-heptadecenoic acid, α-linolenic acid,11-eicosenoic acid,eicosadienoic acid, arachidonic acid, heneicosanoic acid, behenic acid, lignoceric acid and docosahexaenoic acid were present in all samples in very small amounts (<0.5%). 3.2. Determination of Carotenoids The carotenoids lutein, zeaxanthin, β-cryptoxanthin, astaxanthin, canthaxanthin, and physalien belong to the group of xanthophyll. The presence of alcohol, ketone, aldehyde,acid, or epoxide groups in their structure makes them less lipophilic. On the other hand,apocarotenal , B-carotene and lycopene belong to a group of carotenoids which contain only a hydrocarbon chain without any functional group; therefore, they are very lipophilic [37].The extraction procedure was adapted to the different polarity of these two groups. Ethanol and acetone were applied for more polar carotenoids, while hexane, which is a nonpolar solvent, was used for the extraction of nonpolar carotenoids [32]. The amounts of each carotenoid content expressed as micrograms per gram of dry weight (ug/g DW) are presented in Figure 2 and the statistica l parameters (min, max, median, SD) are given in Table 4. Cultivars Figure 2. The amounts and standard errors of carotenoid s in analyzed seeds of apple cultivars grown in Norway. Table 4. Statistica l parameters for carotenoids ug/g DW Lut Zea Cry Ast Apo Can Phy B-Car Lyc Min 0.046 0.007 0.001 0.005 0.004 0.001 0.004 1.375 0.079 Max 0.936 0.109 0.025 0.062 0.024 0.029 0.151 25.800 5.370 Median 0.127 0.014 0.007 0.012 0.009 0.004 0.048 7.300 0.384 St. dev. 0.136 0.018 0.004 0.010 0.004 0.004 0.031 4.438 0.924 1List of abbreviations is given in Appendix A . The dominant carotenoids in t he carotenoid profile of investigated seeds from ap-ple cultivars are B-carotene and lycopene in a range of 1.370-25.800 ug/g DW and 0.080-5.370 ug/g DW, respectively. The cultivars with the highest content of these pig- ments in seeds were Sureple Gron (25.800 and 2.034 ug/g DW,respectively), Furuholm (21.800 and 2.754 ug/g DW, respectively), and Laxton Exquisite (17.120 and 5.370 ug/g DW, respectively) while the lowest amounts were in the cultivars Garborg (1.415 and 0.1176 ug/g DW, respec t ively) and Quinte (1.375 and 0.1677 ug/g DW, respectively). At the same time, the cultivars Garborg and Quinte had the lowest determined amount of total carotenoids. Most other carotenoids (lutein, zeaxanthin, cryptoxanthin, astaxanthin,apocarotenal, and physalien) were present in much lower amounts. Among them, a lutein in slight l y higher amounts was found in seeds of cultivars Herrasaleple (0.936 ug/g DW),Sureple Gron: (0.698ug/gDW), and Aroma (0.694ug/gDW). 3.3. Determination of Tocopherols The amounts of each tocopherol content i n cul t ivar seeds expressed as micrograms per gram of dry weight (ug/g DW) are given in Figure 3 and the statistical parameters (min, max, median, SD) are given in Table 5. In investigated residues after oil extraction,the dominant tocopherol isomers were a- and y-tocopherols (mean values 0.539 and 0.258 ug/g DW, respectively) while β-and 6-tocopherols were detected in lower amounts (mean values 0.009 and 0.003 ug/g DW, respectively). Culti v ars Figure 3. The amounts and standard errors of tocopherols in investigated seeds of apple cultivars grown in Norway. Table 5. Statistical parameters for tocopherol. ug/g DW 8-Tocopherol Y-Tocopherol β-Tocopherol α-Tocopherol Min 0.001 0.069 0.002 0.022 Max 0.010 0.540 0.022 1.331 Median 0.003 0.238 0.009 0.528 St. dev. 0.002 0.100 0.005 0.238 The largest amounts of y-tocopherol, above 1 ug/g DW, were detected in the cultivars Loeeple (1.331 Hg/g DW), Paradiseple (1.093 ug/g DW) and Sureple Gron (1.067 ug/gDW),while y-tocopherol concentration was the highest in the cultivars Charlamowsky (0.540ug/gDW),Sureple Gron (0.527 ug/g DW) and Loeeple (0.462 ug/g DW). At the same time, the l argest amounts of total tocopherols were detected in the cultivars Loeeple, Sureple Gron and Charlamowsky, 1.811, 1.626 and 1.391 ug/g DW of the residue, respectively. This variation in tocopherol content and composition was attributed to genetic factors. 