方案详情
文
在本研究中,将咖啡因(CA)封装到食品级淀粉基质中,包括膨胀淀粉(SS)、多孔淀粉(PS)和v型淀粉(VS)。采用电子舌法、分子动力学(MD)模拟和CA的体外释放动力学研究了微胶囊的苦味及其抑制机制
方案详情

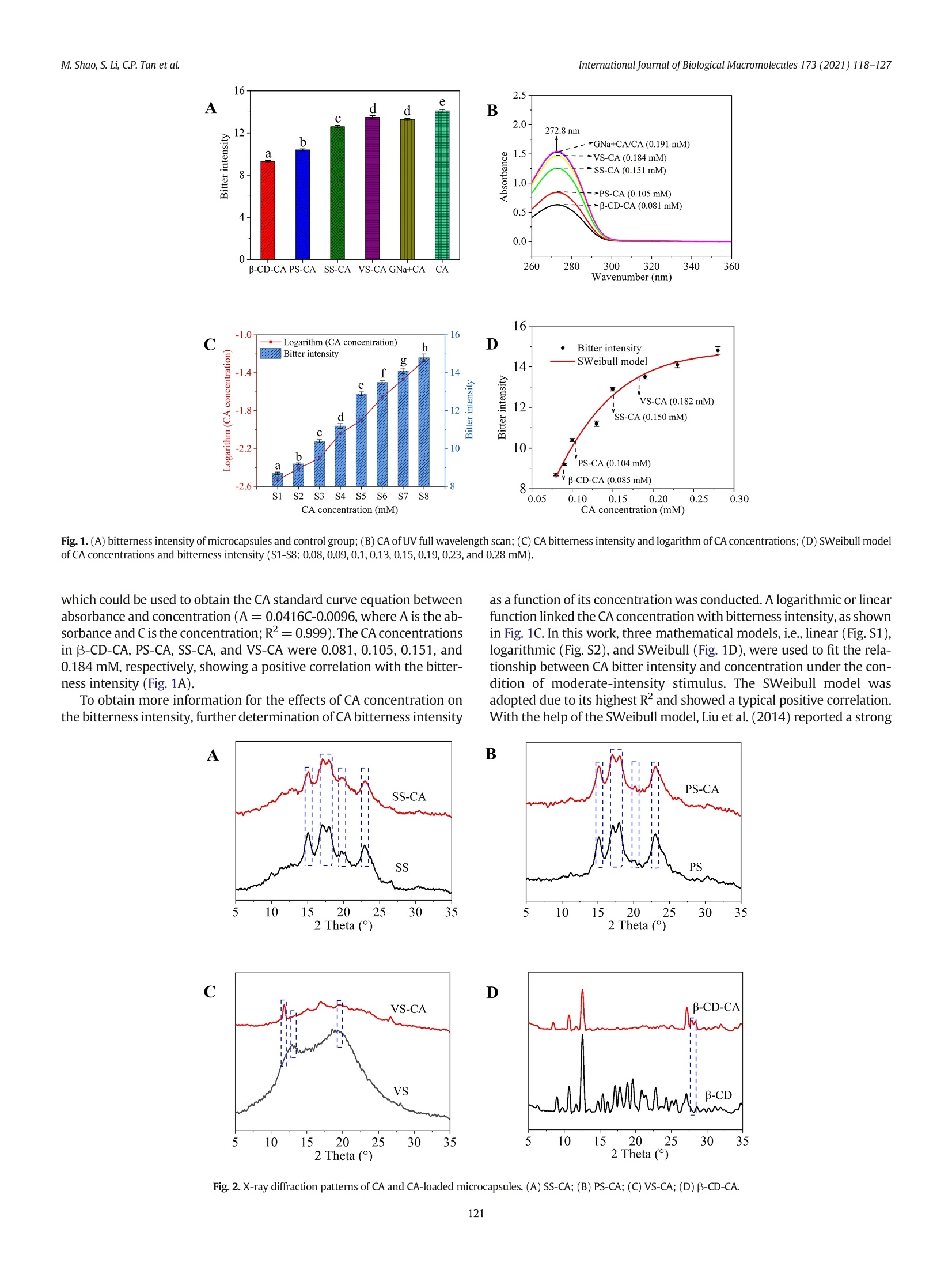
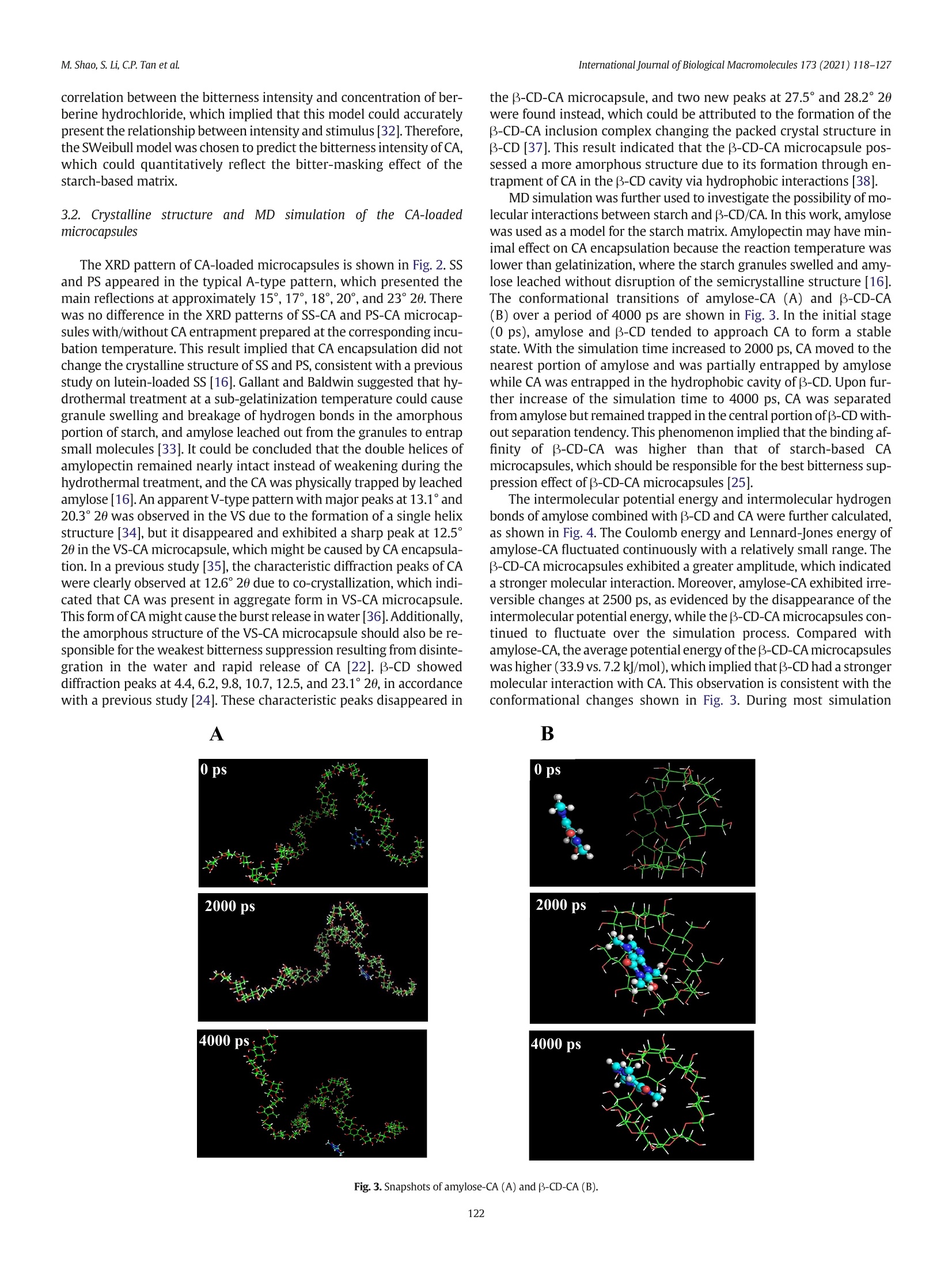
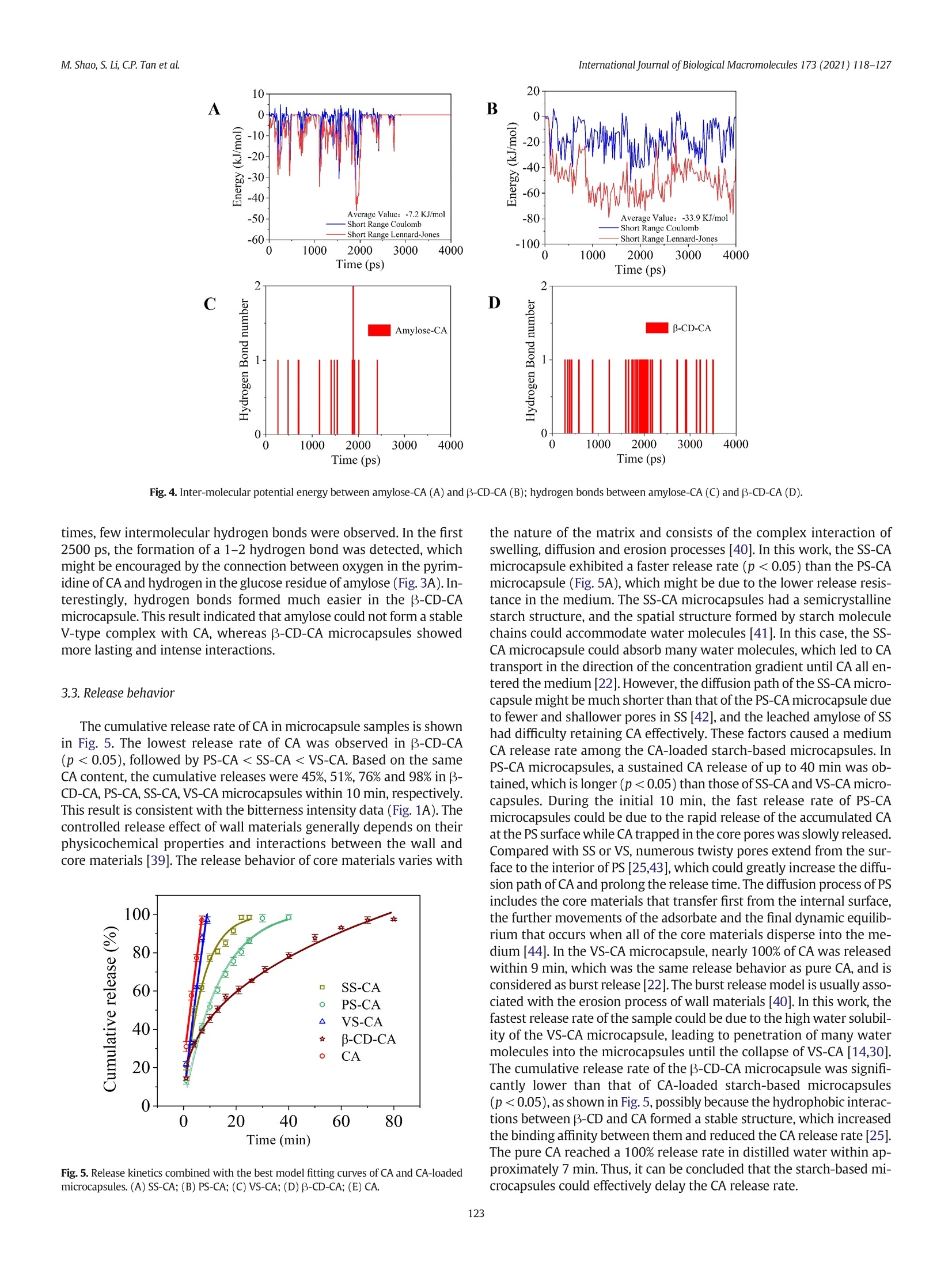
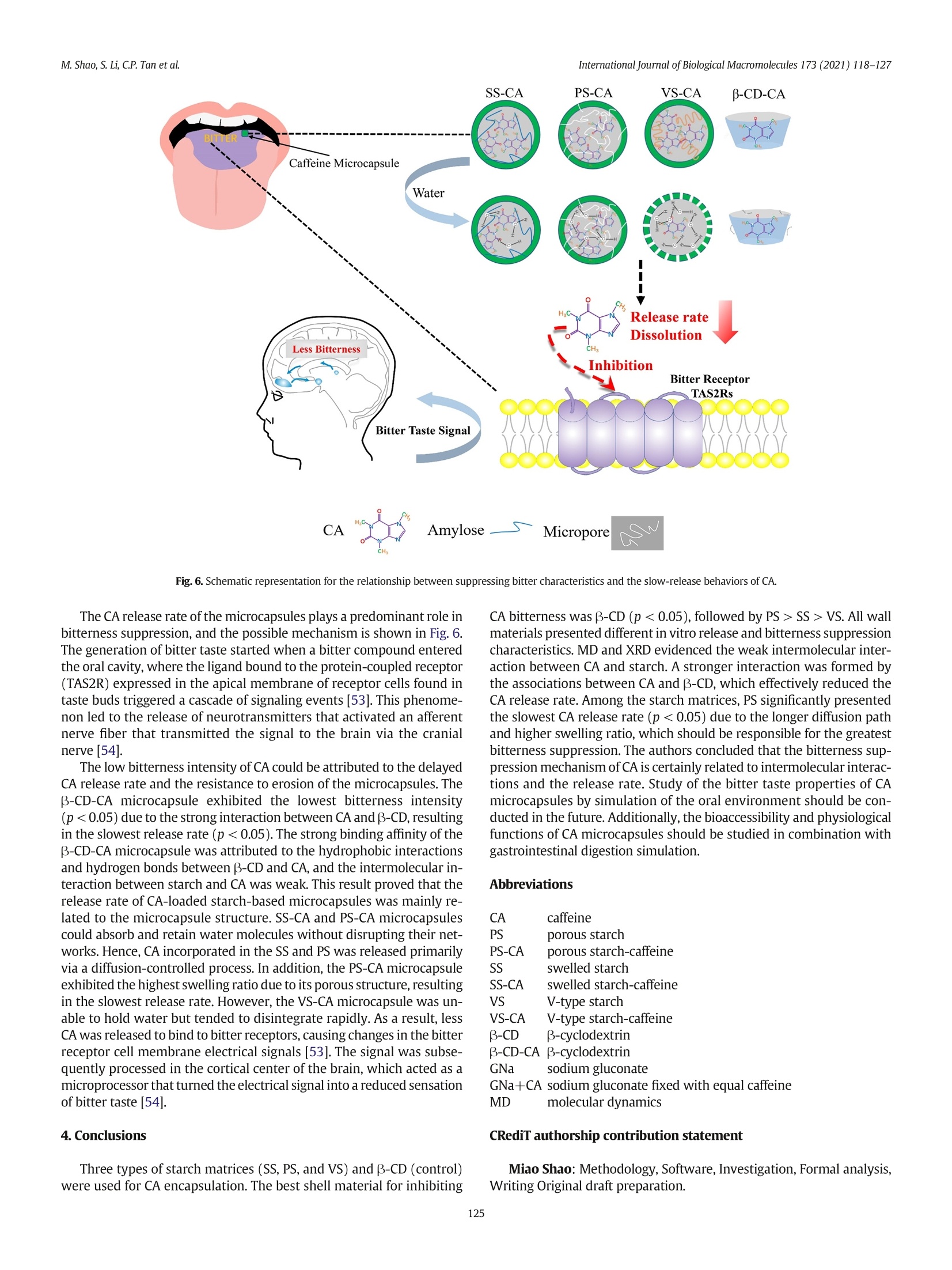
International Journal of Biological Macromolecules 173 (2021) 118-127 Contents lists available at ScienceDirect International Journal of Biological Macromolecules journal homepage: http://www.elsevier.com/locate/ijbiomac Encapsulation of caffeine into starch matrices: Bitterness evaluation andsuppression mechanism Miao Shao a, Songnan Li, Chin Ping Tan , Supaluck Kraithong , Qing Gao,Xiong Fu ad,Bin Zhang,Qiang Huang a.b.d* a School of Food Science and Engineering, Guangdong Province Key Laboratory for Green Processing of Natural Products and Product Safety, South China University ofTechnology, Guangzhou510640, China Sino-Singapore International Joint Research Institute, Guangzhou 511363, China‘Department of Food Technology, Faculty of Food Science and Technology, Universiti Putra Malaysia, Serdang 43400, Selangor, MalaysiaOverseas Expertise Introduction Center for Discipline Innovation of Food Nutrition and Human Health (111 Center), Guangzhou 510640, China ARTICLE INF O Article history: Received 7 October 2020 Received in revised form 13 December 2020 Accepted 7 January 2021 Available online 11 January 2021 Keywords: Caffeine Bitter evaluation Modified starch 1. Introduction Caffeine (1,3,7-trimethylxanthine; CA), a white and water-solublecompound found in many plant species, such as guarana seed and coffeebean, has received increasing attention in the food and pharmaceuticalindustries due to its pharmacological properties [1]. Several in vitro andin vivo studies have demonstrated that CA can stimulate the central ner-vous system, increase alertness and relieve minor fatigue [2]. CA hasbeen widely applied in food and medical products, including beverages,chocolates, and pain-relieving medicines [3]. However, for a single doseof CA, the stimulative effect lasts for only 2-3 h because CA absorption isquite rapid and reaches complete absorption (99%) within 45 min,pro-ducing a quick effect on the central nervous system [4]. Additionally, theextreme bitterness of CA causes an unpleasant aftertaste, which limitsits application in food and drink formulations [5]. Therefore, we needa way to consume CA that can suppress its bitterness as well as providebenefits over a longer period of time. ( * C orresponding author at: School of Food Science and Engineering, Guangdong Province K ey Laboratory for Green Processing of N atural P roducts and Product Safety,South China University of Technology, Guangzhou 510640, China. ) ( E-mail address: qia n gh@s c u t . e du . cn (Q.Huang). ) Encapsulation of CA using odorless/tasteless substances, especiallybiopolymers, can be a better option for suppressing CA bitterness with-out presenting an unrequited flavor/odor [6]. To date, various wall ma-terials have been used to encapsulate CA, such as alginate hydrogelscombined with pectin, carrageenan, chitosan and psyllium [7], B-glucan [8] and B-cyclodextrin (B-CD) [9]. Although these pectinscould suppress CA bitterness, the obtained CA-loaded beads stillshowed some flaws in chewiness during oral processing [7]. Addition-ally, the CA encapsulated by B-glucan was released in an explosiveform within the initial 5 min, which could be attributed to the CA adher-ing to the surface of the wall material [8]. B-CD has been widely appliedfor masking CA bitterness by forming an inclusion complex between thenano-hydrophobic cavity of B-CD and CA [9]. However, the encapsula-tion of CA in different modified starch-based model systems has notyet been studied and could be considered as another excellent optionto control CA release and bitterness. Starch is a major component of our diet and is widely used to encap-sulate bioactive compounds. The ability of native starch to encapsulateactive substances depends on its granule size, shape, solubility, andother properties [10]. Therefore, modification of native starch is neededto improve the controlled release and stability of bioactive componentswhile preventing the perception of undesirable sensory attributes(e.g., bitterness and astringency). Swelled starch (SS) is hydrothermally prepared at sub-gelatinization temperatures. Under this condition,starch granules mildly expand without disrupting their semicrystallinestructure and, via the reverse process, shrink back to their originalform when cooling to room temperature [11,12]. Porous starch (PS)can be prepared using chemical, enzymatic and physical approachesto create or enhance the deep pores and cavities in starch granules,and the high specific surface area can be used to absorb active ingredi-ents [13]. V-type starch (VS) can be produced by alcoholic-alkalinetreatment at elevated temperatures. Under this condition, single helicalamylose crystals are formed by linear alcohol, which is dispersed inwater at ambient temperature and is used to entrap active substances[14,15]. Swelled maize starch granules have been used for encapsula-tion of lutein, and their retention index reached 76% after 21 days ofstorage [16]. Ades et al. [17] used high-amylose, normal, and waxymaize starches for oral controlled release of menthone and menthol,and half of the aroma was released within the first 40 min. In addition,resistant starch was used to encapsulate CA using the freeze-dryingtechnique, and this formulation showed a slow release (47%) in thefirst 5 min [8]. Oliyaei et al.