
炸洋葱圈吸油量的检测
 收藏
收藏
方案详情
文
葵花油聚甘油硬脂酸酯油:洋葱圈油浴油炸替代油炸介质Sunflower Oil-Polyglycerol Stearate Oleogels Alternative Deep-fat Frying Media for Onion Rings
方案详情

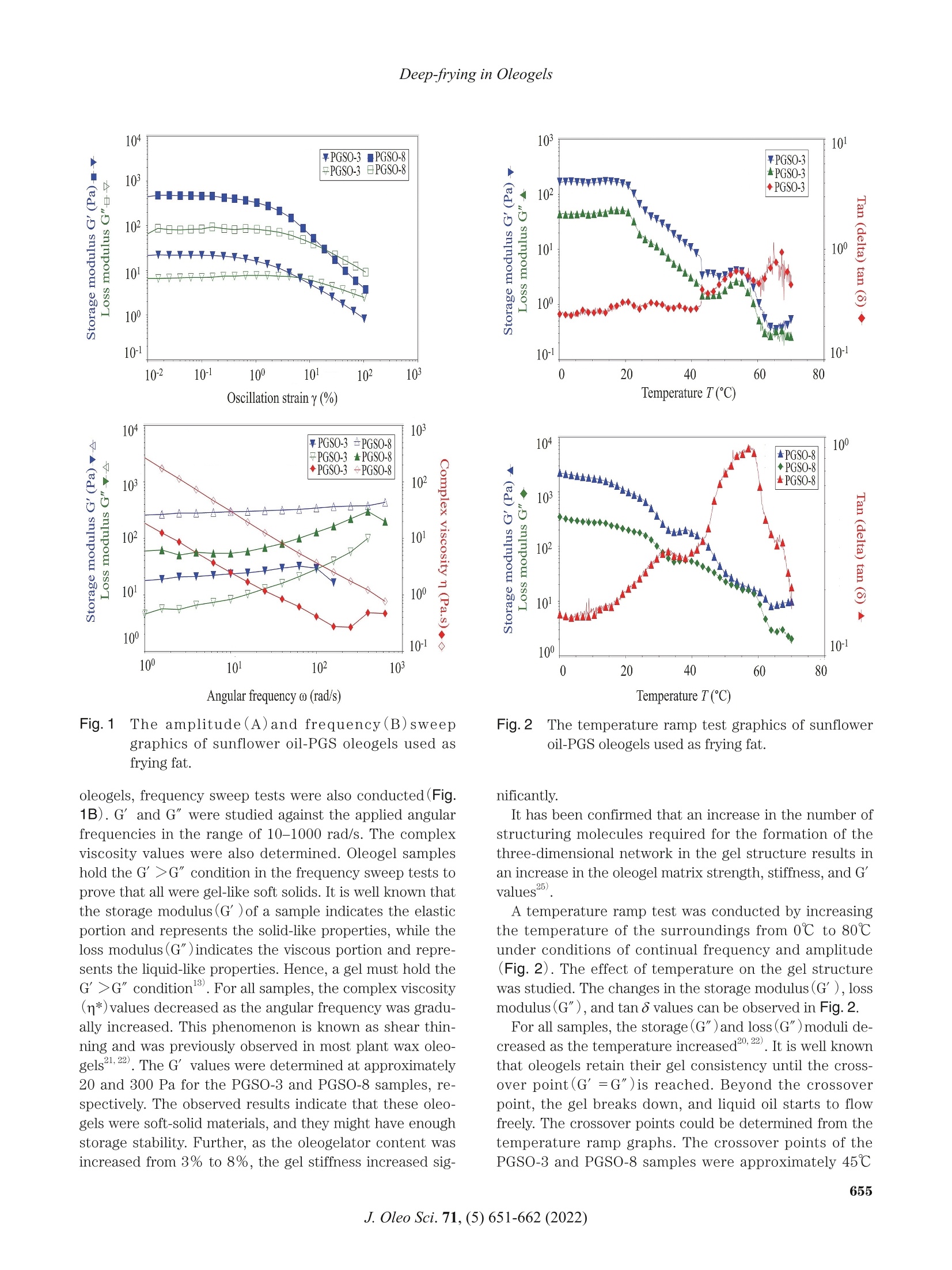
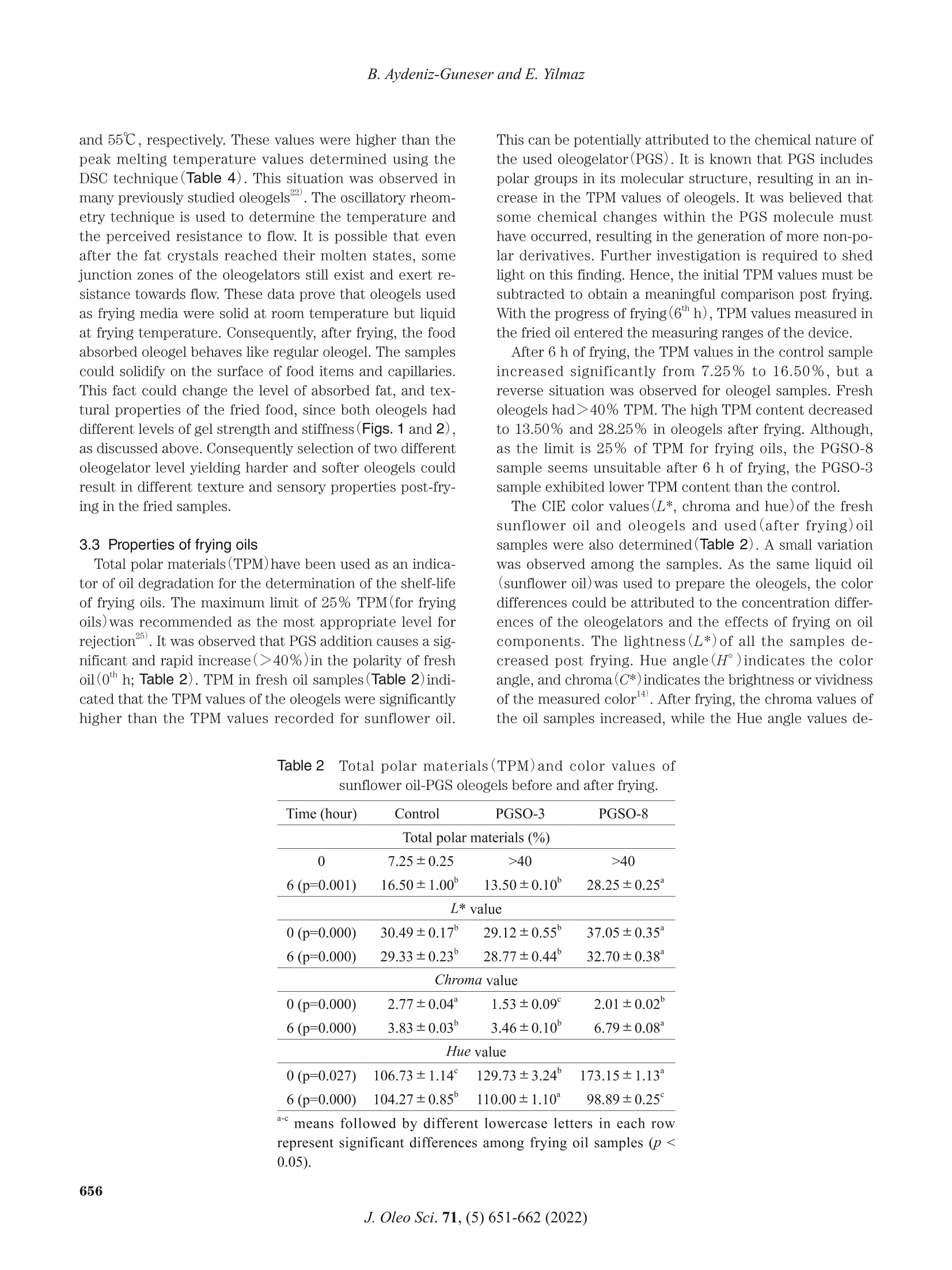
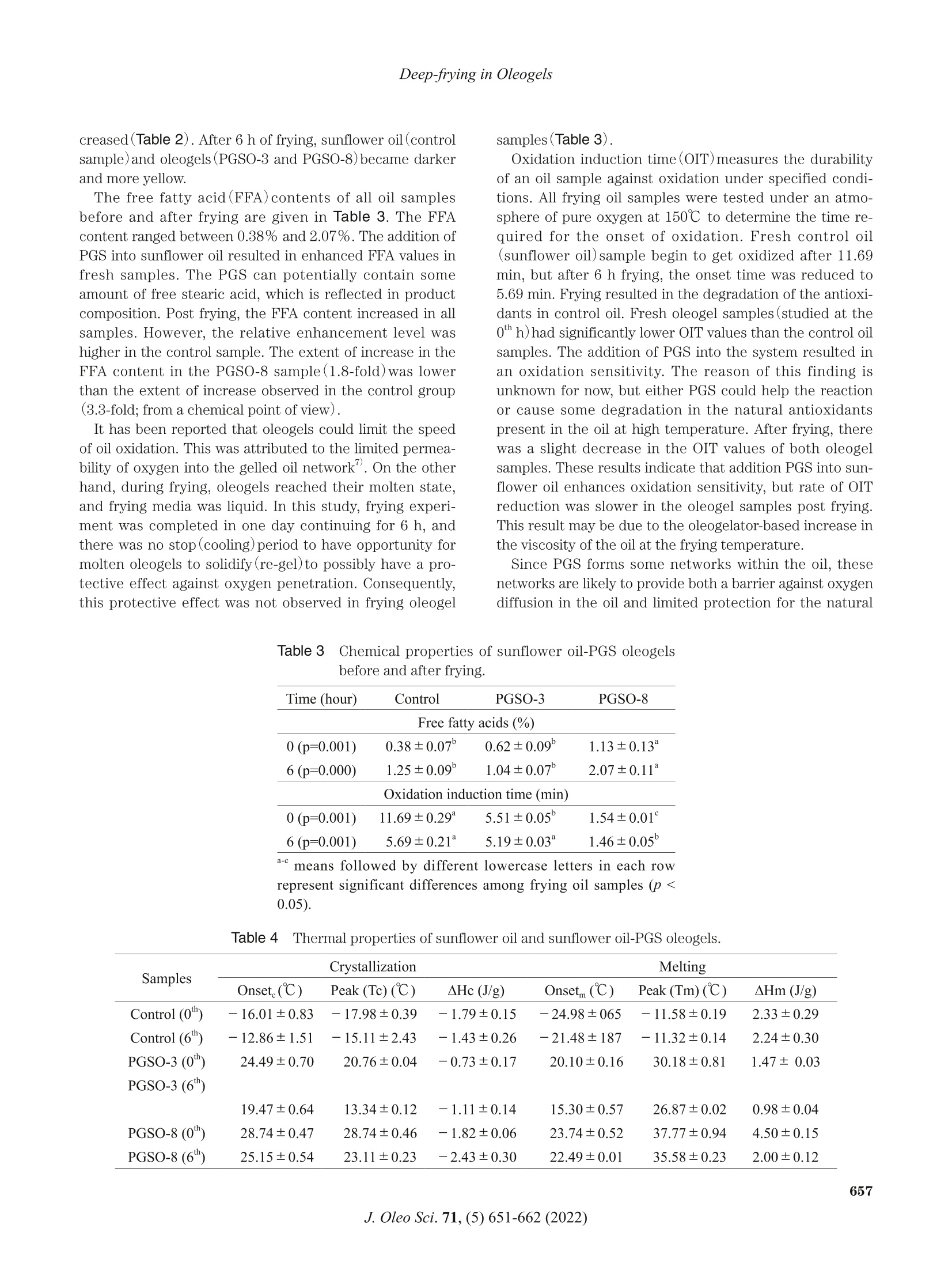
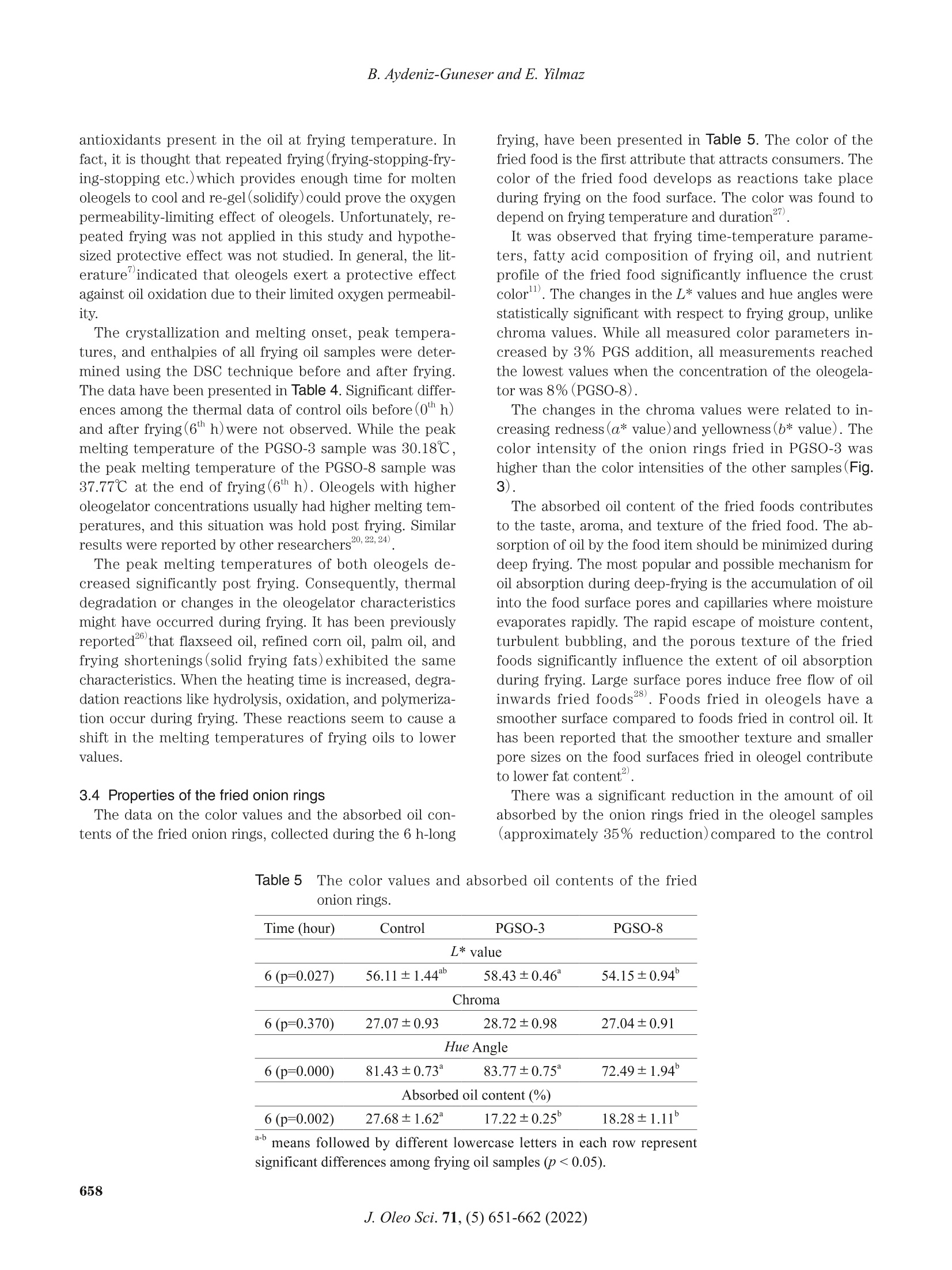
葵花油聚甘油硬脂酸酯油:洋葱圈油浴油炸替代油炸介质Sunflower Oil-Polyglycerol Stearate Oleogels Alternative Deep-fat Frying Media for Onion RingsJOURNAL OFOLEO SCIENCEJournal ofOleo ScienceCopyright C2022 by Japan Oil Chemists'Societydoi : 10.5650/jos.ess21446J. Oleo Sci. 71, (5)651-662 (2022) B. Aydeniz-Guneser and E. Yilmaz Sunflower Oil-Polyglycerol Stearate Oleogels:Alternative Deep-fat Frying Media for Onion Rings Buket Aydeniz-Guneser * and Emin Yilmaz² 'Usak University, Engineering Faculty, Department of Food Engineering, Usak, Turkiye ‘Canakkale Onsekiz Mart University, Engineering Faculty, Department of Food Engineering, Canakkale, Turkiye Abstract: Sunflower oil oleogels consisting of 3% and 8% polyglycerol stearate (PGSO) were studied as an alternative frying media for onion rings. The physicochemical properties and thermo-rheological characteristics of oleogels were provided. The sensorial quality and texture profiles of onion rings fried in oleogels were compared with those fried in contro l sunflower oil . Free fatty acids at the end of 6 h were determined, and decreasing trend was reported in order as PGSO-8, control (sunflower) oil, and PGSO-3.The oxidation induction t ime for PGSO-8 was significantly lower (1.46 min) than those of the control and PGSO-3 samples following frying. Compared to the control group, the onion ring samples fried in oleogels absorbed approximately 33-37% less oil. It was thought that this reduction would help consumers to less total calorie and weight gain from the fried products. There were no negative effects of oleogel usage on the L* value, aroma, crispness/texture, and overall acceptability scores for the onion ring samples fried in the oleogels. Key words: deep frying, oleogel, onion ring, rheology, sensory analysis 1 Introduction Deep-fat frying is characterized by a series of physic a l (reduction of water act i vity, color , crust format i on, etc.)and chemical changes (enzyme inact i vation, Maillard reac -tions, st a rch ge l atinization , protein denaturation , etc.)in the fried foods. During frying, frying oils and the fried products undergo several irreversible reactions such as degradation, polymerization, and oxidation. Some of these changes can potentially result in adverse h e alth effects". Hydrogenated fats or natural saturated fat (palm oil )a re widely used as frying media in the fast -food industry. It is important to develop frying fats that do not i ncrease the cholesterol content in the body and the risk of cardiovas -cular diseases. Researchers working in the field of food technology are trying to address these problems . Devel-oping innovative frying techniques to produce healthier products that have less oil content and calories but are tasty and sme l l good are still trending research areas. The controlling of oil absorpt i on could be achieved with the mo d ifications o f frying media and process parameters ° Oleogelation is an emerging strategy that is used to structure liquid oils into solid -like consistency to ch a nge the physical nature of the oi l without modifying the chemi -cal composition. Oleogelation processes are based on the formation of self-assembled three-dimensional networks .The networks are formed by a dding the oleogelators for the physical entrapment of the li q uid oil 4,5. Oleogelat i on i s a he a lthy technique as trans and saturated f a tty acids are not formed. Thus , oleoge l s can be potent ia lly used for for-mulat i ng healthy l ipi d -based food formulations due to their low saturated fatty acids content , preventive effects on oi l oxidation6) In oleoge l science, general l y 10%(w/w) of oleogelator addition is considered as the upper limit for practica l appli -cations (av a ilability and price of the oleogelator, sensory aspect s of the products etc .)4,6). Based on the gelling prop-ert i es, high or low molecular weight oleogelators such as hydrophobic ce l lulose derivatives, sorbitan esters, waxes,and monoglycerid e s are widely used for the development of oleogels with desired propert i es4". Polyglycerol stearate (PGS), an o l igomer of glycerol , is a product formed by the esteri f ication of stearic acid in the presence of a polyglyc-erol molecule . PGS esters, which are characterized by a n e utral odor and t a ste, are w a ter dispersible and oil soluble. They are used as additives dur i ng the preparation of v a rious food formulations as they exhibit surface-active and whipping properties8,9) Keskin U s lu and Yilmaz have defined the oleogel forma- *Correspondence to: Buket Aydeniz-Guneser , Usak University, Engineering Faculty, Department of Food Engineer i ng, Usak, Turkiye E-mail: buket .guneser@usak.edu.t r ORCID ID: https://orcid.org/0000-0003-2197-5504 ORCID ID: https://orcid.org/0000-0003-1527-5042(EY) Accepted January 29, 2022 (received for review December 28,2021) Journal of Oleo Science ISSN 1345-8957 print /ISSN 1347-3352 online tion abi li ty and oleogel properties of PGS as the oleogel a-tor . Enhanced oleogel features such as enhanced stability a n d softer texture were observed in the prepared oleogels. Oleogels l imit oi l oxidation, prevent oil l e akage from en-capsules , and form edible films. It is important