3.4. Principal Component Analysis (PCA) PCA resulted in a four-component model, which explains 49.72% of total variance (PC1 21.70%, PC2 12.78%, PC3 8.35%, and PC4 6.88%). The cultivars Bramley Seedling,Herrasaleple, Knuteple, and Quinte are outside the T2 Hotelling limits. In the scores plot of data, cultivar Bramley Seedling had the highest positive value of PC1, and the loadings plot indicates that the highest values of amounts (max of range) of linoleic acid (66.73%),omega-6 fatty acids (66.98%) and polyunsaturated fatty acids (63.87%) are discriminative factors, which influence the position of cultivar Bramley Seedling outside of T2 Hotelling limits. Cultivar Herrasaleple is recognized as the outlier due to having the highest amounts of carotenoids: lutein and zeaxanthin (0.936 and 0.109 ug/g DW, respectively). In cultivar Knuteple, the highest amount of omega-3 fatty acids (1.26%) was detected. Cultivar Quinte was characterized with the highest value (13.75%) of saturated fatty acids (SFA). In Figure 4,scores and loading plots of PC1 and PC2 are given. Figure 4. PC1-PC2 Score plots. (a) The classes are marked based on ripening time; (b) The classes are marked based on country of origin; (c) Loading plot. Please see t he Appendix A for the list of abbreviations. 4. Discussion 4.1. Oil and Fatty Acids The results are at the same level as published data regarding the fatty acid profiles and the content of triacylglycerols. Large variations in the total oil contents among studied cultivars are reported to be genotype dependent. Gornas et al. [13] studied crab and dessert apple seeds and reported seed oil yield in a range from 12.06 to 27.49% dry weight, and it was significantly dependent on the cultivar. In another study, Matthaus and Ozcan [38]obtained 21.9% and 25.6% of seed oil content of the two apple cultivars Golden Delicious and Starking, respec t ively, while Lei-Tian et al. [20] reported apple seed oil with a range from 20.6% to 24.3%. The most dominant fatty acids in the i nvestigated apple seeds were l inoleic acid (59.37-67.94%), oleic acid (20.68-29.00%), and palmi t ic acid (5.78-8.33%). Arain et al. [18]found oil content in the seeds of cultivars Royal Gala, Red Delicious and Golden Delicious from 26.8% up to 28.9%. In addition, Bada et al. [39] found the content of oil ranged from 19.67 to 22.73% of seeds from seven apple species from Asturias (Spain) and linoleic acid was reported as the main component i n Limon Montes (60.78%) and Riega (60.01%).Linoleic acid was dominant in Royal Gala (45.1%), Red Delicious (47.8%), and Golden Delicious (40.5%) [13]. In other studies [29,40], the Fuji and New Red Star seed oils mainly consisted of l inoleic acid (50.7-51.4g/100 g) and oleic acid (37.49-38.55g/100 g). These authors investigated dessert and cider apples (Malus domestica Borkh.) cultivars of different origin. Quantitatively, the oils were rich i n l inoleic acid (32.5 to 49.7 g/100 g) and oleic acid (15.1-33.3 g/100 g), while the contents of saturated fatty acids were up to ten times lower. The potential benefit of increasing dietary intake of l inoleic acid is connected to cardiovascular and mental health, and anti-cancerogenic and anti-diabetic effects [40].Oleic fatty acid consumption has been related to improved pancreas and liver secretory activity, protecting the cells f rom inflammation and fighting against coronary heart disease,rheumatoid arthritis, and cancer [41]. Linoleic, oleic and palmitic acid, the rest of the fatty acids determined in this study, had minor quantities, which supports the f indings of Arain et al. [18] who also identified l inolenic, palmitoleic, heptadecanoic, and 11-ecosenoic fatty acids in trace amounts (<1%). Moreover, apple cultivars showed pronounced differences in yields, numbers, and weights of their seeds [29] and this was also the case for the investigated samples presented herein. 