[18] reported that encapsulation of fucoxan-thin with PS improved stability and achieved a sustained release of 60%within 6 h. Numerous attempts have been made to suppress CA bitterness:(i) peripheral interactions in which a compound (e.g., whey protein) an-tagonizes the CA bitter taste receptor, (ii) central cognitive interactions inwhich one strong taste or aroma (e.g.,sodium and zinc) reduces the per-ception of CA bitterness in the brain, and (iii) encapsulation (e.g.,B-CD)by which CA is physically prevented from interacting with the activesites [5,6,9]. However, the scientific details of the interactions betweenCA and starch-based wall materials,which are an important key to con-trolling CA bitterness, are still rarely explored. In this work,SS, PS, andVS were used as encapsulants, and B-CD was used as a control. The abilityof the starch-based wall material to suppress CA bitterness was evaluatedusing an electronic tongue. The release behavior of CA-loaded microcap-sules was assessed to investigate the mechanism of suppressing the bit-terness of CA. Furthermore, the microcapsule structure was studiedusing X-ray diffractometry (XRD) and molecular dynamics (MD) to ex-plain the different release behaviors of microcapsules. This study revealsa promising pathway for use of the starch matrix as a targeted-deliveryand controlled-release carrier of hydrophilic active flavors. 2. Materials and methods 2.1. Materials Normal maize starch (NMS) was purchased from Dacheng Company(Changchun, China). PS was provided by Ingredion (Bridgewater,New Jer-sey, USA). CA (24.8% purity) originating from guarana extract was ac-quired from Dele Company (Shanghai, China). Beta-CD (purity 99%) andthe CA standard (99% purity) were purchased from Sigma-Aldrich (Shang-hai, China). All other chemicals used in this study were of analytical grade 2.2. Preparation of caffeine-loaded microcapsules SS was prepared following a previous study with a minor modifica-tion [16]. To prepare the SS suspension, NMS (5 g) was dispersed in dis-tilled water (100 mL) before heating at 60 ℃ in a water bath underconstant stirring for 2 h. An amount of 100 mg CA (guarana extractwith 24.8% purity) in 100 mL distilled water was added dropwise tothe SS suspension. The system was subjected to the same incubationtemperature and stirring time to obtain an SS-CA mixture. . VS was prepared by alcoholic-alkaline treatment [19]. NMS (20g dryweight basis;DW) was suspended in 140 g of 40% ethanol before addi-tion of NaOH (28 g). The mixture was mixed at 150 rpm for 15 min (at35℃), and 14 g of 40% ethanol was slowly added. The resulting solutionwas centrifuged at 2000g for 10 min and washed twice with 40% etha-nol. The recovered starch was resuspended again in 40% ethanol and neutralized with HCl (3 M in absolute ethanol). The mixture waswashed with 80% ethanol followed by absolute ethanol and oven-dried at 45 ℃ for 20 h. The dried material was gently crushed in ahand agate mortar and passed through a 150-mesh sieve. VS (5 g)was dispersed in distilled water (100 mL) and heated in a water bath(35℃). The same CA solution (guarana extract with 24.8% purity) wasadded to the VS suspension under constant stirring for2 h to obtain aVS-CA mixture. The PS-CA mixture was prepared at a lower temperature than theSS-CA preparation to avoid PS swelling. PS (5 g) was dispersed in100 mL distilled water and heated in a water bath (35℃). PS-CA mix-ture was obtained by adding the same CA solution (guarana extractwith 24.8% purity) into the PS suspension with constant stirring for 2 h. The obtained SS-CA, VS-PA, and PS-CA mixtures were slowly cooledto room temperature. The resulting suspension was centrifuged at2500g for 10 min, and the precipitate was washed three times using dis-tilled water to remove unencapsulated CA. The precipitate was dried at30 °C overnight, and SS-CA, PS-CA, and VS-CA microcapsules were ob-tained. The B-CD-CA microcapsule (control) was prepared under thesame conditions as the PS-CA microcapsule. 2.3. Bitterness evaluation Each type of CA microcapsule containing 1 mg (DW) CA wasweighed and transferred to 200 mL of distilled water followed by con-stant stirring at 25 C for 10 min. The resulting suspension was immedi-ately filtered through filter paper with a pore size of 3 um. The bitternessof the filtered solution was evaluated using a TS-5000Z taste sensingsystem equipped with a COO sensor and a reference electrode (i-Tongue,Insent Co.,Japan).The machine detected the samples four times auto-matically,and theaverage value of the final three groups was obtained.Positive values indicate that the taste is stronger than that of the controlgroup. The CA standard solution (0.5%,w/v) and a solution of CA stan-dard (0.5%,w/v) mixed with sodium gluconate (GNa) at a ratio of 1:1(w/w) were used as controls [20]. In this work, GNa was used as a con-trol to compare the differences in bitterness suppression between bitterblockers and encapsulation. 2.4. Determination of CA concentration The CA concentration was determined according to a previous re-port with minor modifications [8]. The obtained filtered CA solution inSection 2.3. was used to determine the CA concentration by ultravioletspectrophotometry at 272.8 nm. 2.5. X-ray diffractometry The samples were equilibrated in a 100% relative humidity chamberat 25 C for 24 h prior to X-ray analysis (D8 Advance, Bruker, Germany).The X-ray source was Cu-Ka radiation (入=0.154 nm) with voltagesand currents of 40 kV and 40 mA, respectively. The sample powderwas packed tightly in a rectangular glass cell and scanned over a rangeof5-35° 20 at a rate of 2°/min [16]. 