to test them a s frying media for deep-fat fry i ng . Oleogels are solids at room temperature. However , they retain all fatty acids present in the st a rting l iquid oil . Hence, they can be poten-ti a lly used for frying. Oleogels can help to increase oil sta-bility during frying. Oleogel-fried foods absorb lower amounts of fat 211) The objective of this study was to evaluate sunflower oil oleogels prepared with polyglycerol stearate oleoge l ator at two concentrations (3 and 8% w/w )against control(sun-f l ower oil ) for onion r i ng f rying. A low (3%) and a high(8%)concentration of oleoge l ator were selected to ver i fy the in-fluence of d ifferent concentrations on the structuring of oleogels and their impa c t against frying. Changes in frying oils after frying and the qual i ty of the onion r i ngs fried i n three frying medias were studied using various analytica l techniques. 2 Experimental Procedures 2.1 Materials Polyglycerol stearate (PGS, Finamul PGE 2000, creamish powder, max acid value of 5.0 mg KOG/g oil, saponi f ication value of 130-150 mg KOH/g oi l and the melt i ng point of 52-59℃)was purchased from Fine Organics Inc . (Mumbai,India). Refined sunflower oil s (0.08% of free fatty acids, 1meq O /kg oi l of peroxide value, 2.0% stearic , 4.5% palmit-ic , 38.5% oleic , and 55.0% l inoleic acids)used as deep frying media were purchased from Bunge Veget a ble Oil Company (Tekirdag, Turkiye ). Frozen pre-fried onion rings (Nimet Frozen Products, Ist a nbul , Turkiye) were pur -chased from a local store . A l l other chemicals and reagents used in this study were of analytical grade and purchased from Merck(Darmsta d t , Germany ) and Sigma Chem. Co.(St . Louis, MO, USA). 2.2 Preparation of oleogels Polyglycerol stearate(PGS ) as the oleogelator was added to refined sunflower oil a t two different concentrations(3and 8%, w/w ). Calculated amounts of PGS and oi l were mixed before heating i n a water bath at 80℃ for a h a lf hour to completely dissolve the oleoge l ator. The homogenous mixture was cooled down to ambient temperature for gel formation. After 24-hour , the solidified oleogel was used for deep frying of the onion rings. 2.3 Oleogel formation time (OFT) OFT was defin e d a s the time required for an oleogel to solidify at a given temperature and the ver if ication of the oleogel formation was v i sually confirmed using the inverted tube method 2.5 g of the oleogel was weighed i nto a glass tube(16×100 mm)and melted in a water bath at 80℃ for 30 min. Melted oleogel was taken to the ambient tempera-ture and meant i me the digital stopwatch(Thomas Scient i f-ic, Traceable Digital Stopwatch, model 8788T56, Swedes-boro, NJ, USA, resolution of 0,01 s)was started. Once oleogel solidifie d , the t i me elapsed was recorded as oleogel formation t i me . Sample tubes were t i lted 180℃ to observe th e flow, and if there was no flow it was d e cided that the gel formation was completed. 2.4 Oil binding capacity (OBC) OBC is a parameter indicating the percent amounts of entrapped liquid oil with a known concentration of an oleo-gelator . Oleogel samples were melted (80℃ for 30 min),and 1 mL of each melted sample was transferred into tared Eppendorf tubes. The tubes were stored a t ambient tem-perature overnight for full gelation. Finally, the tubes were weighed and cent r i f uged at room temperature (10,000 rpm for 15 min)before drainage of the released oil on the paper cloth. The tubes were weighed again, and the OBC wa s cal-culated gravimetr i cally 12. 2.5 Rheological and thermal measurements of oleogels A DHR 2 rheometer (TA Instruments, USA)equipped with a Peltier system(±0.1℃)under the lower plate donated with cross -hatched parallel pl a te geometry(中=40mm, gap 0.9±0.1 mm) was used for characteriz i ng the oleoge l samples. A ll rheological parameters were measured at 10℃ as the samples were solid-like at the spec i fied tem-perature. An amplitude sweep test was first conducted in the strain range of 0.01-100% under a frequency of 1 Hz to determine the linear viscoelastic region(LVR)for each sample. The LVR is def i ned as the region where a plate a u for the storage (G’)and loss (G")moduli from the stress sweeps must be observed. By definition, rheologic a l mea -surements must be carried out within the LVR of each sample . Frequency sweep tests were completed a t the LVR strain ranges of 0.016-0.232%. The frequency was varied in the range of 0.1-100 Hz at 10℃. The storage modulus(G’)and loss modulus (G")values of the samples were determined. The effects of temperature on the struc-ture of oleogel were observed by conducting a temperature ramp test . The test was accomplished by heat i ng the samples from 0℃ to 80℃ at a heat i ng rate of 1℃/mi n (at 1Hz)within the l i mit of LVR. The thermal parameters (crystall i zation and melt i ng onset, peak temperatures and enthalpies ) of control oil and oleogels prepared were assessed with a Perkin-Elmer 4000Series Different i al Scanning Calor i meter (DSC ) (Groningen,The Netherlands). Thermal analyses were completed for fresh samples, as well as after 6 h of frying. The i nstrument was previously calibrated with Indium a nd Zinc. Around 10 mg of each sample was placed into the aluminum pans (volume capac i ty of 40 uL, part number: 02190041,Perkin-Elmer, Shelton, CT , USA )and sealed. The temperature program: heat samples from 20℃ to 100℃ at 10℃/min rate; cool samples to -30℃ at 10℃/min rate and keep 3min at that temperature for ful l crystallization; and f i nally heat samples again to 100℃ at 5℃/min rate. The Pyris 1Manager Software of the instrument was used for the cal-culations. 