4.2. Carotenoids The examination of the carotenoids in plants is mainly focused on the photosynthetic tissues and fruits. In seeds, they limi t the levels of free radicals and reduce peroxidase activity, thus reducing seed ageing and loss of seed viability [42]. I t has been noticed that during germination, the carotenoid content f irst increases slightly, and after the end of germination, it continues to grow sharply, even ten times more than the initial carotenoid content. This process is accompanied by a change in the presence of carotenoids, which reduces the presence of xanthophylls. There are few studies which compare the carotenoid contents of wild and domesticated plant species. Recent research has shown that wild legume seeds have higher levels of total carotenoids compared to their domesticated relatives. This is attributed to a side effect of selection for other desired traits, such as seed propagation mechanisms, seed storage and taste selections [43]. In the investigated press cake from apple seeds grown in Norway, the highest level of carotenoids were B-carotene and lycopene. B-carotene is a thermolabile orange pigment,and oxygen sensitive, and in most cases, i t is connected with heart disease (i t lowers LDL-cholesterol) and cancer. It is also a precursor of vitamin A, which is necessary for vision and cel l proliferation. On the other hand, diets rich in lycopene are associated with lower risk of cancer and all kind of degenerative diseases [44,45]. Unlike seeds, some authors found higher amounts of carotenoids in the flesh and espe-cially in the peel of cultivars with different external coloration (green, yellow and red) [46].Also, Fromm et al. [47] reported carotenoid concentration from 0.10 to 1.58 mg/100 g oil i n seed oils recovered from six cultivars of apples. Amariz et al . [48] determined 3.9 ug/g of total carotenoids in apple seeds. 4.3. Tocopherols In previously investigated apple seed oils, the most abundant isomer was B-tocopherol;therefore, it is expected that the residue after oil extraction will not be rich in this toco-pherol14,47,49. In the pressed cake of the studied apple cultivars that are grown i n Norway, α-tocopherols were the most abundant, followed by y-tocopherols. Although it is well known that o-tocopherol i s t he most abundant i n leaves, whereas y-tocopherol is dominant in seeds, some species such as sunflower, olive, safflower or grape store c-tocopherol as the main tocopherol form in seeds [24]. According to Gornas et al. [14], seeds from the apple cultivars Antej'and 'Beforest’were analyzed and c-tocopherol ranged f rom 17.22 to 25.79 mg/100 g dry weight basis (dwb),B-tocopherol between 7.53 and 29.05 mg/100 g dwb, y-tocopherol up to 13.82 mg/100 g dwb and 6-tocopherol within the range 0.16-10.79 mg/100 g dwb. The α:B:y:6 tocopherol ratio in those two cultivars was 1.7:1.5:1.3:1.0 and 2.1:2.0:1.3:1.0, respectively. All i somers (c-,B-, y-and 8-) of tocopherol belong to t he vitamin E active compounds.Their importance is reflected in the protection of polyunsaturated fatty acids against peroxidation [37]. They have various positive effects on human health, especially against cancer, heart disease, and other chronic ailments. Unfortunately, according to Fernandez-Marin et