方案详情
文
本研究首次报道了芒果、番石榴、香蕉水果的挥发性气味特征分析,这些水果在中国普遍商业销售和种植的。采用顶空固相微萃取(HS-SPME)和porapak Q相结合的气相色谱-质谱分析(GC-MS)方法对各品种果实的挥发性成分进行了鉴定。从芒果、番石榴和香蕉果实中分别鉴定出10、15和23种挥发性成分/化合物。
方案详情

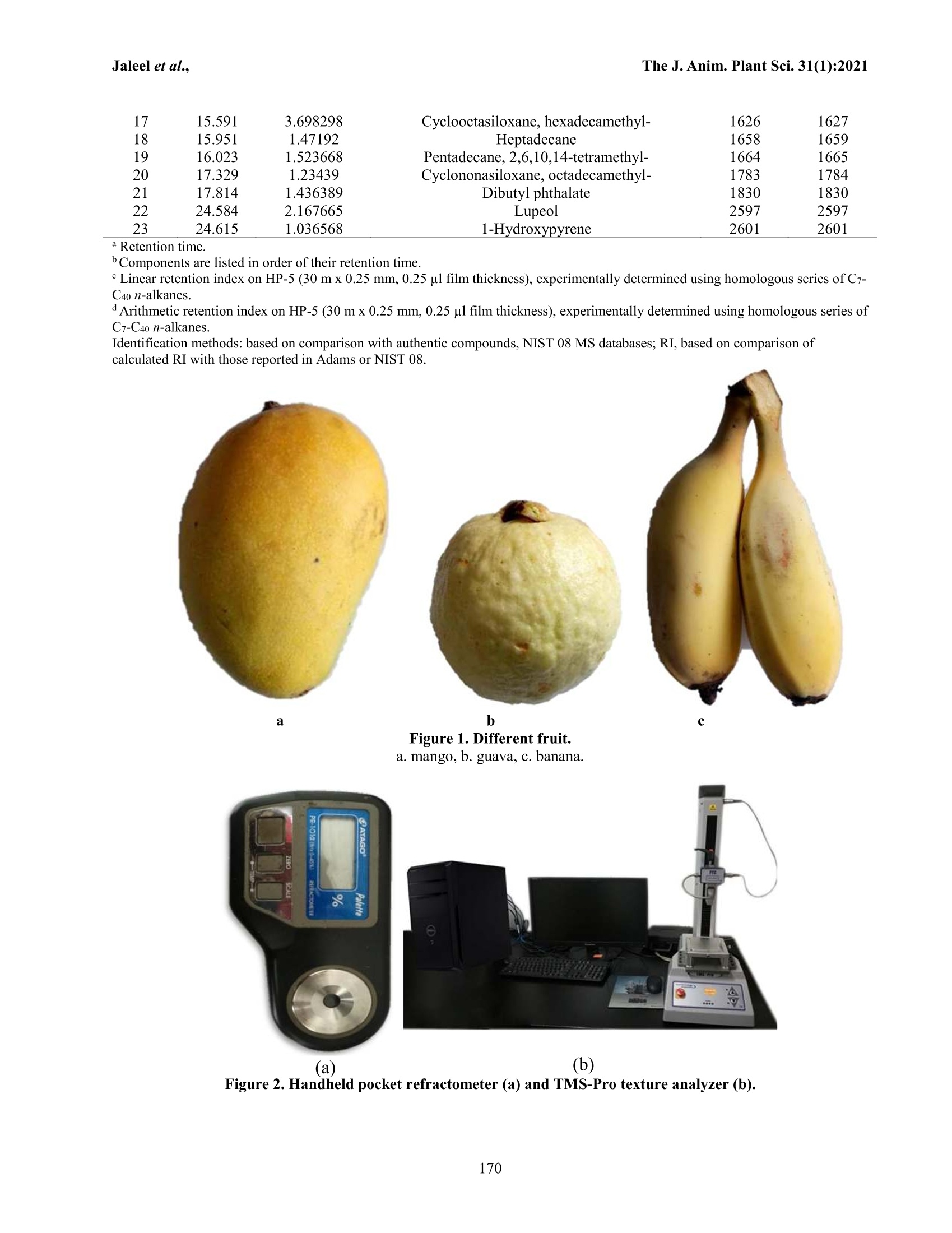
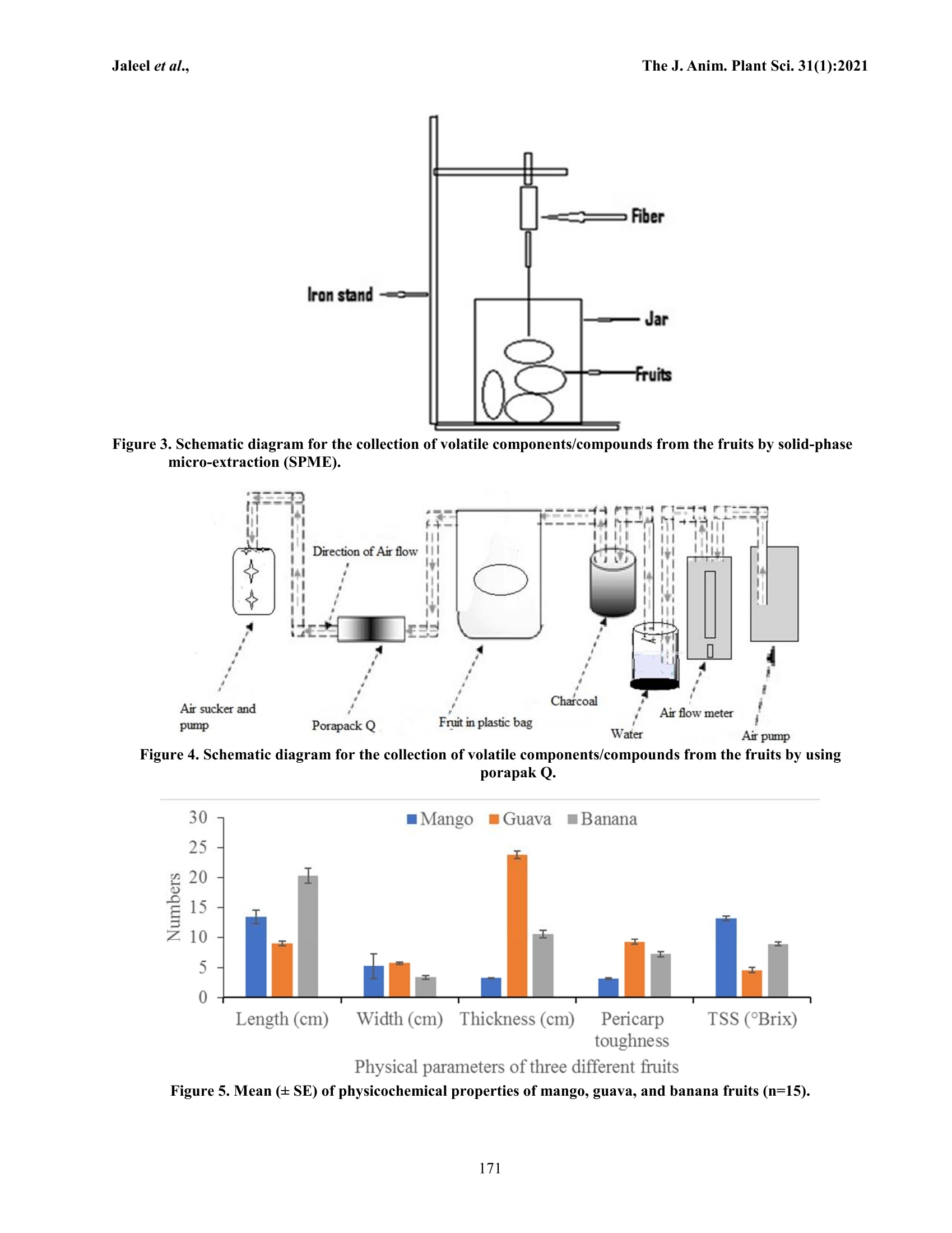
The Journal of Animal & Plant Sciences, 31(1): 2021,Page: 166-174ISSN (print): 1018-7081; ISSN (online): 2309-8694 The J. Anim. Plant Sci.31(1):2021Jaleel et al.. USING GCMS TO FIND OUT THE VOLATILE COMPONENTS IN THE AROMA OFTHREE DIFFERENT COMMERCIAL FRUITS IN CHINA W. Jaleel1.2,3,4*, Q.Li14, Q. Shit, G. Qi, M. Latif, S. Ali, N. A. Yasin’, L. Lyu and Y. Hel.2,3,* Key Laboratory of Bio-Pesticide Innovation and Application, Guangdong Province, Guangzhou 510640, ChinaEngineering Research Center of Biological control, Ministry of Education, Guangzhou 510640, ChinaDepartment of Entomology, College of Agriculture, South China Agricultural University, Guangzhou 510642,Guangdong Province, China. 4Plant Protection Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou, China. Department of Zoology Division of Science and Technology, University of Education Lahore. Hubei Insect Resources Utilization and Sustainable Pest Management Key Laboratory, College of Plant Science and Technology, Huazhong Agricultural University, Wuhan 430070, P. R. China. RO (II) Wing, New Campus, University of the Punjab, Lahore-54590, Punjab, Pakistan. Corresponding author’s email: yrhe@scau.edu.cn, waqar4me@yahoo.com ABSTRACT The agricultural sector is an essential source of income and food for humans. Fruits are important sources of nutrients,vitamins, essential elements, and as well as the source of income for humans. Volatile components make the aroma offruit and fruit species and cultivars. However, the knowledge and information about volatile components or volatileprofiles are essential, because these aroma components make fruit delicious to humans and as well as attract or repelinsect pests. And the identification of volatile components from fruits is very important to a better understanding of theimpact of climatic conditions. To the best authors knowledge, in this study, we reported the first time volatile profile ofunidentified cultivar of mango (Mangifera indica L. Hanana Datai Nong Mang), guava (Psidium guajava Linn. ChinaPear), and banana (Musa acuminata L. Dwarf banana or Fen Jiao) fruits and these fruits are commercially selling andgrowing in China. The Headspace Solid-phase Microextraction (HS-SPME) and porapak Q coupled with gaschromatography-mass spectrometry analysis (GC-MS) were used to identify the volatile profiles of each fruit cultivar. Atotal of 10, 15, and 23 volatile components/compounds were identified from mango, guava, and banana fruits,respectively. The major constitution of volatile components obtained from mango, guava, and banana was 3-carene,caryophyllene, and cycloheptasiloxane, tetradecamethyl-. Key words; Banana, 3-carene, caryophyllene, cycloheptasiloxane, components, tetradecamethyl-, GC-MS, guava,mango, volatile components. INTRODUCTION The agricultural sector is an important source offood, e.g., fruits, vegetables, and crops, as well as incomefor humans (FAO, 2016;FAO,2017). Mango (Mangiferaindica L), guava (Psidium guajava Linn), and banana(Musa spp) are most important tropical, sub-tropical, andtemperate fruits and kept most important nutrients,vitamins, essential elements that are good for humanshealth (Paniandy et al., 2000; Joseph and Priya, 2011;Gautam and Gotame, 2020). Each fruit has its volatilecomponents that have a great impact on making thearoma of fruits. The attraction of insect pests to volatilesthat is elicited from their host, e.g., fruits have beenstudied (Cruz-Lopez et al., 2006; Siderhurst and Jang,2006; Alagarmalai et al.,2009; El-Sayed et al. 2009;Siderhurst and Jang, 2010; Biasazin et al., 2014; Jayanthiet al., 2014; Jaleel et al., 2019; Ali et al., 2020; Jaleel etal.,2020). Mango fruits are also called “King of fruits."Mango fruits have rich nutrient sources as well as alivelihood for millions of people (Mitra, 2014; Sial et al.,2015).Volatiles components from a different cultivar ofmango fruits have been identified in Africa, Australia,India, UnitedStates, Sri Lanka, andVenezuela(Rodriguez et al., 2013; Souza et al., 2018). Differentmango cultivars still have not been used on a commerciallevel that exhibiting a wide range of flavors (MacLeodand Pieris, 1984; Damodaram et al., 2015; Galal et al.,2017). In the Coche mangoes, the predominant volatilecomponents were 3-carene, b-selinene, terpinolene, andlimonene (Malo et al., 2012; Xiao-Wei et al., 2016). 