2.6 Onion rings frying A ll frying trials were performed using household fryers (Premier PDF 5615, Premier Electronic s Ltd., I stanbul,Turkiye ). Both the contro l(sunflower oil)and oleogels s t ructured with polyglycerol stearate were heated up to 180℃ and kept a total of 6 h at that temperature during frying experiments. Before start i ng frying, each fryer was f i lled with 1.5 L of fresh sunflower oils or the oleoge l s , and started to heat . When frying temperature (180℃)was reached, f rozen onion r ings (100 g)were thrown i nto the heated oil without waiting for them to thaw. Frozen onion rings (100 g)were fried for only 5 min in per hour (at a fry i ng cycle) unt il a total of 500 g fried onion rings were ob-t a ined in each fryer. Then fryers were held at 180℃ unt i l the next hour . Frying of totally 500 g frozen onion rings was performed in all frying cycles . The fryers were stopped a t the end of the 6 h frying. Oi l samples and the fried onion rings were collected a t the end of fryi n g and stored i n a re-frigerator unt il the analyses. All frying experiments were duplicated under the same conditions. 2.7 Quality assurance tests of frying oil samples Total Polar Material (TPM)levels i n frying oil samples were determined using a frying oil measuring tester (Testo 270, Testo Inc., Germany)at minimum 80℃ oi l tempera -ture. Before the measurement, the tester sensor was cali -brated with the reference oil(3.5% TPM)according to the instructions guide . Instrumental color values (L*, a* and b* values ) were re-corded for the oils and fried onion rings samples by a color-imeter (CR-400, Minolta Camera Co., Osaka, Japan). Four replications were performed on different surface locations of randomly selected fried onion ring samples . Also, hue angle (H )and chroma (C*) values were c a lculated from the L*, a*, and b* parameters according to Equations (1),and (2)14). All color measurements were reported as mean values . The free fatty acids (linoleic %) of oil samples were de-termined following AOCS Ca 5a-40 method . All chemical measurements were performe d in triplicate for each frying oi l sample. The oxidation induction t i me (OIT ) was also measured with a Perkin-Elmer 4000 Series Differentia l Scanning Cal-or i meter (DSC ) (Groningen , The Nether l ands)for both fresh and used oil samples . Around 10 mg of sample was weighed into standard aluminum pan(vo l ume capacity of 40 uL, part number : 02190041, Perkin-Elmer, Shelton, CT,USA)but not sealed , and analyzed against an empty pan by modi fi ed method of Tan et al.16). Un d er const a nt nitrogen (99.9% pure )flow(50 mL/min), the sample was f i rst heated from 20℃ to 150℃ by 10℃/min rate. After holding 1 min under nitrogen at 150℃, the gas switched to oxygen (99.9% pure )with 50 mL/min f low unt i l the line curved .The Pyris 1 Manager Software of the instrument was used for the calculations 2.8 Quality assurance tests of the f ried onion ring sam-ples The oil uptake of the fried onion rings was analyzed ac-cording to a solvent extrac t ion procedure . Before oil ex-traction from the fried samples, the samples were ground with a blend e r (Waring blender 8011EG , War i ng Commer-cial, USA )and the absorbed oi l contents were calculated from the percentage weight using petroleum ether as solvent with automatic Soxtherm apparatus (Gerhardt Sox -therm Manager SX, Germany) The textural profile analysis (TPA) of the fried onion rings was assessed with a texture analyzer (Brookfield CT3,Brookfield Engineering Labs Inc ., Massachusetts,USA)equipped with TA25/1000 probe (50.8 mm diameter which fully covering fried sample). Each sample was placed cen-trally on the platform and the test was replicated at least 4t i mes. All texture measurements were carried out within 10 minutes after the end of each frying time. TPA condi -tions followed as load cell : 4.5 kg; test type: TPA; test speed: 1.5 mm/s; target type: d e formation %; trigger loa d :5 g. I nstrumental parameters of food texture including h a rdness , resilience, adhesiveness, cohesiveness , stringi -ness length, springiness, gumminess, and chewiness were recorded. Color , aroma , crispness , t a ste/flavor and overall accept-ability of the frie d onion rings were evaluated with sensory analysis . A consumer test was performed by volunteer con-sumers selected from acad e micians and students. All vol -unteer consumers were provided an information sh e et and a consent form. In the consent form, the panelists were in-formed that the fried onion ring was edible and polyglycer-ol stearate (PGS) as the oleogelator was food grade. All par-ticipant s read and accepted the terms of the consent form before conduc ti ng the test. Sensory evaluations were con-ducted with 50 volunteers on al l fried onion rings obtained from twice replicated frying experiments . All fried onion rings in ea c h fryer were mixed at the end of the frying and were served to the consumers individually, coded with three -digit figures. The fr i ed samples were served to the panelists within 10 minutes after taking from the fryer at suitable consumption temperature (min 40℃). Warm water and expectorat i on cups were available for rinsing the mouth between evaluations. Sensory evaluations were carried out with a 9-point hedonic scale (1=extremely dis-liked, 9=extremely l iked)according to Me i lga a rd et al.The mean scores of the sensory a ttributes collected by the hedonic scale were c a lculate d . Considering that consumers wi ll prefer a golden yellow fried product rather than a very light yellow or extremely burnt /dark yellow fried product ,9-point was chosen for the golden ye l low color. 