al. [50], a decrease in concentration of carotenoids in legume seeds has been observed because of domestication, where modern cultivars are showing much lower levels of carotenoids compared to wild relatives, so such a trend should be i nvestigated in apple wild genotypes and cultivars too. 4.4. Principal Component Analysis Based on ripening time and based on country of origin, some conc l usions could be drawn. According to the graph of loadings (Figure 4c), going along the PC1 axis, in the upper left part of the scores graph, cultivars are grouped according to their total amount of saturated fatty acids, as well as by individual content of saturated (palmitic, lignoceric,behenic, arachidonic, 11-eicosenoic, heneicosanoic) and some unsaturated omega 3 fatty acids (palmitoleic and docosahexaenoic) in their seeds. These cultivars were ripened in August and September. In the lower left part of the scores graph cultivars are grouped with similar amounts of saturated fatty acids (stearic, arachidic, and heptadecanoic), total amounts of monounsaturated fatty acids, omega 9, and with a similar amount of oleic acid, which is monounsaturated. These cultivars were ripened from August to October.In the upper right part of the scores graph, cultivars are grouped with similar amounts of omega 3 and x-linolenic, as with the total amount of PUFA, omega 6 fatty acids,and linoleic acid . These cultivars were ripened from August to October. The cultivars that have similar amounts of carotenoids and tocopherols in the seeds and were ripened from August to November are positioned in the lower right part of the scores graph. Generally, cultivars with negative values of PC1 and PC2 are similar in content of saturated, monounsaturated fatty acids, carotenoids and tocopherols and belong mostly to the group of Norway cultivars. Cultivars with positive values of PC1 and PC2, which have similar content of polyunsaturated fatty acids, have different geographical origins. 5. Conclusions Seeds obtained from apple cultivars grown in Norway (domestic and international)were investigated for the f irst time, in order to determine fatty acids, carotenoids and tocopherols. Taking into account geographical origin, in general , Norwegian cultivars are distinguished by a similar content of saturated, monounsaturated fatty acids, carotenoids and tocopherols. In addition, cultivars with similar content of saturated and monounsat-urated fatty acids ripen mainly in August and September. On the other hand, varieties )that have a similar content of polyunsaturated fatty acids are of different geographical origin and ripening t ime. Generally, seeds from the investigated apple cultivars could be separated into three different groups: one with similar amounts of omega 3 and c-linolenic acids, the second having similar total amounts of polyunsaturated fatty acids, omega 6fatty acids, and linoleic acid, and the third group of seeds being characterized by similar amounts of carotenoids and tocopherols. The fatty ac i ds profile could not provide a clear separation between the cultivars, but total oil content could distinguish between the cul- tivars. Therefore, seeds f rom several Norwegian apple cultivars (Hakonseple, Kviteple,Tolleivseple, Vinterrosenstrips, and Tokheimseple) could be further recommended for processing. Seeds from cultivar Sureple Gron can be underlined due to its high levels of B-carotene, lycopene and total tocopherols. Altogether, our findings could be considered valuable when a strategy to re-evaluate waste is created. This is the first study regarding effective reuse of by-products from the apple industry by producing oil and a defatted oil cake rich in tocopherols and carotenoids.Based on oil content analysis and favorable fatty acid