2.6. Molecular dynamics simulation MD simulation was performed by a GROMACS 5.1.4 program. All sim-ulations were carried out using the GROMOS 56A6CARBOR force field [21].Both the molecular model and force field were provided by Shanghai In-stitute of Technology (Shanghai, China).The structure file of CA was ob-tained from http://zinc.docking.org/. In the system, CA and B-CD/amylose (36 glucose residues) were embedded in a dodecahedron boxwith dimensions of 130 Ax30Ax30A and filled with 1000 water mol-ecules. In this work, SS-CA and PS-CA microcapsules were prepared atsub-gelatinization temperatures, and for VS, the original amylopectinwas transformed into a single helix. Thus, the system can be used to investigate the interactions between starch and CA. The simulation (en-ergy-minimized model) was further conducted by annealing using theNPT ensemble (100 ns) at 60 °C until equilibrium was reached. The sim-ulations of the system were run for 4000 ps with a step size of 0.001 ps.The short-range Lennard-Jones, Coulomb interaction energy, and hydro-gen bonds were analyzed. The Lennard-Jones and Coulomb measures rep-resent the energy change of van der Waals and electrostatic forces,respectively. The hydrogen bonds were determined as follows: the max-imum distance of the acceptor atom to the donor atom is 0.35 nm, and theangle of the hydrogen donor-acceptor is less than 30°. 2.7. Release kinetics The release kinetics of the microcapsules were studied following themethod described by Belscak-Cvitanovic et al. [7] with slight modifica-tions. Each type of microcapsule containing 50 mg of CA was suspendedin distilled water at a ratio of 1:40 (w/w) before continuous agitation ata stirring speed of 100 rpm at25C. The suspension (2 mL) was removedat specific time intervals and immediately replaced by 2 mL of freshwater. The samples were centrifuged at 4000g for 20 min, and the super-natants were used to determine the CA concentrations. The cumulativerelease (%) of CA was calculated using the following equation. where Mo and Mt are the amounts of CA in the initial samples and thecumulative amount in the release medium at each sampling time (t), re-spectively. For a better understanding of the release characteristics ofCA, four different models were applied to fit the obtained release data:(i) zero-order kinetics model, (ii) first-order kinetics model, (iii)Higuchi model, and (iv) Ritger-Peppas model [22]. Each equation isshown below. where k is the release constant of different models, and n is the releaseexponent. The correlation coefficient (R-) refers to the fitting degree be-tween the release model and data. 2.8. Microcapsule swelling and erosion studies The microcapsule samples (0.5g) were placed into 40 mL of distilledwater before agitation at 100 rpm at 25℃ for 1 h. The swollen micro-capsules were removed from the suspension, dried on filter paper andweighed (W). The percentage of water uptake or swelling rate was es-timated at each time point using the following equation. [23] where W is the weight of the microcapsules at a defined time and W isthe initial weight. After the swelling study, the wet samples were driedin an oven for 48 h until a constant weight (Wa) was achieved. The per-centage of microcapsule erosion was estimated from the followingequation. where Wo is the initial weight of the microcapsule and Wa is the micro-capsule weight after drying. 2.9. Statistical analysis All experiments were performed at least in duplicate unless other-wise specified, and only XRD analysis was conducted with a single mea-surement. Mean comparison was performed with Duncan's multiplerange tests by SPSS 19.0 software (SPSS, Inc., Chicago, IL). The resultsare presented as means ± standard deviations (SD). The significant dif-ference was set at p<0.05. 3. Results and discussion 3.1. Bitterness evaluation and CA concentration Fig. 1A presents the bitterness intensity of CA and CA-loaded micro-capsules. The lowest value was found in B-CD-CA (p<0.05), followedby PS-CA0.05), indicating that encapsulating CA into starch-based wall ma-terials and directly adding bitter blockers had similar effects on sup-pressing CA bitterness. Fig. 1B shows the UV spectrum in the full wavelength scan of CA-loaded starch-based microcapsules as a function of concentration. Themaximum absorption wavelength of CA was observed at 272.8 nm, Fig.1. (A) bitterness intensity of microcapsules and control group; (B) CA of UV full wavelength scan; (C) CA bitterness intensity and logarithm of CA concentrations; (D) SWeibull modelof CA concentrations and bitterness intensity (S1-S8:0.08,0.09,0.1,0.13,0.15,0.19, 0.23, and 0.28 mM). which could be used to obtain the CA standard curve equation betweenabsorbance and concentration (A=0.0416C-0.0096, where A is the ab-sorbance and C is the concentration;R²=0.999). The CA concentrationsin B-CD-CA,PS-CA,SS-CA, and VS-CA were 0.081, 0.105, 0.151, and0.184 mM, respectively, showing a positive correlation with the bitter-ness intensity (Fig.1A). To obtain more information for the effects of CA concentration onthe bitterness intensity, further determination of CA bitterness intensity as a function of its concentration was conducted. A logarithmic or linearfunction linked the CA concentration with bitterness intensity,as shownin Fig. 1C. In this work, three mathematical models, i.e.,linear (Fig. S1),logarithmic (Fig. S2), and SWeibull (Fig. 1D), were used to fit the rela-tionship between CA bitter intensity and concentration under the con-dition of moderate-intensity stimulus. The SWeibull model wasadopted due to its highest R² and showed a typical positive correlation.With the help of the SWeibull model,Liu et al.(2014) reported a strong Fig. 2.X-ray diffraction patterns of CA and CA-loaded microcapsules. (A) SS-CA; (B) PS-CA; (C) VS-CA;(D) B-CD-CA. correlation between the bitterness intensity and concentration of ber-berine hydrochloride, which implied that this model could accuratelypresent the relationship between intensity and stimulus [32]. Therefore,the SWeibull model was chosen to predict the bitterness intensity of CA,which could quantitatively reflect the bitter-masking effect of thestarch-based matrix. 