3-carene has been reported in the aroma of mango fruits(Tamura et al., 2000;Vithana et al., 2018). 3-carene,acetic, butyric, hexanoic acids, andethyl3-hydroxybutyrate have been reported from mango fruits(Sakho et al., 1985; Kondo,2018). Thirteen mangovolatiles components were detected, e.g., acetaldehyde, acetone, methanol, ethanol, a-pinene,caryophyllene, 3-carene, b-pinene, myrcene, limonene, terpinolene, a-copaene, and r-cymene (Baldwin et al.,1999; Pino andMesa,2006; Shaw,2017). Guava fruits have been originated from tropicalAmerica (Shen et al., 2008; Singh et al., 2018). GuavafruitsShaveeVvolatilearomaticc components,e.g.,antioxidants, vitamins, lutein, zeaxanthine, and lycopene.Leaves, shoots, and fruits of guava are useful in medicinefor the treatment of various human diseases (Zahoor et al.,2017). Polysaccharides, vitamins, minerals, enzymes,essential oils, proteins (Jordán et al., 2001; Deo andShastri, 2003; Bodini et al., 2019), sesquiterpenoid,triterpenoid acids, alkaloids, glycosides, steroids, tannins,and saponins are essential compounds of guava fruit (Xuet al.,2017). Bananas are very important source of humandiets and income. Genus Musa. Linnaeus has more than100 common names around the world (Arvanitoyannisand Mavromatis,2009; Asif and Kaur, 2018). Jordán et al.(2001)) have reported 43volatilee components andprominent were 1-methyl butyl isobutyrate 2,3-butanedioldiacetate. 2-hydroxy-3-methylethylbutyrate, methylbutyrate, ethyl 3-hydroxyhexanoate, 2-pentanol acetate,isoamyl acetate,2-methyl-1-propanol,3-methyl-1-butanol,3-methylbutanal, acetal,isobutylacetate.hexanal, ethyl butyrate, 2-heptanol, and butyl butyrate.Banana fruits have full acceptance, among other fruits,due to nutritional value (Zhu et al., 2018). More than 350volatile components/compounds have been identified inthe aroma of banana fruits, e.g., esters, alcohols, andcarbonyl compounds are the most important volatilecomponents (Girard et al.,1997; Jordán et al., 2001; Pinoand Febles, 2013; Zhu et al., 2018). Volatile components,e.g., 3-methyl butyl acetate, isoamyl butanoate, andisoamyl isovalerate, were reported as key components ofbanana “fruity”odor (Schwab et al., 2008). Identificationof phytochemicals is the most important study; becausephytochemicals play an important role in attraction orrepellant of insects (Forister et al., 2015; Jaleel et al.,2018b). Volatile or aromatic compounds are importantfor the quality of fruit and can vary among fruit cultivars(Zhu et al., 2018). Information about volatile componentsor volatile profile is very important because these aromacomponents are responsible for fruit quality. And theidentification of volatile components from fruits is veryimportant to a better understanding of the impact ofenvironmental conditions. In this study, we reported firsttime volatile profile of unidentified cultivar of mango(Mangifera indica L. Hanana Datai Nong Mang), guava(Psidium guajava Linn. China Pear),and banana (Musaacuminata L. Dwarf banana or Fen Jiao) fruits and thesefruits are commercially selling and growing in China. MATERIALS AND METHODS Fruit: Ripe mango (M. indica: Hanana Datai Nong Mang:guava((P. guajava: ChinaPear: and banana(M.acuminate: Dwarf banana or Fen Jiao) were purchasedfrom the orchard located in Guangzhou, Guangdong,China. Fruits were washed and dried and then used forexperimental purposes (Figure 1). Fruits characteristics: Based on detailed discussion witha sailor, all fruits were bagged in the field before thestage of ripening to avoid the attack of wild insect pests.Total:soluble solidss(i(TSS or Brix) of fruits;Vweremeasured using handheld pocket refractometer pal-1(ATAGO, PR-101a, Brix 0-45%, Tokyo Tech. Japan)(Figure 2a), and the pericarp toughness or firmness offruits were measured by a TMS-Pro texture analyzer(FTC-TV,USA) with probe (1 mm diameter) (Jaleel etal.,2018c) (Figure 2b). Measurements