2.9 Statistical analysis Frying experiments in ea c h group were replicated twice.All physico -chemical analyses in frying oil and the fried onion ring samples were c a rrie d out a t least twice for e a ch replicate samples. Textural measurements of the fried onion r i ngs and the rheological curves of oleogel samples were the mean values of three measurements . The experi -mental data in tables were presented as mean ±standard error, excluding sensory properties data(mean±stan d ard deviation). Al l collecte d data were analyzed by variance a nalysis (ANOVA) with Tukey 's mult i ple compar i son test using Minitab ver. 16.1.1st a tistical package programs at 5% significance level . Sensory dat a were evaluated by non-parametric Krusk a l -Wallis a nd Dunn's test . 3 Results and Discussion 3.1 Gel formation times and oil binding capacities of the oleogels Oleogel formation times and oil binding capacities of polyglycerol stearate oleogel(PGSO) samples were deter-mined , and the findings have been presented in Table 1.The gelation process involves the structuring of a cont i nu-ous three-dimensional network that traps the mobile phase. Oleoge l formation time (OFT)depends on the balance between the soluble and insoluble fractions and can be defined as the minimum time required to ful l y solid-ify oleogel 2,20) As cle a rly observed in Table 1, there w e re distinct oleogel formation time differences between oleogels pre -pared under conditions of two different oleogelator addi - tion levels. It was observed that at ambient temperatures,the enhancement of the oleogelator level from 3% to 8%resulted in a remarkable decrease in the OFT from 21.30min to 12.17 min. This indicates that if the con c entration of the oleogelator increa s es, OFT generally decreases I t was stated that OFT would depend on the oleogelator ad-dition level , melting temperature and duration, cooling rate, a mbient temperature, and oi l type. Low OFT values h e l p i n s a ving t i me under real conditions10,20) Keskin Uslu a nd Yilmazreported that the PGS a ddition at 5% concentration to sunflower oils resulted the oleogel formation time of 4.3 min. Under these conditions, an oil binding capacity (OBC) of 99.9%, similar to our f i ndings,was recor d ed. OBC is an important criterion for the determination of oleogel functionality and can be def i ned as the percent a ge of liquid oil entrapment ability of oleogels. Higher OBC values are desirable for preparing oleogels. Under condi -tions of high OBC, less amounts of the oleogelators could be ut i lized to create stable oleogels 22,23) Slight differences among the samples for OBC values were also observed (Table 1). We observed that OBC increased as the oleo-gelator concentration increased from 3% to 8%. Al l o l eogel samples formulated w i th PGS exhibited OBC v a lues above 99%. The results agreed wel l with previously reported results for various oleogel a tors22,24). I t was concluded that polyglycerol stearate was a good gel -forming oleogelator . 3.2 Rheological properties of the oleogels Rheological characteristics of the fresh oleogels were de-termined to describe the flow behavior of frying media . An ampl i tu d e sweep test was conducted to determine the linear viscoelastic region(LVR)strain values of each sample. It i s well knownthat valid rheological measure-ments could only be conducted at the LVR of each sample.Th e LVR str a in values of PGSO-3 a nd PGSO-8 were deter -min e d as 0.25% and 0.26%, respectively (Fig. 1A). For both samples , within the region of the LVR strain, the storage modulus(G’)values were higher than those of the loss modulus (G")values. Under conditions of G’>G", the sample is considered to be in the gel state. The storage moduli of the PGSO-3 and PGSO-8 samples were 20.78 Pa and 423.05 Pa , respec ti vely, and the loss moduli were 7.40Pa and 86.69 Pa , respectively in the LVR region (Fig .1A ). To observe long-term stabil i ties and inner strengths of Table 1 Oleoge l formation times and oil binding capacities of sunflower oil -PGS oleogels. Samples Oleogel Formation Time (min) Oil Binding Capacity (%) PGSO-3 21.30±1.20° 99.40±0.06° PGSO-8 12.17±0.08° 99.92±0.02° a-bmeans followed by different lowercase letters in each column represent signi f icant di f ferences (p <0.05). 104 ¥PGSO -3 ■PGSO-8 F PGSO-3 B PGSO-8 103 HHHHH 中 HHHH o o o 1022808800000008g 10 VV VVVVVVVVV 100 10 102 10 100 10 102 10 Osc i l l at i o n s t ra in y (%) A ngul a r f r e quenc y o (ra d /s) Fig.1 The amplitude (A )and frequency(B)sweep graphics of sunflower oil-PGS oleogels used as frying fat . oleogels, frequency sweep tests were also conducted(Fig.1B). G’ and G" were studied against the appl i ed a ngul a r frequencies in the range of 10-1000 rad/s. The complex viscosity values were also dete r mined. Oleogel samples hold the G’>G" condition in the frequency sweep tests to prove that all were gel-l i ke soft solids. It is wel l known that the storage modulus (G ’) of a sample indicates the elast i c portion and represents the solid-like properties, while the loss modulus (G")indicates the viscous por t ion and repre-sents the liquid-like properties. Hence, a gel must hold the G’>G"condit i on 3. For all samples, the complex