3.2. Crystalline structure and MD simulation of the CA-loadedmicrocapsules The XRD pattern of CA-loaded microcapsules is shown in Fig. 2. SSand PS appeared in the typical A-type pattern, which presented themain reflections at approximately 15°, 17°, 18°, 20°, and 23°20. Therewas no difference in the XRD patterns of SS-CA and PS-CA microcap-sules with/without CA entrapment prepared at the corresponding incu-bation temperature.This result implied that CA encapsulation did notchange the crystalline structure of SS and PS, consistent with a previousstudy on lutein-loaded SS [16]. Gallant and Baldwin suggested that hy-drothermal treatment at a sub-gelatinization temperature could causegranule swelling and breakage of hydrogen bonds in the amorphousportion of starch, and amylose leached out from the granules to entrapsmall molecules [33]. It could be concluded that the double helices ofamylopectin remained nearly intact instead of weakening during thehydrothermal treatment, and the CA was physically trapped by leachedamylose [16]. An apparent V-type pattern with major peaks at 13.1°and20.3° 20 was observed in the VS due to the formation of a single helixstructure [34], but it disappeared and exhibited a sharp peak at 12.5°20 in the VS-CA microcapsule, which might be caused by CA encapsula-tion. In a previous study [35], the characteristic diffraction peaks of CAwere clearly observed at 12.6° 20 due to co-crystallization, which indi-cated that CA was present in aggregate form in VS-CA microcapsule.This form of CA might cause the burst release in water [36].Additionally,the amorphous structure of the VS-CA microcapsule should also be re-sponsible for the weakest bitterness suppression resulting from disinte-gration in the water and rapid release of CA [22]. B-CD showeddiffraction peaks at 4.4, 6.2, 9.8, 10.7, 12.5, and 23.1° 20, in accordancewith a previous study [24]. These characteristic peaks disappeared in Fig.3. Snapshots of amylose-CA (A) and B-CD-CA(B). the B-CD-CA microcapsule, and two new peaks at 27.5° and 28.2° 20were found instead, which could be attributed to the formation of theB-CD-CA inclusion complex changing the packed crystal structure inB-CD [37]. This result indicated that the B-CD-CA microcapsule pos-sessed a more amorphous structure due to its formation through en-trapment of CA in the B-CD cavity via hydrophobic interactions [38]. MD simulation was further used to investigate the possibility of mo-lecular interactions between starch and B-CD/CA. In this work, amylosewas used as a model for the starch matrix. Amylopectin may have min-imal effect on CA encapsulation because the reaction temperature waslower than gelatinization, where the starch granules swelled and amy-lose leached without disruption of the semicrystalline structure [16].The conformational transitions of amylose-CA (A) and B-CD-CA(B) over a period of 4000 ps are shown in Fig. 3. In the initial stage(0 ps), amylose and B-CD tended to approach CA to form a stablestate. With the simulation time increased to 2000 ps, CA moved to thenearest portion of amylose and was partially entrapped by amylosewhile CA was entrapped in the hydrophobic cavity of B-CD. Upon fur-ther increase of the simulation time to 4000 ps, CA was separatedfrom amylose but remained trapped in the central portion of B-CD with-out separation tendency. This phenomenon implied that the binding af-finity of B-CD-CA was higher than that of starch-based CAmicrocapsules, which should be responsible for the best bitterness sup-pression effect of B-CD-CA microcapsules [25]. The intermolecular potential energy and intermolecular hydrogenbonds ofamylose combined with B-CD and CA were further calculated,as shown in Fig. 4. The Coulomb energy and Lennard-Jones energy ofamylose-CA fluctuated continuously with a relatively small range. TheB-CD-CA microcapsules exhibited a greater amplitude,which indicateda stronger molecular interaction. Moreover, amylose-CA exhibited irre-versible changes at 2500 ps, as evidenced by the disappearance of theintermolecular potential energy, while the B-CD-CA microcapsules con-tinued to fluctuate over the simulation process. Compared withamylose-CA, the average potential energy of the B-CD-CA microcapsuleswas higher (33.9 vs. 7.2 kJ/mol), which implied that B-CD had a strongermolecular interaction with CA. This observation is consistent with theconformational changes shown in Fig. 3. During most simulation times, few intermolecular hydrogen bonds were observed. In the first2500 ps, the formation of a 1-2 hydrogen bond was detected, whichmight be encouraged by the connection between oxygen in the pyrim-idine of CA and hydrogen in the glucose residue of amylose (Fig. 3A). In-terestingly, hydrogen bonds formed much easier in the B-CD-CAmicrocapsule. This result indicated that amylose could not form a stableV-type complex with CA, whereas B-CD-CA microcapsules showedmore lasting and intense interactions. 