were taken andrecorded at three different locations on each fruit. Fifteenreplications were done for each fruit. Volatiles collection: Collection of volatiles from thesurface of mango, guava, and banana were done by twowaysassolid-phase micro-extraction (SPME)andporapak Q (80-100 mesh; Alltech, Deerfield, IL, USA) inthe laboratory of Plant Protection Research Institute,Guangdong Academy of Agricultural Science,Guangzhou, China. The solid-phase micro-extraction (SPME) fiber(assembly 100 um PDMS, fused silica 24 Ga, the manualholder 3 pk red) was used for the collection of fruitsvolatiles compounds, First, the SPME fiber was pre-conditioned in a GC injector (250°C) for 30 min. A fruitwas placed in a 500 ml clear straight-sided jar with ascrew-top lid fitted with a Teflon liner. The SPME needleinserted through a small hole in the lid, and the fiberexposed till 1 h (Figure 3), the fiber was removed fromthe jar and then immediately inserted into the GCinjection port to desorb volatiles. The whole procedurewas repeated fifteen times for each fruit (mango, guava,andbanana). Collection of volatiles from mango, guava, andbanana was done using porapak Q. Before collection ofsamples, the porapak Q tube preconditioned at 280°C for30 min and washed with dichloromethane, then driedunder charcoalpurified nitrogen. Porapak Q wasconnected with an air pump, that have connection with anair flow meter (AFM) (for controlling the flow of airthrough the system), water bottle, charcoal, plastic bag(Oven bag, Turkey size, 482×596 mm), and air sucker(Figure 4). In order to activate the charcoal, it waspreheated at 200 C for 3 hrs. The charcoal flask wasfollowed by oven bag containing a specified amount (2kg) of the sample (fruits). Air after passing through theoven bag then passed through the porapak Q, theabsorbent material inside the porapak Q. The adsorption of the volatile compoundswas done after 24 hrs.Volatiles eluted from the adsorbents of porapak Qwiththe help of 1 ml CH2Cl2 and then stored at-80C in therefrigerator. With the help of a microsyringe (1000microlitres) the volatile compounds/components werecollected from the porapak Q into the glass tube (capacity2 ml). The whole procedure was repeated fifteen timesfor each fruit. The 0.1 ul was taken from a sample offruits and used for the analysis. Gas chromatography-mass spectrometry (GC/MS):The GC-MS quantitative and qualitative analysis wereperformed on an Agilent 7890N gas chromatograph,coupled with an Agilent 5975 C mass selective detector(GC-MS) that equipped with an HP-5 MS, capillarycolumn (30 m x0.25 mm ID, film thickness 0.25 um,Agilent TTechnologies, USA). The temperatureewasprogrammed from 45 (held for 1 min) to 280°℃ at 10 °C/min. The solvent delay was kept 5 min, while the injectortemperature was used at 250 °℃ and helium gas was usedas the carrier. Electron ionization mass spectra wererecorded from m /z 29°C to 280 °C at 70 eV with ironsource temperature at 230°C Compounds identification: Quantitative and qualitativeconstituents’analysis of fruits were done on the basis oftheir retention times (Rt) and mass spectra in thecomputer library (NIST. 11). The quantity of each fruitcomponents was compared by measurements as usingarea of the peak. RESULTS Fruits characteristics: Characteristics like length (cm), Table 1. Volatile components from the mango. width (cm), thickness (cm), total soluble solids (TSS) orbrix firmness/hardness (N) of and mango, guava, andbanana, are shown in table 1. The pericarp toughness ofmango fruit was lower than those of the other two testedfruits (banana and guava). While the Brix level of mangowas higher than those of the other two tested fruits(Figure 5). GC-MS analyses of mango: The volatile components ofmango were given in table 1. Overall, 99.99 % ofcomponentsiswere identified. The major dominatingvolatile/aromatic components were 3-carene, a-pinene,and butyl caprylate, which accounts (86.62 %) of totalcomponents.While other minor constitutes, whichmakeupthe balance sfilled withhffollowingcomponents e.g. cyclopentasiloxane decamethyl,cyclohexasiloxaneddodecamethyl-, hexanoiccacid, 3-hexenyl ester, (Z: 1-tridecene, tetradecane, humulene, and1-limonene (Table 1). GC-MS analyses of guava: The GC-MS analyses ofvolatile components of guava are presented in table 2.Overall, 99.99 % of components were recognized. Themajor dominating components were caryophyllene, a-pinene.ggamma.