composition, Norwegian apple seeds were shown to be a good source of fatty acids. Its oil has t he potential to be used as an edible oil, which is valuable in the search for unconventiona l sources of health beneficial products. In addition, having in mind the fact that defatted oil cakes received l ittle attention in general , studying other plant seeds is a new way to identify new sources important for good nutri t ion. Supplementary Materials: The following are available online a t https://www.mdpi.com/article/10.3390/foods10081956/s1, Table S1: T he oil content and f atty acid composit i on of apple cultivars, Table S2: Composition of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), polyunsatu-rated fatty acids (PUFA), Omega-3 fatty acids (Omega 3), Omega-6 fatty acid s (Omega 6), Omega-9fatty acids (Omega 9) Author Contributions: Conceptualization, M.F.A. and M.M.; Methodology, M.N., I.C., T.T. and K.L.;Formal analysis, K.L., S.S. and T.T .; Investigation, M.M., M.N. and M.F.A.; Data Curation, M.N., K.L.,I .C., S.S. and T .T.; Writing-original draf t preparation, K.L., M.N.,S.S. and M.F.A.; Writing-review and editing, M.F.A., M.N. and M.M.; Supervision, M.M.; project administration, M.M.; Funding Acquisition M.M. All authors have read and agreed to the published version of the manuscript. Funding: This study was funded by The Research Council of Norway (project No. 280376). Institutional Review Board Statement: Not applicable. Informed Consent Statement: Not applicable. Data Availability Statement: All data are presented in this manuscript. Acknowledgments: The authors would like to thank Oddmund Froynes and Marianne Hotle, NIBIO Ullensvang for harvesting the fruits and conducting the lab work. Conflicts of Interest: The authors declare no conflict of interest . Appendix A List of abbreviations. Lut (Lutein), Zea (Zeaxanthin), Cry (β-Cryptoxantine), Ast (Astaxanthin), Apo (Apocarotenal), Can (Canthaxanthin), Phy (Physalien), beta Car (β-Carrotene), Lyc (Lycopene), 8T (8 Tocopherol), yT (y Tocopherol), BT(B Tocopherol),oT (x Tocopherol), Tac (Tocopheryl acetate), Oi l (Oil content (%)), Palm (Palmitic acid (C16:0)),Pal (Palmitoleic acid (C16:1(n-7))), Heptdec (Heptadecanoic acid (C17:0)), cis-10-Heptdec (cis-10- Heptadecanoic acid (C17:1)), Stear (Stearic acid (C18:0)), Oleic (Oleic acid), Linoleic (Linoleic acid (C18:2 cis 9,12) n-6), α-Linoleic (x-Linolenic acid (C18:3 cis 9,12,15)n-3),Arach (Arachidic acid (C20:0)), 11-Eicos (11-Eicosenoic acid (20:1 cis-11) n-9), C20, (Eicosadienoic acid (C20:2 cis11,14)n-6), Arachi (Arachidonic acid (C20:4 cis-5,8,11,14) n-6), Heneicos (Heneicosanoic acid (C21:0)), Behenic (Behenic acid (C22:0)), Lignoceric (Lignoceric acid (C24:0)),Docosahex (Docosahexaenoic acid (C22:6 cis 4,7,10,13,16,19) n-3), SFA (saturated fatty acids), MUFA (monounsaturated fatty acids), PUFA (polyunsaturated fatty acids),Omega 3 (Omega-3 fatty acids), Omega 6 (Omega-6 fatty acids), Omega 9 (Omega-9 fatty acids). References 2. Fotiric Akěic , M.; Mutic, J .; Tesic, Z.;Meland, M. Evaluation of frui t mineral contents of two apple cultivars grown in organic and integrated production systems. Acta Hortic. 2020,1281,59-66. [CrossRef] 3FaoStat . 2019. Available online: ht tp ://www.fao.or g/faostat/en/#data/QC (accessed on 25 June 2021). 4 Gerhauser, C. Cancer chemopreventive potential of apples, apple juice, and apple components . Planta Med. 2008, 74,1608-1624.[CrossRef ][PubMed] 5. Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr . 2011,2, 408-420. [CrossRef ] 6. Statista. 2021. Available online: https://www.statista.com/statistics/423198/production-volume-of-ap pl e-juice-eu/ (accessed on 21 June 2021). 7 AICV (The European Cider & Fruit Wine Association). European Cider Trends 2020; The European Cider & Fruit Wine Association:Brussels, Belgium, 2020. 