3.3. Release behavior The cumulative release rate of CA in microcapsule samples is shownin Fig. 5. The lowest release rate of CA was observed in B-CD-CA(p<0.05), followed by PS-CA < SS-CA 0.89 “supra II" type transport [45]. The nvalue is influenced by the diffusion, swelling, and erosion process of mi-crocapsules, which could explain the release mechanism [22]. In thiswork, PS-CA (n =0.51) exhibited non-Fickian transport, whereas SS-CA (n=0.39) and B-CD-CA (n=0.38) displayeda Fickian transportmechanism, which indicated a diffusion-controlled process [46]. Non-Fickian transport can be observed during the in vivo simulation of mi-crocapsules prepared with natural and synthetic polysaccharides [47],which implied that PS-CA was an intermediate model between diffusionand wall material relaxation related to the porous structure. The Fickiantransport was mainly controlled by diffusion, consistent with the finestructure of SS-CA and B-CD-CA. The initial high release rate might beattributed to the migration of CA to the SS and PS surfaces during storage [48],which might reduce the effective path of diffusion. While CAdiffused into the medium, the different concentrations between the in-ternal and external CA-loaded microcapsules decreased, resulting in aslower release rate of CA. The VS-CA microcapsules showed “supra II"-type transport (burst effect), which released CA through wall materialerosion or degradation within the dissolution medium [22]. Previousstudies suggested that the burst effect might occur when the formed in-clusion complex tended to disintegrate in the medium [49]. This obser-vation indicated that a considerable percentage of CA was released in ashort time due to disintegration of VS. 3.4. Swelling and erosion studies Table 2 shows the rehydration behavior of the CA-loaded microcap-sules. The PS-CA microcapsule exhibited the highest swelling ratio(p<0.05), which might be encouraged by its porous structure [44].Many studies have proven that the swelling power of native starchcould be enhanced after pore formation because the amorphous regionsof native starch are disrupted during the pore-forming process [50].Consequently, the macromolecular segment movement of starch in-creases gradually, allowing starch volume to increase, which is condu-cive to continuing infiltrating of water molecules [51]. At a higherswelling ratio, a wall material that can highly expand and recover itsshape after swelling commonly presents greater resistance to dissolu-tion [52]. Notably, SS-CA and PS-CA microcapsules showed a low ero-sion ratio that could be caused by dimensional networks formed bystarch molecules with high molecular weights, flexibility, and Table 2Swelling and erosion ratio of CA-loaded microcapsules. Sample Rehydration (%) Swelling ratio Erosion ratio SS-CA 27.56 ±1.46° 4.29±1.17 PS-CA 39.03±2.91° 6.39±1.05 VS-CA 17.48±1.62” 31.55±2.04° B-CD-CA 27.24±1.33° Means in a column with different superscript letters are significantly different(p<0.05). interchain spaces in which the networks might extremely absorb andretain water without disrupting granule structure [41]. However, theVS-CA microcapsule had the largest erosion ratio (p<0.05), whichwas due to the disruption of the starch semicrystalline structure leadingto the partial dissolution of the microcapsule in water [36]. These resultsconfirmed that SS and PS could retain CA by providing a barrier againstdiffusion, which is also consistent with release kinetics. 3.5. Possible mechanisms for suppressing CA bitterness Encapsulating CA into the starch matrix can reduce its dissolutionand release rate in water. The ability to resist disintegration and CA re-lease highly relies on the starch structural characteristics and the molec-ular interaction between CA and starch. SS-CA microcapsules wereformed by the adsorption and physical entrapment of amylose [16],exhibiting a medial release rate (Fig. 5A). This molecular interaction be-tween amylose/long branch chains of amylopectin and hydrophobiccompounds in an aqueous medium was driven by hydrophobic and/orvan der Waals interactions between the guest compound and theinner hydrophobic core of the starch chain [52]. However, in thiswork, weak interactions between amylose and CA were evidenced bythe MD simulation results (Figs. 3A and 4A), and no V-type peak charac-teristic was detected in the XRD analysis (Fig. 2A). This result indicatedthe insufficient driving force of forming the V-type crystalline structureand the weak bonding force of SS-CA, which could be responsible for thefaster release rate compared to that of the PS-CA microcapsule. As pre-sented in Fig.5B, the slowest CA release rate was observed in the PS-CA microcapsule (p<0.05) due to the CA diffusion rate (Table 1) andhigh swelling ratio (Table 2). In other words, PS could retain more CAwithin its porous structure, thus delaying CA diffusion [28]. VS-CA microcapsules were formed during the recrystallization pro-cess of VS without the XRD characteristic peak of starch (Fig.2C), indi-cating that the semicrystalline structure of starch was disrupted|[36].The VS-CA microcapsule exhibited high erosion but a low swellingratio in water (Table 2), which was attributed to its partial dissolutionand the rapid release of CA [22]. The stable B-CD-CA microcapsule struc-ture was proven by the crystalline structure formation (Fig.2D) and thestrong interaction between B-CD and CA (Figs. 3B and 4B), whichshould be responsible for the slowest release rate of CA. Sample Mathematical model Zero order First order Higuchi Ritger-Peppas SS-CA Q=2.61t+ 40.13 In(100-Q)=-0.14t + 4.61 Q=18.21t1/2+12.0 InQ=0.39Int + 3.36 (R²=0.86) (R²=0.98) (R²=0.95) (R²=0.98);n = 0.39 PS-CA Q= 2.35t +22.83 In(100-Q)=-0.07t+4.63 Q=17.38t1/2-2.86 InQ=0.51Int + 2.77 (R²=0.89) (R²=0.99) (R-=0.98) (R²=0.99); n = 0.51 VS-CA Q=10.40t +7.62 In(100-Q)=-0.04t + 5.64 Q=39.92t1/2-27.06 InQ=0.91Int + 2.71 (R²=0.96) (R²=0.94) (R²=0.89) (R²=0.96); n= 0.91 B-CD-CA Q=0.92t + 33.43 In(100-Q)=-0.05t + 4.57 Q=10.30t1/2+10.98 InQ=0.38Int + 2.92 (R²=0.89) (R²=0.99) (R²=0.97) (R²=0.98); n=0.38 CA Q=10.61t+23.24 In(100-Q)=-0.21t + 4.83 Q=40.77t1/2-11.73 InQ= 0.61Int + 3.37 (R²=0.99) (R²=0.96) (R²=0.98) (R²= 0.96); n = 0.61 Fig. 6. Schematic representation for the relationship between suppressing bitter characteristics and the slow-release behaviors ofCA. The CA release