-terpinene,,aandda-copaene,whichaccounts (56.90 %) of total constitutes. While otherminor constitutes, which make up the balance was filledwith following components e.g. Eucalyptol,cyclohexasiloxane dodecamethyl-, aromandendrene,humulene, cycloheptasiloxane tetradecamethyl-., cis-a-bisabolene, epizonarene, phenol2,,2'-methylenebis[6-(1,1-dimethylethyl)-4-methyl-, diisooctyl phthalate.eicosane, and octadecanal (Table 2). Peak# RT(Paniandy et al.) Relative % Components name KI(Exp) AI(Exp) 1 6.778 77.07666 3-Carene 984 986 2 8.342 4.253949 a-pinene 1003 1003 3 9.449 2.823219 Cyclopentasiloxane, decamethyl 1121 1122 4 12.142 2.687374 Cyclohexasiloxane,dodecamethyl- 1290 1291 5 12.642 0.19506 Hexanoic acid, 3-hexenyl ester, (Z) 1397 1397 6 13.232 5.314137 Butyl caprylate 1459 1460 7 13.942 1.147518 1-Tridecene 1559 1559 8 14.156 2.928431 Tetradecane 1650 1651 9 15.876 2.056999 Humulene 1656 1657 16.373 1.516658 l-limonene 1678 1679 aRetention time. Components are listed in order of their retention time. Linear retention index on HP-5 (30 m x0.25 mm, 0.25 pl film thickness), experimentally determined using homologous series of C7-C40 n-alkanes. ( Arithmetic r e tention index on H P -5 (30 m x 0.25 mm , 0.25 ul f ilm thickness), ex p erimentally determined using homologous series of C7-C40 n-alkanes. ) ( Identificatio n methods : based on comparison w ith a uthentic c ompounds, N I ST 08 MS d atabases; RI, b ase d on c o mparison of calculated RI with those reported in Adams or NIST 08. ) GC-MS analyses of banana: The GC-MS analyses ofvolatile components of banana are given in table 3.Overall, 99.99 % of volatile components were recognized.The major dominating components werecycloheptasiloxane, tetradecamethyl-, phenol, 2-ethyl-6-methyl-,tridecane. hexadecane.nonadecane. andtetradecane, which accounts (64.74%) of total constitutes.While other minor constitutes, which makeup the balance WaSfilled with following componentss e.g. menthylacetate. cyclohexasiloxane, dodecamethyl-,1-triethylsilyloxyheptadecane, beta.-curcumene. ethylchrysanthemate, dodecane, 2-cyclohexyl-, pentadecane,4-methyl--,. cedrol.pentadecane,小cyclooctasiloxane,hexadecamethyl-, heptadecane, pentadecane, 2,6,10,14-tetramethyl-, cyclononasiloxane, octadecamethyl-,dibutyl phthalate, lupeol, and 1-hydroxypyrene (Table 3). Table 2. Volatile components from the guava. Peak# RT Relative % Components name" KI(Exp) AI(Exp) 1 5.933 5.817697 a-Pinene 932 935 2 5.985 6.684064 gamma.-Terpinene 936 939 3 7.519 2.660957 Eucalyptol 1034 1037 4 11.774 1.247745 Cyclohexasiloxane, dodecamethyl- 1322 1323 5 12.651 4.521598 a-Copaene 1387 1388 6 13.255 38.08295 Caryophyllene 1434 1435 7 13.51 3.667704 Aromandendrene 1454 1455 8 13.696 2.971869 Humulene 1469 1470 9 14.016 1.32376 Cycloheptasiloxane, tetradecamethyl- 1494 1494 10 14.227 3.006783 cis-a-Bisabolene 1511 1512 11 14.503 3.332218 Epizonarene 1534 1535 12 23.309 3.415814 Phenol, 2,2'-methylenebis[6-(1,1-dimethylethyl)- 4-methyl- 2434 2434 13 24.247 17.1801 Diisooctyl phthalate 2553 2554 14 25.365 3.443879 Eicosane 2693 2693 15 29.301 2.642863 Octadecanal 3443 3444 a Retention time. Components are listed in order of their retention time. Linear retention index on HP-5 (30 m x 0.25 mm, 0.25 ul film thickness), experimentally determined using homologous series of C7- C40 n-alkanes. Arithmetic retention index on HP-5 (30 mx 0.25 mm, 0.25 ul film thickness), experimentally determined using homologous series ofC7-C40 n-alkanes. Identification methods: based on comparison with authentic compounds, NIST 08 MS databases; RI, based on comparison ofcalculated RI with those reported in Adams or NIST 08. Table 3. Volatile compounds from the banana. Peak# 1 RT Relative % Components name" KI(Exp) AI(Exp)" 11.155 0.846196 Menthyl acetate 1278 1279 11.236 3.951046 Cyclohexasiloxane, dodecamethyl- 1283 1284 11.312 3.218066 1-Triethylsilyloxyheptadecane 1289 1289 11.335 1.695342 Trimethylsilylestrone 1290 1291 12.296 4.00518 Tetradecane 1361 1362 12.343 6.014059 Hexadecane 1364 1366 12.753 1.4022 beta.