10. Attri, D.; Joshi, V.K. Optimiza t ion of apple pomace based medium and fermentation conditions for pigment production by Chromobacter species. J. Food Sci. Technol. 2006,43,484-487. 12. Sharma, R.C.; Joshi, V.K. The Apple: I mprovement, Production and Postharvest Management; Chadha, K.L., Awasthi , R.P., Eds.;Malhotra Publishing: New Delhi, India, 2001; pp. 428-483. 13. Gornas,P.; Rudzinska, M.; Segl i na, D. Lipophilic composition of eleven apple seed oi l s: A promising source of unconventional oil from i ndustry by-products. Ind. Crop. Prod.2014,60,86-91. [CrossRef ] 14. C Gornas, P . Unique variabi l ity of tocopherol composition in various seed oi l s recovered from by-products of apple i ndustry: Rapid and simple determination of all four homologues (a, , y and 8) by RP-HPLC/FLD. Food Chem. 2015,172,129-134.[CrossRef ][PubMed 1 15. Stanojevic , M.; Trifkovic, J.; Fotiric Aksic, M.; Rakonjac, V.; Nikolic, D.; Segan, S.; Milojkovic-Opsenica, D. Sugar Profile of Kernels as a Marker of Origin and Ripening Time of Peach (Prunus persicae L.). Plant . Foods Hum. Nutr. 2015,70, 433-440. [CrossRef ] 16. Yu, X.; Voort, F.R.; Li , Z.; Yue, T. Proximate composition of the apple seed and characterization of its oil . Int. J. Food Eng. 2007, 3,1556-3758. [CrossRef | Lu, Y.; Foo, L.Y. Constitution 18 of some chemical components of apple seed. Food Chem. 1998, 61,29-33. [CrossRef ]Arain, S.; Sherazi, S.T.H.; Bhanger, M.I.; Memon, N.; Mahesar, S.A.; Rajput, M.T . Prospects of fatty acid profile and bioactive composition from l ipid seeds for the discrimination of apple varieties with the application of chemometrics. Grasas Aceites 2012,63,175-183. 19. Yukui, R.; Wenya, W.;Rashid, F; Qing, L. Fatty acids composition of apple and pear seed oils. I nt. J. Food Prop. 2009, 12,774-779.[CrossRe f 20. Lei-Tian, H.;Zhan,P.; Li, K. Analysis of components and study on antioxidant and antimicrobial activities of oi l i n apple seeds.Int. J . Food Sci. Nutr. 2010, 61,395-403. 21. Walia, M.; Rawat, K.; Bhushan, S.; Padwad, Y.S.; Singh, B. Fatty acid composition, physicochemical properties, antioxidant and cytotoxic activity of apple seed oil obtained from apple pomace. J. Sci. Food Agric . 2014, 94, 929-934. [CrossRef ] 22. Jaszczak-Wilke, E .;Polkowska, Z.; Koprowski, M.;Owsianik, K.; Mitchell , A.E .; Balczewski , P. Amygdalin: Toxici t y, Anticancer Activity and Analytical Procedures for Its Determination in Plant Seeds . Molecules 2021, 26,2253. [CrossRef] [PubMed ] 23. Shalini, R.; Gupta, D.K. Utilization of pomace f rom apple processing industries: A rev i ew. Food Sci. Technol. 2010, 47,365-371.[CrossR e f | 24. Fritsche, S.; Wang,X.; Jung, C. Recent Advances in our Understanding of Tocopherol Biosynthesis in Plants: An Overview of Key Genes, Functions, and Breeding of Vitamin E Improved Crops. Antioxidants 2017, 6, 99. [CrossRef ] 25. Sattler, S.E.; Cahoon, E.B.; Coughlan, S.J .; DellaPenna, D. Characterization of tocopherol cyclases from higher plants and cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant. Physiol. 2003,132,2184-2195. [CrossRef ] 26. Saldeen, K.; Saldeen, T . Importance of tocopherols beyond a-tocopherol: Evidence from animal and human studies. Nutr. Res.2005,25,877-889. [CrossRef ] 27. Bri t ton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids, Vol . 5. In Nutrition and Health; Birkhauser Verlag: Basel , Switzerland , 2009;pp. 1-431. Norwegian Agriculture Agency. 2021. Available online: https://www.landbruksdirektoratet.no/nb (accessed on 25 June 2021).29 Fromm, M.; Bayha, S.; Carle, R.; Kammerer, D.R. Comparison of f atty acid profiles and contents of seed oils recovered from dessert and cider apples and further Rosaceous plants. Eur. Food Res. Technol. 