rate of the microcapsules plays a predominant role inbitterness suppression, and the possible mechanism is shown in Fig. 6.The generation of bitter taste started when a bitter compound enteredthe oral cavity, where the ligand bound to the protein-coupled receptor(TAS2R) expressed in the apical membrane of receptor cells found intaste buds triggered a cascade of signaling events [53]. This phenome-non led to the release of neurotransmitters that activated an afferentnerve fiber that transmitted the signal to the brain via the cranialnerve [54]. The low bitterness intensity of CA could be attributed to the delayedCA release rate and the resistance to erosion of the microcapsules. TheB-CD-CA microcapsule exhibited the lowest bitterness intensity(p<0.05) due to the strong interaction between CA and B-CD, resultingin the slowest release rate (p<0.05). The strong binding affinity of theB-CD-CA microcapsule was attributed to the hydrophobic interactionsand hydrogen bonds between B-CD and CA, and the intermolecular in-teraction between starch and CA was weak. This result proved that therelease rate of CA-loaded starch-based microcapsules was mainly re-lated to the microcapsule structure. SS-CA and PS-CA microcapsulescould absorb and retain water molecules without disrupting their net-works. Hence, CA incorporated in the SS and PS was released primarilyvia a diffusion-controlled process. In addition, the PS-CA microcapsuleexhibited the highest swelling ratio due to its porous structure, resultingin the slowest release rate.However, the VS-CA microcapsule was un-able to hold water but tended to disintegrate rapidly. As a result, lessCA was released to bind to bitter receptors, causing changes in the bitterreceptor cell membrane electrical signals [53]. The signal was subse-quently processed in the cortical center of the brain, which acted as amicroprocessor that turned the electrical signal into a reduced sensationof bitter taste [54]. 4. Conclusions Three types of starch matrices (SS, PS, and VS) and B-CD (control)were used for CA encapsulation. The best shell material for inhibiting CA bitterness was B-CD (p<0.05), followed by PS > SS> VS. All wallmaterials presented different in vitro release and bitterness suppressioncharacteristics. MD and XRD evidenced the weak intermolecular inter-action between CA and starch. A stronger interaction was formed bythe associations between CA and B-CD, which effectively reduced theCA release rate. Among the starch matrices, PS significantly presentedthe slowest CA release rate (p <0.05) due to the longer diffusion pathand higher swelling ratio, which should be responsible for the greatestbitterness suppression. The authors concluded that the bitterness sup-pression mechanism of CA is certainly related to intermolecular interac-tions and the release rate. Study of the bitter taste properties of CAmicrocapsules by simulation of the oral environment should be con-ducted in the future. Additionally, the bioaccessibility and physiologicalfunctions of CA microcapsules should be studied in combination withgastrointestinal digestion simulation. Abbreviations caffeine porous starch PS-CA porous starch-caffeine SS swelled starch SS-CA swelled starch-caffeine VS V-type starch VS-CA V-type starch-caffeine B-CD B-cyclodextrin B-CD-CA B-cyclodextrin GNa sodium gluconate GNa+CA sodium gluconate fixed with equal caffeine MD molecular dynamics CRediT authorship contribution statement Miao Shao: Methodology, Software, Investigation, Formal analysis,Writing Original draft preparation. Songnan Li: Investigation. Chin Ping Tan: Writing-Review & Editing. Supaluck Kraithong: Writing-Review & Editing. Qing Gao: Investigation, Writing-Review & Editing. Xiong Fu: Writing-Review & Editing. Bin Zhang: Investigation, Writing-Review & Editing. Qiang Huang: Conceptualization, Supervision, Writing-Review &Editing, Funding acquisition, Validation. Declaration of competing interest The authors declare no conflict of interest. Acknowledgments The authors are thankful for all supporting from the National Key Re-search and Development Program of China (2017YFD0400502) the Na-tional Natural Science Foundation of China (31771929), the NaturalScience Foundation of Guangdong Province (2019A1515012174), andthe 111 Project (B17018), China. Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijbiomac.2021.01.043. ( References ) ( [1] F . C . S chimp l, J. F . Si lva, J .F . C. Gon c a l v es , P.M a zz a fera, G u a rana : r e v i si t i n g a h i g hly caf - f ei nated pl a n t fr om t h e A m a z on, J . Et h n op h a rm a c ol. 1 50 ( 1 ) ( 20 1 3) 14 - 31. ) [2] A. Nehlig,J. Daval, G. Debry, Caffeine and the central nervous system: mechanismsof action, biochemical, metabolic and psychostimulant effects, Brain Res. Rev. 17(2)(1992)139-170. ( [3] 1 P . Q ui n lan ,J . La ne , L . Aspinall , Eff e cts o f h o t t ea, c of fe e a n d w ate r in g estion on phy s - i o logical r e s po n s e s and m o od: t h e r o le o f caffeine,water an d bever a ge ty p e, P sych o - pha r mac o logy 1 34 ( 2) (1 9 9 7) 1 6 4 - 1 73. ) ( [4] ] P .J. Gre e n,J . S u l s , T he ef fe ct s o f c affe i ne o n a m b u lato r y b lo o d pr e s sur e, he ar t ra t e, a n d m o o d in co f fee dri n kers, J our n a l of Beh a v i oral M edici n e 19 (2) ( 1 996)11 1 - 1 2 8 . ) ( [5] K . Ten ney, J . Ha yes, S . E u s t o n , R. E lias,J . C o u pl an d, Bin ding of caffe i ne and q uin i n e b y w h ey pr o tei n an d th e e ffect o n b i t terne s s, J. F o od Sc i . 82 ( 1 ) (201 7) 50 9-51 6. ) ( [6] R . S .J. Keas t , Mod i fic a tion of t h e bi tt ern e ss o f c aff e in e , Fo od Q u a l. Pr e fer . 