-curcumene 1395 1395 12.957 10.2603 Phenol, 2-ethyl-6-methyl- 1411 1411 13.04 2.58074 Ethyl chrysanthemate 1417 1418 13.128 6.297894 Nonadecane 1424 1425 13.581 29.51357 Cycloheptasiloxane, tetradecamethyl- 1460 1461 14.299 3.560385 Dodecane, 2-cyclohexyl- 1517 1518 14.374 1.760229 Pentadecane, 4-methyl- 1524 1524 14.809 8.604666 Tridecane 1560 1561 15.119 1.546458 Cedrol 1586 1586 15.378 2.174767 Pentadecane 1608 1608 15.591 3.698298 Cyclooctasiloxane, hexadecamethyl- 1626 1627 15.951 1.47192 Heptadecane 1658 1659 16.023 1.523668 Pentadecane, 2,6,10,14-tetramethyl- 1664 1665 17.329 1.23439 Cyclononasiloxane, octadecamethyl- 1783 1784 17.814 1.436389 Dibutyl phthalate 1830 1830 24.584 2.167665 Lupeol 2597 2597 24.615 1.036568 1-Hydroxypyrene 2601 2601 a Retention time. Components are listed in order of their retention time. · Linear retention index on HP-5 (30 m x 0.25 mm, 0.25 ul film thickness), experimentally determined using homologous series of C7-C40 n-alkanes. d Arithmetic retention index on HP-5 (30 m x 0.25 mm, 0.25 ul film thickness), experimentally determined using homologous series ofC7-C40 n-alkanes. Identification methods: based on comparison with authentic compounds, NIST 08 MS databases; RI, based on comparison ofcalculated RI with those reported in Adams or NIST 08. b C Figure 1. Different fruit. a.mango, b. guava, c. banana. (a) (b) Figure 2. Handheld pocket refractometer (a) and TMS-Pro texture analyzer (b). Figure 3. Schematic diagram for the collection of volatile components/compounds from the fruits by solid-phasemicro-extraction (SPME). Figure 4. Schematic diagram for the collection of volatile components/compounds from the fruits by usingporapak Q. Figure 5. Mean (±SE) of physicochemical properties of mango, guava, and banana fruits (n=15). DISCUSSION Volatile or aromatic compounds can vary amongfruit cultivars (Zhu et al., 2018). Volatile components offruit are very important for taste (Jayanthi et al., 2012;Malo et al., 2012; Song et al., 2019). The predominantvolatilele components were determined in the Cochemangoe.g.3-carene.,b-selinene, terpinolene, andlimonene (Malo et al.,2012;Jimenez et al., 2019). The 3-carene is major aromatic compound in mango fruits andpulp (Tamura et al., 2000; Wetungu et al., 2018) and alsoimportant attractive components for insect pests (Jaleel etal., 2019). The 3-carene was major constitute in mangoamong other constitutes e.g. acetic, butyric and hexanoicacids; and ethyl 3-hydroxybutyrate (Ghatak et al., 2018).Thirteen volatiles components were reported in mangousing GCMSe.g. acetaldehyde, acetone, methanol,ethanol, a-pinene, caryophyllene, 3-carene, b-pinene,myrcene, limonene, terpinolene, a-copaene, and r-cymene(Baldwin et al., 1999; Pino and Mesa, 2006; Maldonado-Celis et al., 2019). In our study,3-carene, a-pinene, andbutyl caprylate were the main volatile components inmango odors. Each volatile component has a specificaroma to call or repel the insect pests (Jang and Light,1991; Jaleel et al., 2018a; Jaleel et al.,2018c). Volatilecomponents of fruits are very important to attract thefemale adults of insect pests (Jaleel et al., 2018b). 3-carene, caryophyllene, and humulene were reported mainvolatile components in guava fruits (McQuate et al.,2017). In our study, caryophyllene, a-pinene, gamma.-terpinene,,anda-copaenewere thhee mair volatilecomponents in the order of guava fruit. Schwab et al.(2008) reported the major portion of 3-methyl butylacetate, isoamyl butanoate, and isoamyl isovalerate inbanana fruits. And concluded that, the 3-methyl-1-methylhexyl-2- methyl propyl or isobutyl: 3-methyl butyl orisoamyl: and hexyl esters of acetic, butanoic, andisovaleric acids are the main source of aroma in bananafruits (Wetungu et al., 2018; Zhu et al., 2018). Butylacetate, isoamyl acetate, ethyl acetate, butyl butanoate,and isoamyl isobutanoate were main components inbanana fruit (Zhu et al.,2018; Zhang etal.,2019). In ourstudy, cycloheptasiloxane, tetradecamethyl-, Phenol, 2-ethyl-6-methyl-, Tridecane, Hexadecane, Nonadecane,and Tetradecane were the major portion of aromatic orvolatile components in banana fruits. In conclusion, themain major constitution of volatile component obtainedmango, guava, and banana was 3-carene, caryophyllene,and