2012, 2 3 4 , 1 rOSS Ref 30. ISO 12966-2:2017 Gas Chromatography of Fatt y Acid Methyl Esters-Part 2: Preparation of Methyl Esters of Fat t y Acids; I nternational Organization for Standardization: Geneva, Switzerland, 2017. 31. ISO 12966-4:2015 Animal and Vegetable Fats and Oils -Gas Chromatography of Fatty Ac i d Methyl Esters -Part 4: Determination by Capillary Gas Chromatography; International Organization for Standardization: Geneva, Switzerland, 2015. 34. Gebregziabher, B.S.; Zhang, S.; Qi, J.; Azam, M.; Ghosh, S.; Feng, Y.;Huai, Y.; Li, J; Li, B.; Sun, J. Simultaneous Determination of Carotenoids and Chlorophylls by the HPLC-UV-VIS Method in Soybean Seeds. Agronomy 2021, 11,758. [CrossRef ] 35. Cozzolino, D.; Power, A.; Chapman, J . Interpreting and Reporting Principa l Component Analysis in Food Science Analysis and Beyond. Food Anal. Methods 2019, 12,2469-2473. [CrossRef ] 36. Sarbu, C.; Nascu-Briciu, R.D.; Kot-Wasik, A.; Gorinstein, S.; Wasik, A.; Namiesnik, J . Classification and fingerprinting of kiwi and pomelo fruits by multivariate analysis of chromatographic and spec t roscopic data. Food Chem. 2012, 130, 994-1002. [CrossRef ] 37. Butnariu, M. Methods of Analysis (Extraction, Separation, Identification and Quantification) of Carotenoids from Natural Products. J. Ecosys. Ecograph. 2016,6,193-211. [CrossRef ] 38. Matthaus, B.; Ozcan, M.M. Oil Content, Fatty Acid Composition and Distributions of Vitamin-E-Active Compounds of Some Frui t Seed Oils. Antioxidants 2015, 4, 124. [CrossRef ] 39. Bada, J.C.; Leon-Camacho, M.; Copovi, P.; Alonso, L. Characterization of apple seed oil with Denomination of Origin f rom Asturias, Spain. Grasas Aceites 2014, 65,27-34. 40. Marangoni,F.; Agostoni , C.; Borghi , C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.;Manzato, E.; Pirillo,A.; et al. Dietary l inoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292,90-98.[CrossRef] [PubMed ] 41. Piccinin, E.; Cariello, M.; De Sant i s, S.; Ducheix, S.; Sabba, C.; Ntambi, J.M.; Moschetta, A. Role o f Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation o f Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283.[CrossRef ] [PubMed ] 42. Howitt, C.A.; Pogson, B.J. Carotenoid accumulation and function in seeds and non-green tissues. Plant . Cell. Environ. 2006, 29,435-445. [CrossRef ] 43. Fernandez-Marin, M.; Miguez, F .; Méndez-Fernandez, L.; Agut, A.; Becerril, J.M.; Garcia-Plazaola, J .I.; Kranner, I.; Colville, L.Seed Carotenoid and Tocochromanol Composition of Wild Fabaceae Species Is Shaped by Phylogeny and Ecological Factors.Front . Plant . Sci . 2017, 8,1428-1443. [CrossRef ] [PubMed ] 44. Van Breemen, R.B.; Dong, L.;Pajkovic, N.D. Atmospheric pressure chemical ionization tandem mass spectrometry of carotenoids.Int. J . Mass Spectrom. 2012,312,163-172. [CrossRef ] 45. Mezzomo, N.; Ferreira, S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem.2016,2016,3164312. [CrossRef ] 46. Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Mendez, D. Chlorophyll and carotenoid pigments i n the peel and f lesh of commercial apple fruit varieties. Food Res. Int. 2014,65,272-281.[CrossRef ] 47. Fromm, M.; Bayha, S.; Kammerer, D.R.; Carle, R. Identi f ication and quantitation of carotenoids and tocopherols in seed oils recovered from different rosaceae species. J. Agric. Food Chem 2012,60,10733-10742. [CrossRef ] 48. Amariz, A.; de Lima, M.A.C .; Alves, R.E. Qual i ty and antioxidant potential of byproducts from refining of fruit pulp. Food Sci.Technol. 2018, 38,203-209. [C rossRef ]
确定










还剩13页未读,是否继续阅读?
产品配置单
中国格哈特为您提供《75个苹果品种种子含油率的检测》,该方案主要用于种子中营养成分检测,参考标准《GB 5009.6 食品中脂肪的测定》,《75个苹果品种种子含油率的检测》用到的仪器有格哈特全自动快速索氏提取SOXTHERM、德国加液器MM、滤纸筒
该厂商其他方案
更多