1 9 ( 5 )(20 0 8) 465- 47 2. ) [7A]. Belscak-Cvitanovic, D. Komes, S. Karlovic, S. Djakovic, I. Spoljaric, G. Mrsic, D.Jezek, Improving the controlled delivery formulations of caffeine in alginate hydro-gel beads combined with pectin, carrageenan, chitosan and psyllium, Food Chem.167(2015)378-386. ( [8] N . N o o r, A. S ha h , A . G a n i , A . G a n i , F .A. M as ood i, M icro enc a ps u l atio n o f ca ff ei n e l oa ded i n p olysaccharid e ba sed del i very syste m s, Food H ydrocoll . 8 2 ( 20 1 8 ) 3 12 -32 1 . ) ( [9] S . Pa n da, M . Na yak, T a s te mas king of c a ff eine b y i n cl u s ion co mple x a ti o n o f β - c y cl o dextr i n , Jo u r n a l of P h a r m ace ut ical Ad va nce d Res ea rc h 1 (4)(201 8 )224- 22 8 . ) [10] F. Zhu, Encapsulation and delivery of food ingredients using starch based systems,Food Chem.229 (2017) 542-552. ( [11] F.D . C h a n g , X . X. He, Q . Huan g, The p hysic o ch em ica l p r o p erties o f sw el le d m aiz e s t arch granu l e s complexed wit h l a ur i c acid, Food Hy d roco l l. 32 (2) (20 1 3 ) 3 6 5-3 72 . ) ( [12] J .A . P ut s eys, L . L a mber ts, J. A . Del c o u r, Am ylose - in clu si o n c omp l e xes: f o rm atio n, i de n t i ty a nd p hy sico- c he mical p r o p er ti e s, J. Cere al Sc i . 5 1 ( 3 ) ( 2 0 1 0)238-2 4 7 . ) ( [1 3 ] G . C hen,B . Zhang, H y drolysis of gra n ular corn s t arch w i t h c o nt ro l l e d p o re siz e , J. Ce - r eal Sci. 56 ( 2) ( 20 1 2) 3 16-3 2 0. ) ( [ 1 4] J . Ch e n , J . J an e, Effe cti v e ness o f g r an u l a r c ol d - w a te r-so l u ble s t a r c h as a co n t r oll e d - r e l e ase m a t ri x , Cerea l C hem . 7 2 (3 ) ( 1 995) 26 5- 26 8. ) ( [ 1 5] M . A. W h ittam, P. D . O r fo r d, S . G. R i n g ,S.A . Clar k , M . L . P a r k er , P. Cai rns, M . J. M i le s , A que ous d issol ut io n o f cr y st al lin e a nd amor p h o u s am ylose a lc o ho l c omp le x e s, I nt . J . Bi o l . M ac ro mol . 1 1 (6) ( 198 9 ) 33 9- 34 4. ) [16] S.N.Li, C. Wang,X. Fu, C. Li, X.W.He, B. Zhang, Q. Huang, Encapsulation of lutein intoswelled cornstarch granules: structure, stability and in vitro digestion, Food Chem.268(2018) 362-368. ( [17] H . A des, E . K es s elm a n, Y . U n gar, E . Shim on i, C o mp l e xatio n with s t ar c h f o r e n ca ps u - l ation a n d c ont r oll e d re lea s e of men t h o n e and m e n t hol, L WT Foo d Sc i. T e c hnol . 45 (2) ( 20 1 2) 2 77 - 288 . ) ( [ 1 8]N . O l i y a e i, M .M o o s avi- N asa b ,A.M . Tam ad do n , M . Faz ae l i , En c a ps u l a t io n o f f u cox a n- t hi n i n bi n a r y matr i ces of po rou s star c h a nd h a ll oys i t e , F o o d H ydro c oll . 1 00 (20 1 8 ) 1 0 5 458. ) ( [19] L . F . S hi , X . Fu , C . P. Ta n , Enc ap s u la t i o n o f e t hy le n e gas i n t o g r a n ul ar c old - wat er- s olu b l e s t a r c h : s truct ure an d rele a se k in e ti c s, J.Agric . F o od Ch e m . 6 5 ( 1 0 )( 2 0 1 7)21 8 9- 21 97. ) [20] L.L. Wu,M. Zhang, Y.P. Liu, Q. Sun, Characteristics and release of monosodium gluta-mate microcapsules obtained by spray drying, Dry. Technol. 37 (11)(2019)1340-1351. ( [21] L . L. C he n g , T. Fe n g, B.Y. Z h ang, X . Zhu, B. Ha m a k er, H . Z ha n g, O . C am pa n e l la, A m o- lec ular d ynam i cs s i m ul ati on s t u dy on the con fo rm a tional sta b i li t y of a my l os e - linoleic ac id c o mp l ex i n wate r , C ar bo h yd r . Poly m . 1 96 ( 15 ) ( 20 1 8 ) 56 -65 . ) ( [22] F .P. F l ores, F. Ko n g, I n v i t r o r ele a se ki neti c s o f mic ro encap s ul at e d m a ter ia l s a n d the e ffec t of t he foo d matrix, A n n u . Re v . Food S ci .T e chno l . 8 ( 1 )(201 7 ) 23 7 -259. ) ( [23] C . Dima,M. C ot a r l e t, P. Alexe, S . Di ma ,M icroe nc apsul at i o n o f es se nt ial o i l o f pi ment o [ P i men t ad i oic a (L) M e r r.] by ch i tosan/k- c a rr a g een a n com plex coace r vation m et hod, I nnov. Fo o d S c i. E m e r g. Tech no l . 2 2 ( 2 014) 203-2 1 1. ) ( [24] K . Siva k um a r , G. P arina m ach i vayam, M. Mu ra li Kr i s h n a n, S . Ch a kr ava r t y , A. Bha rathi, Prepa r a t i o n , char a cte r i zat i o n an d m o lec ul ar m o d elin g studies of t h e bet a-c yclodextri n incl u sion co mpl e x with be n zo g uana m i n e an d its an a lyt i ca l app li cation a s c he m os e n so r f o r t he s e lect i v e sen s i n g of C e 4+, S pec tr ochi m. A c ta A M o l. B i omo l . Spe ct ros c. 200 ( 2 0 1 8 ) 2 12 -2 25. ) ( [25] J . N .C o u pl an d , J. E. Ha ye s , Phys ic a l a p p ro ach e s to m aski n g bitt e r t aste : le s sons f rom f o od a nd p h a r m a c eu ti c a ls , P ha r m.R e s . 31 (1 1 ) ( 20 14)2 9 21-2 93 9. ) ( [26] G . A s tr ay, J .C . M ej ut o, J. Mor a l e s, R . R ia l -O t e ro , J. Sim a l- G an da r a, Fac t o r s c o n trol l in g f lavors bin ding cons t a nts to cyclodextrins a n d t he i r app l ic a tions in f oo d s , Foo d Res . I n t . 4 3 ( 4 ) ( 20 1 0) 1 2 12 -1218. ) ( [27] B . Zha ng , D . C u i , M . L i u , H. Gong, Y . H u a ng, F . Ha n , C orn p o r ou s starc h : p r e parat i on, c haract eri z a ti on a nd adsorp t i o n p ro p e rty , In t . J. Bi ol . Ma c r om o l . 5 0 ( 1) ( 2 0 12 ) 2 5 0 -25 6 . ) ( [28] Y .Y . L i, X. H. Z hao , L . L . Wang, Y.J.L i u , W. W . W u, Z. Ch e n, Q .Z hang , J . H. Y a ng, P rep ara - t ion, ch ar a cter i za t ion an d in v i tr o e va l uati o n of melato n i n -l oaded por o us s tar c h f o r en ha nc e d b i oa v a i labili ty, C a rbo h ydr . P oly m . 2 02 ( 2 01 8 ) 1 25 -1 33. ) ( [29] S . N . Li, B. Zhang, C. Li, X. Fu, Q. H uang, P i ckering em u lsion gel s tab i lized by o ctenylsuccinate quinoa starch granule as lutein c a rrier: R ole of the gel network, Food Chemistry 305 ( 2020).125476. ) ( [30] S . H ed a yati, F. S hahid i , A . K oochek i , A . F arahnaky, M. M ajzoob i , P hys i c a l p roper t ies o f p re g el a t i ni z ed a nd g r an u la r c o ld water sw e l li ng m aize s t ar c hes at d i f f ere nt p H v al ues , In t. J . B i o l. M a cr o mol . 