cycloheptasiloxane, tetradecamethyl-respectively. Acknowledgments: This research was supported by theNational Key Research and Development Program ofChina (2018YFD0201300), the Guangdong ProvincialSpecial FundforModern Agriculture IndustryTechnology Innovation Teams, Department ofAgriculture and Rural Affairs of Guangdong Province (No. 2019KJ125), the Innovation Team of ModernAgricultural Industry Generic Key Technology R and Dof Guangdong Province (2019KJ134), the National KeyR and D Program of China (2017YFC1200600), and theOpen Fund of the Guangxi Key Laboratory of Biologyfor Crop Diseases and Insect Pests, China (2016-KF-3).The funders had no role in study design, data collectionand analysis, decision to publish, or preparation of themanuscript. REFERENCES ( Alagarmalai, J. , Neste l . D,Dragushich. D, Nemny-Lavy.E, Anshelevich. L, Zada. A and S oroker. V(2009). I dentification o f host attractants f or theEthiopian f ruit fly , Dacus ciliatus Loew. J . Chem. Ecol. 35:542-551. ) Ali, S.,Li. S, Jaleel. W, Khan. M. M, Wang. J and Zhou.X (2020). Using a Two-Sex Life Table Tool toCalculate the Fitness of Orius strigicollis as aPredator of Pectinophora gossypiella. Insects.11(5):275. ( Arvanitoyannis, I. and Mavromatis. A (2009). B a nana cultivars. cultivation practices, and physicochemical properties. C rit. Rev. Food Sci.Nutr.49:1 1 3-135. ) ( Asif, M. and Kaur. A (2018). Biologically a ctivephytochemical contents and biological activitiesof whole Musa acuminata (BANANA) plant. IJRAMPR1: 5-33. ) ( Baldwin, E. A., Burns. J . k, Kazokas. W, Brecht. J. K,Hagenmaier. R. D, B ender. R. J and Pesis. E(1999). Effect of t wo edible c oatings withdifferent permeability characteristics on mango (Mangifera i ndica L .) r ipening during storage. Postharvest. Biol. Tec. 17:215-226. ) Biasazin, T. D., Karlsson. M. F, Hillbur. Y, Seyoum. Eand Dekker. T (2014). Identification of hostblends that attract the African invasive fruit fly,Bactrocera invadens. J. Chem. Ecol. 40: 966-976. Bodini, R. B., Montini. E. M, de Carvalho. C. C, deMoraes L. A, Meirelles. A. J. D. A and deOliveira. A. L (2019). Sensory and CompositionAnalyses of the Aqueous Phases from theConcentration of Guava (Psidium Guava L.) andMango (Mangifera Indica L.) Juices and theProcess-Induced Losses of Vitamin C. 11. TheOpen Food Sci J. 12:44-55 ( Cruz-Lopez, L ., M alo. E. A, T o ledo. J, Virgen. A , DelMazo. A and R o jas. J. C (2006). A new potential attractant for Anastrepha obliqua from Spondias mombin fruits. J. Chem. Ecol. 32:351-365. ) ( Damodaram, K. J. P . , Aurade. R. M , Kempraj. V , Ro y . T.K, S hivashankara. K . S a nd Verghese.A (2015). Salicylic acid induces changes i n mango f r uit ) ( that affect oviposition b ehavior and development of the oriental f ruit Fly, Bactrocera dorsalis. Plos One. 10:e0139124. ) Deo, A. and Shastri. N. V (2003). Purification andcharacterization of polygalacturonase-inhibitoryproteins from Psidium guajava Linn. (guava)fruit. Plant Sci. 164: 147-156. El-Sayed, A. M., Suckling. D. M, Byers. J. A, Jang. E. Band Wearing. C. H (2009). Potential of“lure andkill”in long-termppest managementtaanderadication of invasive species. J. Econ.Entomol. 102:815-835. FAO, F. J.L. P. F (2016). Agriculture Organization, 2014.Food Agriculture Organization of the UnitedNations. FAO, I (2017) W. F. P. The State of Food Insecurity inthe World, 2014. Strengthening the enablingenvironment for food security and nutrition. Forister, M. L., Novotny. V, Panorska. A. K, Baje. L,Basset. Y, Butterill. P. T, Cizek. L, Coley. P. D,Dem. F, Diniz. I. R and Drozd.P (2015). Theglobal distribution of diet breadth in insectherbivores. Procee. Nat. Acad. Sci. 112: 442-447. Galal, O., Galal. H and Aboulila. A (2017). Geneticvariability and molecular characterization ofsome local and imported mango cultivars inEgypt. Egyptian J Genetics Cytology. E. J. Gand Cytology.46:121-138. Gautam, I. P. and Gotame. T. P (2020). Diversity ofNative and Exotic Fruit Genetic Resources inNepal. 6: 44-55. JNARC. Ghatak, B., Ali. S. B, Prasad. A, Ghosh. A, Sharma. P,Tudu. B, Pramanik. P and Bandyopadhyay. R(2018). Application of polymethacrylic acidimprinted quartz crystal microbalance sensor fordetection of 3-Carene in mango. IEEE Sensors.18:2697-2704. Girard, B., Kopp. T. G, Reynolds. A. G and Cliff. M(1997). Influence of vinification treatments onaroma constituents and sensory descriptors ofPinot noir wines. Am. J. Enol. Viticult. 