v i scosity (n*) values decreased a s the angular frequency was gradu-ally increased. This phenomenon i s known as shear thin-ning and was previously observed in most plant wax oleo-ge ls 21,22). The G’ values were determined at approximate l y 20 and 300 Pa for the PGSO-3 and PGSO-8 samples , re-spectively . Th e observed results indicate that these oleo -gels were soft -solid materials , and they might have enough storage stabil i ty . Further , as the oleog e lator content was increased from 3% to 8%, the gel st i f fness i ncreased sig- The temperature ramp test graphics of sunflower oil-PGS oleogels used as fry i ng fat . ni f icantly. It has been confirmed that an increase in the number of structuring molecules required for the formation of the three-dimensional network in the gel stru c ture results i n an increase in the oleoge l matrix strength , st i ffness, and G values25) A temperature ramp test was conducte d by increasing the temperature of the surroundings from 0℃ to 80℃un d er conditions of cont i nual frequency and ampl i tude (Fig.2). The effect of temperature on the gel structure was studied. The changes i n the storage modu l us (G’), loss modulus (G"), an d t an 8 values can be observed in Fig. 2. For al l samples, the storage(G ")and loss (G")moduli de-creased as the temperature increased20,22). I t i s well known that oleogels retain the i r gel cons i stency until the cross-over point(G’=G")is reached. Beyond the crossover point, the gel breaks down, and liquid oil starts to flow freely. The crossover points could be d etermined from the temperature ramp graphs. The crossover points of the PGSO-3 and PGSO-8 samples were approximately 45℃ and 55℃, respectively. These values were higher th a n the peak mel t ing temperature values d etermined using the DSC technique(Table 4). This situation was observed in many previous l y studied o l eogels . The oscillatory rheom-etry technique is used to d e termine the temperature and the perceived resistance to flow. It is possible that even after the fat crystals reached their molten states, some junct i on zones of the oleogelators st i l l exist a nd exert re-sistance towards flow. These data prove that oleogels used as fry i ng media were solid at room temperature but l iquid a t frying temperature. Consequently, after frying, the food absorbed oleogel behaves l ike regular oleogel . The samples could solidify on the surface of food items and capillaries.This fact could change the level of absorbed fat, and tex-tural properties of the fried food, since both oleogels had different levels of gel strength and stiffness (Figs. 1 and 2),as discussed above. Consequently selection of two different oleogelator level y i elding harder and softer oleogels could resu l t in different texture and sensory properties post -fry-ing in the f ried samples. 3.3 Properties of f rying oils Total polar materials(TPM) have been used as an indica-tor of oi l degradation for the determination of the shelf-life of fry i ng oi ls . The maximum l i mi t of 25% TPM (for frying oils) was recommended as the most a ppropr i ate level for rejection25. I t was observed that PGS a ddition causes a sig-nificant and rapid i ncrease (>40%)in the pol a rity of fresh oil (O"h h ; Table 2). TPM in fresh oil samples (Table 2)i n di -cated that the TPM values of the oleogels were significantly higher than the TPM values recorded for sunflower oil . This can be potent i ally attributed to the chemical nature of the used oleogelator (PGS). It is known th a t PGS i nc l udes polar groups in its molecular structure, resulting in an in-crease in the TPM values of oleogels. It was believed that some chemical changes within the PGS molecule must have occurred, resulting in the generation of more non-po-lar derivatives. Further investigation is required to shed light on this finding. Hence, the initial TPM values must be subtracted to obtain a meaningful comparison post frying.With the progress of f rying (6*h),TPM values measured i n the fried oil entered the measuring ranges of the device. After 6 h of frying, the TPM values in the control sampl e increased significantly from 7.25% to 16.50%, but a reverse situation was observed for oleogel samples. Fresh oleogels had ≥40% TPM. The high TPM content decreased to 13.50% and 28.25% in oleogels after frying. Although,as the limit is 25% of TPM for frying oils, t he PGSO-8s a mple seems unsuitable after 6 h of fry i ng, the PGSO-3sample exhibited lower TPM content than the control . The CIE color values (L*, chroma and hue ) of the fresh sunflower oil and oleogels and used (after frying ) oil samples were also determined(Table 2). A small variation was observed among the samples. As the same liquid oil (sunflower oil ) was used to prepare the oleogels, the color differences could be attributed to the concentration differ -ences of the oleogelators and the effects of frying on oil components . The lightness(L*) of al l the samples de -creased post frying. Hue angle (H°)i ndicates the color angle, and chroma (C *)indicates the brightness or vividness of the measured color4. A fter f rying, the chroma values of th e oi l samples increased, while the Hue angle values de- Table 2Total polar materials(TPM)and color values of sunflower oi l -PGS oleogels before and after fry i ng. Time (hour) Control PGSO-3 PGSO-8 Total polar materials (%) 0 7.25±0.25 >40 >40 6(p=0.001) 16.50±1.00° 13.50±0.10° 28.25±0.25° L* value 0 (p=0.000) 30.49±0.17° 29.12±0.55° 37.05±0.35° 6(p=0.000) 