91 ( 20 1 6 ) 730 - 7 35 . ) ( [31] P . A . S. B r e sl in, G. K . B eau c h a m p, S uppre s si on of b i t t e r ne ss b y sod i um: v a ri at io n a mo n g b itte r t as t e s ti mu li , C he m . Sen se s 20 (6) ( 1 99 5)609 -62 3 . ) ( [32] R .X. L i u ,X . D . Zhang, L. Zhang, X . J . Ga o, H .L . L i , J.H. Shi, X.L. L i , B itt er n ess i nt e n s i t y p r e- d iction o f be rbe r in e hydrochl o rid e us in g an e le c t ro n ic t o ngu e a n d a G A -BP n eural n e twork, Ex pe r ime n t a l and T h e r a p e uti c M ed i c i n e 7 ( 6 ) (20 14 )1 69 6 - 1 702 . ) ( [33] D. J . G allant, B . Bou c he t , P.M. B a l dwin, Mic rosco p y of s tarch: e v i denc e o f a ne w level o f gr a nu le o rganiza ti on , C arbo h ydr . Pol y m . 3 2 ( 97 ) ( 1 9 9 7) 177 - 191 . ) ( [34] J . J a ne , S. A .S. Cr ai g , P. A . Sei b, R. C. Hos e n e y, C h a r ac t e r i zation o f g r a nul a r co l d w a t e r - s ol ub l e s t a r c h , S t arc h -Sta r ke 38 (8 )( 1986 ) 2 5 8 -2 63. ) ( [35] J . Chung, W. Kim , Ef fe c ts o f s om e p o ly m er i c a d di ti ves o n t h e cocr y sta l li za t i on o f c af- f eine, J. Crys t . Growth 3 3 5 ( 1 )(201 1 ) 1 06 - 109. ) [36] J. Singh, N. Singh, Studies on the morphological and rheological properties of gran-ular cold water soluble corn and potato starches, Food Hydrocoll. 17 (1)(2003)63-72. [37] S. Prabu, M. Swaminathan, K. Sivakumar, R. Rajamohan, Preparation, characteriza-tion and molecular modeling studies of the inclusion complex of caffeine with p-cyclodextrin, J.Mol. Struct. 1099 (5)(2015)616-624. ( [38] J . F. He,F. X. Guo, L. L, H . B. Chen,J. Chen, Y. Q, Cheng, Z. P. Zheng, Investigating the oxyresveratrol β -cyclodextrin and 2-hydroxypropyl-B-cyclodextrin complexes: the effects on o xyresveratrol s o lution, stability, and antibrowning ability on fresh grape j uice, LWT-Food Science a n d T e chnology 100 (2018) 263-270. ) ( [39] M . E f e ntakis, S. Poli t is, C omparativ e e v a l ua t ion of va r ious struc t ure s in p o lymer o nt r olled d ru g deli v er y sys t e m s a n d the eff e c t o f t h ei r mo rphol og y an d ch arac t eris- ti cs on drug release, E ur.P oly m . J. 4 2 (5 ) (20 0 6 ) 1 1 83- 1 1 9 5. ) ( [40] S . Sumathi, A. R. R ay, Release behaviour of drugs from Tamarind Seed Po l ysaccha- r ide tablets, Journal of Pharmacy & Pharmaceutical Sc i ences 5 (1) (2 0 02)12-18. ) ( [ 41] C . M a de ruel o, A . Z a r zuelo, J . M . L an a o , C ri t i c a l fac t o r s i n t h e r elea s e o f dru g s f rom sus t ain e d r e l e as e hydrophilic m a t r ic e s, Jo ur n al o f C o nt ro l le d R el e ase O f ficial Jo ur n al of th e C on tro lled R e le a s e S oc i e t y 1 5 4 (1 ) ( 20 11) 2 - 19 . ) [42]L. Roman, M.M. Martinez, C.M. Rosell, M. Gomez, Changes in physicochemical prop-erties and in vitro starch digestion of native and extruded maize flours subjected tobranching enzyme and maltogenic a-amylase treatment, Int. J. Biol. Macromol. 101(2017)326-333. [43] R. Cheng,X. Cheng, B. Xiang, S. Ou, Y. Li, Fabrication of modified porous starch for theremoval of vanadate from aqueous solutions, Desalination & Water Treatment 53(8)(2015)2100-2105. [44] J. Chen, Y. Wang, J. Liu, X. Xu, Preparation, characterization, physicochemical prop-erty and potential application of porous starch: a review, Int. J. Biol. Macromol.148 (2020)1169-1181. ( [ 45] C . D i m a , L . P a t ra s cu , A. C a ntaragiu, P . A l e xe, S. D i ma, Th e k in e t ic s of t he swelling p ro- cess and t h e r e lease m e cha n i sm s of Cori a ndr u m s at i v um L. e s s ential o i l f r om ch it o - s an/alg i n a t e / i n u li n m icr o c ap su l es , Foo d Chem . 19 5 (2016 ) 39 -4 8. ) ( [46] E . R . B al ma yor, E . T . B a ran, H . S. A ze v e d o , R .L. Reis , Inject ab le biode g radable s tar c h/ c hitosan de l ive r y s y s te m f or t he s ust a ined r e lease o f g e n t amicin to trea t bo n e i nfe c- t i o ns , Carbohydr . Pol ym. 8 7 (1 ) (2 0 12) 3 2 - 3 9 . ) ( [47] S . Argin, P. K o fin a s, Y .M . Lo, T h e ce ll re l e ase k i n etics an d th e s well i ng b ehav i or of p hy si c a l ly c r osslin k ed x a nt h a n-c h ito s an h yd r og els in s i m u l a te d gas tr oint e st i nal condi tio ns , F ood Hy d r o c o l l . 4 0 ( 2 014 ) 1 38- 1 4 4 . ) ( [48] M. A. Oliveira,M. S . R. B as t os, H. C. R. Magalhaes, D. S. G ar r uti, A. S. Egi t o, α, B-citr a lf rom Cymbopogon citratus on ce l lulosic f i lm: RELEASE po t ential an d quality of coalho cheese, LWT-Food Science and Technology 85 (2017 ) 246-251. ) [52] B. Conde-Petit, F. Escher, J. Nuessli, Structural features of starch-flavor complexation in food model systems, Trends Food Sci. Technol. 17 (5)(2006) 227-235. [53] P.A. Temussi, Sweet, bitter and umami receptors: a complex relationship, TrendsBiochem.Sci. 34 (6) (2009) 296-302. [54] M. Sugita, K. Yamamoto, C. Hirono, Y.Shiba, Functional dissection of sweet and bit-ter taste pathways, Journal of Oral Biosciences 55 (2)(2013)66-72. https://doi.org/j.ijbiomac.O Elsevier B.V. All rights reserved. 在本研究中,将咖啡因(CA)封装到食品级淀粉基质中,包括膨胀淀粉(SS)、多孔淀粉(PS)和v型淀粉(VS)。采用电子舌法、分子动力学(MD)模拟和CA的体外释放动力学研究了微胶囊的苦味及其抑制机制。所有CA微胶囊的苦味强度均低于对照。结果表明,淀粉与CA之间的弱相互作用使得ss -微胶囊的CA释放速率适中。PS-CA微胶囊的CA释放时间最长,可达40 min,而VS-CA微胶囊的CA完全释放时间为9 min。CA的释放速率与微胶囊的结构和复水性能有关。CA的苦味强度较低可能是由于CA释放速率较慢和微胶囊的抗侵蚀性。本研究结果对通过抑制生物碱类化合物的苦味来改善淀粉基微胶囊(口服靶向给药系统)有价值。单位:华南理工大学
确定






还剩8页未读,是否继续阅读?
产品配置单
北京盈盛恒泰科技有限责任公司为您提供《咖啡因中苦味评价和抑制机制检测方案(感官智能分析)》,该方案主要用于其他饮料中理化分析检测,参考标准--,《咖啡因中苦味评价和抑制机制检测方案(感官智能分析)》用到的仪器有电子舌、日本INSENT味觉分析系统(电子舌)
相关方案
更多
该厂商其他方案
更多