48: 198-206. Jaleel, W., He. Y and Lu. L (2019). The response of twoBactrocera species (Diptera: Tephritidae) tofruit volatiles. J. Asia.Paci. Entomol. 22: 758-765. Jaleel, W.,Lu. L and He.Y (2018a). Biology, taxonomy,and IPM strategies of Bactrocera tau Walkerand complex species (Diptera; Tephritidae) inAsia: a comprehensive review. Environ. Sci.Pollut.Res.25: 19346-19361. Jaleel, W., Yin. J, Wang. D, He. Y,Lu. Land Shi. H(2018b). Using two-sex life tables to determinefitness parameters of four Bactrocera species ( (Diptera: T ephritidae) reared on a semi-artificial diet. Bull. Entomol. Res. 107: 7 07-714. ) ( Jaleel , W. , Tao . X , Wang. D, Lu. L, and He. Y (2018c).Using T wo-Sex Life T a ble Traits to A ssess t he Fruit Preference and F itness o f B a ctrocera dorsalis (Diptera: Tephritidae). J. Eco. Entomol. 111(6):2936-2945. ) Jaleel W., Wang. D, Lei. Y, Qi. G, Chen. T, Rizvi. S. A.H, Sethuraman. V, He. Y and Lu. L (2020).Evaluating the repellent effect of four botanicalsagainst two Bactrocera species on mangoes.PeerJ.e8537 Jang, E. B. and Light. D. M (1991). Behavioral responsesof female oriental fruit flies to the odor ofpapayas at three ripeness stages in a laboratoryflight tunnel (Diptera: Tephritidae). J. InsectBehav.4:751-762. Jayanthi, P. D. K., Kempraj.V, Aurade. R.M.Venkataramanappa. R. K, Nandagopal. B,Verghese. A and Bruce. T. J (2014). Specificvolatilecompounds from mangoelicitovipositioningravid Bactrocera dorsalisfemales. J. Chem. Ecol. 40:259-266. Jayanthi, P. D. K., Woodcock.C. M, Caulfield. J, BirkettM. A and Bruce. T. J (2012). Isolation andidentification of host cues fifrommango,Mangifera indica, that attract gravid femaleoriental fruit fly, Bactrocera dorsalis. J. Chem.Ecol.38:361-369. ( Jimenez, L. F . J ., Barros. A. H and De A v ila Z. I. C (2019). Synthesis an d c characterization n ofFe55Co45 magnetic nanoparticles by polyol andgreen c hemistry m ethod. R. Phy. 15: 1 02785. ) Jordán, M. J., Tandon. K, Shaw. P. E and Goodner. K. L(2001). Aromatic profile of aqueous bananaessence and banana fruit by gaschromatography- mass spectrometry (GC-MS)and gas chromatography-olfactometry (GC-O).J.Agric. Food Chem. 49: 4813-4817. Joseph, B and Priya. M (2011). Review on nutritional,medicinal and pharmacological properties ofguava (Psidium guava Linn.). Int. J. Pharma Bio.Sci. 2:53-69. Kondo,T (2018). Disintegrable capsule, manufacturingmethod forssame,andsmokingddevicecontaining said disintegrable capsule: GooglePatents. MacLeod, A. J., and Pieris. N. M (1984). Comparison ofthee volatilecomponentsoof some mangocultivars. Phytochem. 23:361-366. Maldonado-Celis. M. E., Yahia. E. M, Bedoya. R,Landazuri. P, Loango. N, Aguillon. J, Restrepo.Baand Ospina J. C. J (2019). Chemicalcomposition of mango (Mangifera indica L.)fruit: nutritional and phytochemical compounds.F. P. Sci. 10. ( Malo, E. A., Gallegos. Torres. I, Toledo. J ,Valle-Mora. Jand R ojas. J . C (2012). Attraction of th e West Indian fruit fly to mango fruit volatiles. Entomol. Exp. Appl. 142:45-52. ) McQuate, G. T., Sylva. C. D and Liquido. N. J (2017).Natural field infestation of Mangifera casturiand Mangifera lalijiwa by oriental fruit fly,Bactrocera dorsalis (Diptera: Tephritidae). Int. J.Insect Sci. 9:1179543317717735 ( Mitra, S. K ( 2014). Mango p r oduction i n t h e w o rld-present situation and future prospect. XXIX .International Horticultural Congress 011 Horticulture: Sustaining Lives, Livelihoods andLandscapes (IHC2014):IV1111.287-296. ) Paniandy, J. C., Chane-Ming. J and Pieribattesti J. C(2000). Chemical composition of the essentialoil and headspace solid-phase microextraction ofthe guava fruit (Psidium guajava L.). J. Essent.Oil Res. 12: 153-158. ( Pino. J. J . A and F ebles. ;. Y (2013). Od our-activecompounds in banana fruit cv. G iant C avendish. Food Chem. 141: 795-801. ) ( Pino, J. A and M esa. J (2006). Contribution of volatile compounds t o m ango ( M angifera i ndica L .) aroma. F lavour Fragr. J.21:207-213. ) ( Rodriguez, A., Alquezar. B and P ena. L (2013). F ruitaromas i n m ature f l eshy f r uits a s s ignals