29.33±0.23° 28.77±0.44° 32.70±0.38° Chroma value 0 (p=0.000) 2.77±0.04* 1.53±0.09° 2.01±0.02° 6(p=0.000) 3.83±0.03° 3.46±0.10° 6.79±0.08° Hue value 0 (p=0.027) 106.73±1.14 129.73±3.24° 173.15±1.13° 6(p=0.000) 104.27±0.85° 110.00±1.10° 98.89±0.25° a -C means followed by different lowercase letters in each row represent significant differences among frying oi l samples (p<0.05). creased (Table 2). After 6 h of frying, sunflower oil (control sample )and oleogels (PGSO-3 and PGSO-8)bec a me darker and more yellow. The free fatty acid (FFA)content s of all oil samples before and after frying are given in Table 3. The FFA content ranged between 0.38% and 2.07%. The addit i on of PGS into sunflower oi l resulted in enhanced FFA v a lues in fresh samples. The PGS can potential l y contain some amount of free stearic a cid, which is reflected in product composition. Post frying, the FFA content increased i n all samples. However, the relative enhancement level was higher in the control sample. The extent of increa s e in the FFA content in the PGSO-8 sample (1.8-fold) was lower than the extent of incre a se observed in the control group (3.3-fold; from a chemica l point of view ). It ha s been reported that oleogels could limit the speed of oi l oxid a tion. This was attributed to the limited permea -bility of oxygen into the gelled oil network . On the other hand, during frying, oleoge l s reached their molten state,and frying media was liquid. In this study, frying experi -ment was completed in one day continuing for 6 h , and there was no stop(cooling)period to have opportunity for molten oleoge l s to solidify (re-ge l)to possibly have a pro-tective effect against oxygen penetration. Consequently,this protective effect was not observed in frying oleogel samples (Table 3). Oxidation indu c tion t i me (OIT)measures the durabi l ity of an oi l sample against oxidation under specified condi-tions. All frying oil samples were tested under an atmo-sphere of pure oxygen at 150℃ to determine the t i me re-quired for the onset of oxidation . Fresh control oil (sunflower oil ) sample begin to get oxidized after 11.69min, but after 6 h frying, the onset t i me was reduced to 5.69 min. Frying resulted in the degrada t ion of the a ntioxi-dants in control oil . Fresh oleogel samples (studied at the o" h) had significantly lower OIT values than the control oil sample s. The addit i on of PGS into the system resulted in an oxidation sensitivity. Th e reason of this finding is unknown for now, but either PGS could help the reaction or cause some degradation in the natura l antioxidants present in the oil at high temperature. After frying, there was a slight decrease in the OIT values of both oleogel samples. These results indic a te that addition PGS into sun-flower oil enh a nces oxidation sensitivity, but rate of OIT reduction was slower in the oleogel samples post fryi n g.This result may be due to the oleogelator-based increase i n the viscosity of the oil at the frying temperature. Since PGS forms some networks within the oil , these networks are li kely to provide both a barrier against oxygen diffusion in the oil and limited protection for the natural Table 3Chemical properties of sunflower oi l -PGS oleogels before and after frying. Time (hour) Control PGSO-3 PGSO-8 Free fatty acids (%) 0 (p=0.001) 0.38±0.07° 0.62±0.09° 1.13±0.13° 6(p=0.000) 1.25±0.09° 1.04±0.07° 2.07±0.11* Oxidation induction time (min) 0 (p=0.001) 11.69±0.29° 5.51±0.05° 1.54±0.01° 6(p=0.001) 5.69±0.21° 5.19±0.03° 1.46±0.05° a-C means followed by different lowercase letters in each row represent significant differences among frying oi l samples (p <0.05). Table 4Thermal properties of sunflower oi l and sunflower oil-PGS oleogels. Samples Crystallization Melting Onset(℃) Peak (Tc) (℃) AHc (J/g) Onsetm(℃) Peak (Tm) (℃) AHm (J/g) Control (0") -16.01±0.83 -17.98±0.39 -1.79±0.15 -24.98±065 -11.58±0.19 2.33±0.29 Control (6*) -12.86±1.51 -15.11±2.43 -1.43±0.26 -21.48±187 -11.32±0.14 2.24±0.30 PGSO-3 (0") 24.49±0.70 20.76±0.04 -0.73±0.17 20.10±0.16 30.18±0.81 1.47±0.03 PGSO-3 (6") 19.47±0.64 13.34±0.12 -1.11±0.14 15.30±0.57 26.87±0.02 0.98±0.04 PGSO-8 (0") 28.74±0.47 28.74±0.46 -1.82±0.06 23.74±0.52 37.77±0.94 4.50±0.15 PGSO-8(6") 25.15±0.54 23.11±0.23 -2.43±0.30 22.49±0.01 35.58±0.23 2.00±0.12 antioxidants present in the oi l at frying temperature. In fact , it is th o ught that repeated f rying (frying -stopping-fry-ing-stopping etc .)which provi d es enough t i me for molten oleogels to cool and re-gel(solidify ) could prove the oxygen permeability-l imiting effect of oleogels. Unfortunately , re-peated frying was not applied in this study and hypothe-sized protective effect w as not studied. In general , the l i t-erature i ndicated that oleogels exert a protective effect against oil oxid a tion d ue to their l imited oxygen permeabil-ity . The crystallization and melting onset , peak tempera -tures, and enthalpies of al l frying oil samp l es were deter-mined using the DSC technique before and after frying.The d a ta have been presented in Table 4. Significant differ-ences among the thermal data of contro l oils before (0" h)a n d after frying (6*h) were not observed. While the peak melting temperature of the PGSO-3 sample was 30.18℃,the peak melt i ng temperature of the PGSO-8 sample was 37.77℃ at the end of frying (6"h). Oleogels with higher oleogelator concentrations usually had higher melting tem-peratures , and this s i tuation was hold post frying. Similar results were reported by othe r researchers20,22,24) The peak melting temperatures of both oleogels d e -creased significantly post frying. Consequent l y , thermal degradation or changes in the oleogel a tor character i stics might have occurred dur i ng fry i ng. It has been previously reported t hat f laxseed oil, refined corn oil, palm oil , and frying shortenings (solid frying fats )exhibited the same characteristics. When the heati n g time is i ncreased , degra -dation reactions like hydrolysis , oxidation, and polymeriza-tion occur during frying. These reactions seem to cause a shift in the melting temperatures of frying oils to lower values . 