ofreadiness for predation a nd seed d i spersal.N . Phy. 1 97:36-48. ) ( Sakho, M., C rouzet. J and S eck. S ( 1 9 85). Vo latile components of African mango. J F o od Sci. 5 0 :548-550. ) ( Schwab, W ., Davidovich-Rikanati. R and L e winsohn. E(2008). B iosynthesis o f plant - derived f l avorcompounds. T h e Plant J. 5 4: 712-732. ) ( Shaw, P. E (2017). Fruits ii: Volatile compounds i n foods and beverages (ed. Routledge, pp. 305-327. ) Shen, S. C., Cheng. F. C and Wu. N. J (2008). Effect ofguava(Psidium guajava Linn.) leaf solublesolids on glucose metabolism in type 2 diabeticrats. Phytother.Res. 22:1458-1464. ( Sial, M., Saeed. Q, Saeed. S, Jaleel . W, Naqqash. M. Nand M ajeed. F ( 2 015). S u sceptibility o f M ango cultivars a gains t larvae of Mango midgeProcontarinina m angicola S h i ( C ecidomyiidae:Diptera). Appl. S ci. Business Eco.2: 1- 7 . ) Siderhurst, M. S. and Jang. E. B (2006). Female-biasedattraction of oriental ffruit fly, Bactroceradorsalis (Hendel), toO ablend of host fruitvolatiles from Terminalia catappa L. J. Chem.Ecol.32:2513-2524. ( Siderhurst , M. S. and Jang. E. B (2010). Cucumbervolatile blend attractiv e t o female melon fly, Bactrocera c ucurbitae ( C oquillett). J. C hem.Ecol. 36: 699-708. ) ( Singh, K. K. and Singh. S. P (2018).A review:Micropropagation of guava (Psidium spp.). J. P and Phytochem. 7: 1 45-150. ) Song, J., Bi. J, Chen. Q, Wu. X, Lyu. Y and Meng. X(2019). Assessment of sugar content, fatty acids,free amino acids, and volatile profiles in jujubefruits at different ripening stages. Food Chem.270:344-3522. Souza, J. M., Leonel. S, Modesto. J. H, Ferraz. R. A andGoncalves. B. H (2018). Fruit Physicochemicaland Antioxidant Analysis of Mango Cultivarsunder Subtropical Conditions of Brazil. J. A Sand Technology.20:321-331. Tamura, H., Boonbumrung. S, Yoshizawa..T andVaranyanond. W (2000). Volatile componentsof the essential oils in the pulp of four yellowmangoes (Mangifera indica L.) in Thailand.Food. Sci. Tech. Res. 6:68-73. Vithana, M. D. K., Singh. Z and Johnson. S. K (2018).Levels of terpenoids, mangiferin and phenolicacids in the pulp and peel of ripe mango fruitinfluenced by pre-harvest spray application ofFeSO 4 (Fe 2+), MgSO 4 (Mg 2+) and MnSO4(Mn 2+). Food Chem. 256: 71-76. ( Wetungu,M . W . ,Omolo. M. V, Tarus. P. K and Segor. F . K (2018). V olatile aroma c hemical c onstituents of fruit pulp of some Kenyan varieties of mango (Mangifera indica L.). A. J. E. O and N P r oducts. 6: 29-36. ) ( Xiao-Wei, M. A., Yang. Y. D, Hong-Xia.W . U , Z h ou. Y . G and Wang. S . B (2016). Diversity Analysis ofVolatile in Mature Fruit s o f Mango Germplasm. Acta Horti. Sinica. 43: 1267-1274. ) ( Xu, C., Liang. Z, T ang. D , Xiao. T, T s unoda. M, Zh a ng. Y, Zhao. L , Deng. S a nd S ong.Y (2017). G a sChromatography-Mass S pectrometry (GC-MS) Analysis of Volatil e Components from GuavaLeaves. J.E.O.B. Plants. 20:1536-1546. ) ( Zahoor, M., Yousaf. Z, Aqsa. T , H aroon. M, S a leh. N,Aftab. A, Javed. S, Qadeer. M, and Ramazan. H(2017).An ethnopharmacological evaluation ofNavapin d andl S hahpur Virkanin district Sheikupura, Pakistan for their herbal medicines. .J . Ethnobiol. Ethnomed. 1 3: 27. ) Zhang, J., Jha. S. K, Liu. C and Hayashi. K (2019).Tracing of Chemical Components of Odor inPeels and Flesh from Ripe Banana on a DailyBasisUsing GC-MS Characterization aindStatistical Analysisfor Quality MonitoringDuring Storage. Food A. M. 12: 947-955. ( Zhu, X., Li. Q, L i. J, Luo. J, Chen. W and L i. X (2018). Comparative s t udy of volatile compounds in the fruit of two banana cultivars at different ripening stages. Molecules. 23: 2456. ) 摘 要:农业部门是人类收入和粮食的重要来源,水果是营养、维生素、必需元素的重要来源,也是人类的收入来源。挥发性成分影响果实的香气和品质,关于挥发性成分或挥发性特征的知识和信息是必不可少的,因为这些香气成分使水果对人类来说美味,并可吸引或驱赶害虫。而果实挥发性成分的鉴定对于更好地了解气候条件对果实挥发性成分的影响具有重要意义。本研究首次报道了芒果、番石榴、香蕉水果的挥发性气味特征分析,这些水果在中国普遍商业销售和种植的。采用顶空固相微萃取(HS-SPME)和porapak Q相结合的气相色谱-质谱分析(GC-MS)方法对各品种果实的挥发性成分进行了鉴定。从芒果、番石榴和香蕉果实中分别鉴定出10、15和23种挥发性成分/化合物。果实硬度检测:TMS-PRO质构仪(美国FTC)文献信息:USING GCMS TO FIND OUT THE VOLATILE COMPONENTS IN THE AROMA OF THREE DIFFERENT COMMERCIAL FRUITS IN CHINA
确定






还剩7页未读,是否继续阅读?
北京盈盛恒泰科技有限责任公司为您提供《三种水果中果实硬度检测方案(质构分析仪)》,该方案主要用于其他水果制品中理化分析检测,参考标准--,《三种水果中果实硬度检测方案(质构分析仪)》用到的仪器有美国FTC-质构仪、FTC-质构仪TMS-PRO
相关方案
更多
该厂商其他方案
更多