3.4 Properties of the fried onion rings The data on the color v a lues and the absorbed oil con-tents of the fried onion rings, collected during the 6 h-long fry i ng, have been presented in Table 5. The color of the fried food is the first attribute that attracts consumers. The color of the fried food develops as reactions take place during frying on the food surface. The color was found to depend on fry i ng temperature and duration It was observed that frying time-temperature parame-ters, fatty a cid composition of frying oil, and nutrient profile of the fried food significantly influence the crust color. The changes in the L * values and hue a ngles were statistically significant with respect to frying group, unlike chroma values. While all measured color parameters in-creased by 3% PGS addition, all measurements reached the lowest values when the concentration of the oleogela-tor w a s 8% (PGSO-8). The changes in the chroma values were related to in-creasing redness (a * v a lue )and yellowness (b* value). The color intensity of the onion rings fried in PGSO-3 was higher than the color intensities of the oth e r samples (Fig.3). The absorbed oil content of the fried foods contributes to the taste, aroma, and texture of the fried food. The ab-sorption of oil by the food i tem should be minimized during deep frying. The most popular and possible mechanism for oi l absorption during d eep-frying is the a ccumulation of oil into the food surface pores and capillaries where moisture evapor a tes rapidly. Th e rapid escape of moisture content ,turbulent bubbling, and the porous texture of the fried foods signi f icantly influence the extent of oi l absorption during frying. Large surface pores induce free flow of oi l inwards fried foods28). Foods fried in oleogels have a smoother surface compared to foods fried in control oi l . It has been reported that the smoother texture and smaller pore sizes on the food surfaces fried in oleogel contribute to lower fat content. There was a significant reduction i n the amount of oil absorbed by the onion rings fried in the oleogel samples (approximately 35% reduction ) compared to the control Table 5 The color values and absorbed oi l contents of the fried onion rings. Time (hour) Control PGSO-3 PGSO-8 L* value 6(p=0.027) 56.11±1.44 58.43±0.46° 54.15±0.94 Chroma 6(p=0.370) 27.07±0.93 28.72±0.98 27.04±0.91 Hue Angle 6(p=0.000) 81.43±0.73° 83.77±0.75° 72.49±1.94 Absorbed oil content (%) 6 (p=0.002) 27.68±1.62° 17.22±0.25° 18.28±1.11° a-b means followed by different lowercase letters in each row represent significant di f ferences among f ry i ng oi l samples (p <0.05). sample. A negative rel a tionship was observed between the oleogelator addition and the absorbed oi l content . Even at the molten state , the oleogels could have higher viscosities than the l iquid control oil to not easily penetrate i nto the capi l laries to reduce oil absorption. Although not proved in this study, another reason could be the reduced aff i nity of the oleogels to adhere to the surf a ce of the onion rings.More investigations to find out the mechanism of less oil absorpt i on are needed. Overall , frying onions in oleogel media could help to reduce calorie intake for the interested consumers. L im et al."evaluated the possibilities of using soybean oil oleogel as a frying media . The effects on the quality param-eters of the fried noodles were also evaluated. It was note-worthy that the fried noodles in soybean oi l oleogels ab-sorbed 16% less oil than the palm and soybean oils selected as the control groups. Based on these findings , the use of oleogels as deep-fry i ng me d ia is recommended to redu c e the c a lorie and oi l contents of the fried foods. In a recent study , rapeseed oil oleogels structured with sunflower wax(5%)as oleogelator were used for frying potato chips. It was reported that the oi l uptake exhibited by potato chips fried in rapeseed oi l -based oleogels was lowe r (11.3%)than the oil uptake exhibited by the control sample f r ied i n refined rapeseed oil(12.4%). Our f indings concur with the literature . The instrumental texture prof i le analysis of the fried onion rings was conducted(Table 6). Hardness/fracturabi li ty indicates the required force to compress the molars of the samples . The values indicated significant differences among the samples. Onion rings fried in oleogels had higher hardness values (3656 g and 3928 g)than the control sample (2569 g). It i s possible that the molten oleogels absorbed by and adhered to the onion rings were solidi f ied post fry i ng. As discussed previously i n oleogel rheology (Figs. 2 and 3)section, hardness of oleo-gels in c rease as oleogel a tor level enhances. Hen c e, higher h a rdness values were recorded for the onion ring samples fried in the oleogels. Resi l ience indicates the flexibility of the samples. Signi fi -cant differences were not observed among the resilience values of the samples . A d hesiveness is a measure of how much a sample sticks to a surface. Simi l ar adhesiveness pa-rameters were recorded for the fried samples. Textural co-hesiveness is defined as the strength of the internal bonds constitut i ng the body of the food product . We observed that the cohesiveness of the fried onion ring samples nega-tively correl a ted with the volume percentage of the oleo-gelator. This might be due to less oi l absorption into the structure of the fried products in oleogels. Springiness is d e fin e d as the rate at which the sample turns back to i ts initial dimension afte r the applied force is removed . The springiness length or stretchabi l ity is the distance a food item is extended by as it is moved away from teeth or pal a te . As shown in Table 6, there were no signi f ic a nt Fig .33Onion rings f ried in sunflower oi l a nd sunflower oil -PGS oleogels (a. control , b. PGSO-3,c. PGSO-8). Table 6 Texture profile analysis result s ofthe fried onion ring samples (6*h ). Textural parameters Control PGSO-3 PGSO-8 Hardness/Fracturability (g,p=0.008) 2569±256° 3656±329° 3928±274° Resilience(p=0.561) 0.15±0.01 0.16±0.02 0.14±0.02 Adhesiveness (mJ,p=0.491) 0.06±0.04 0.02±0.001 0.03±0.001 Cohesiveness (p=0.349) 0.75±0.39 0.38±0.03 0.32±0.02 Stringiness length (mm,p=0.029) 2.29±0.27° 2.47±0.31aD 4.13±0.75° Springiness (mm,p=0.613) 3.59±0.11 3.44±0.12 3.52±0.09 Gumminess(g,p=0.069) 1433±119 942±91 1184±192 Chewiness (mJ,p=0.073) 51.05±5.56 31.81±3.26 41.51±7.21 1-b means followed by different lowercase l etters in each row represent significant differences among f rying oi l samples (p<0.05). di f ferences i n the springiness values of the fried samples.The springiness length increased steadily as the PGS content in c reased from 3 to 8%. The gumminess scores and the energy values required for chewing fried onion rings in oleogels are significantly lower than the gumminess scores and the energy values re-corded for the control sample. It is thought that these properties are desirable for the consumption of the fried pr od ucts . According to Yang et al .2, there is a relationship between the oi l binding capacity, melting temperature, and textural firmness values of oleogels. In other words, the higher melting points and oi l binding capacity values can result in a f i rm oleogel structure . In accordance with the findings of Yang et al .32, the products f ried in PGSO-8oleogel w i th higher oil binding c a pacity (Table 1)and melt i ng temperature (Table 2)exhibited higher hardness values . Some chemical reactions such as protein denaturation,starch gelatinization , Mai l lard reaction, and others may promote changes in the sensory attributes of the fried food items depending on frying conditions . Food products that do not meet the sensory expectations of the consumer will not survive in the global market . Hence, a comparison of the sensory properties of the onion rings would help to ev a luate the suit a bility of oleogels as frying media . The sensory properties of the fr i ed onion rings were evaluated and the d a ta have been summarized in Table 7. The color scores indicated that the most preferred onion rings were i n the control group (Fig. 3) as they were not too dark or l ight yellow i n color . Onion rings fried in oleogel-based frying media were less favored in terms of color.However, this difference was not stat i stically significant.The aroma v a lue of the onion rings fried in PGSO-3 was 8.05. The value was significantly higher than th e v a lues of the other two s a mples. The crispiness is a very important property of the fried food items. Higher crispiness values are usua l ly preferred by the consumers and influ e nce purchasing decisions. The crispiness values of the onion rings fried in the oleogels were higher than the crispiness values of the control sample. This could be attributed to the solidification of the oleogel post frying (when the rings cooled down to room temperature ). The taste scores of the onion r ings f r ied i n PGSO-3 were similar to the score of the control sample.The score of the onion ring fried in PGSO-8 was lower than the score of the control sample. The overall acceptability of the samples fried in oleogels was higher than the accept-abi l ity of the samples fried in control oil . These findings reveal that the PGSO-3 oleogels could be promising and suitable alternatives to conventional deep frying medi a for frying onion rings According to Matthaus et al., potato sticks fried in sunflower wax-based(5%)oleogel s appear less oily. In ad-dition, the researchers found no significant di f ference in the t a ste scores of French fries fried i n different oleogels.These results agree wel l with the results reported herein. 4 Conclusion A frying media influences the aroma and taste of the fried food i tems . Frying media significantly influences the quality limit of the fried product . The objective of this study was to test the suitability of sunflower oi l oleogels as frying media . The comparison wa s made against the liquid stock refined sunflower o i l . The results revealed that oleo-gels could potentially be used as a deep-fat frying media for household, commercial, and industrial applications. The use of oleogel a s a frying medium has helped a chieve lower absorbed fat content, higher overa l l sensory acceptability scores, and a des i rable textural prof i le . The replacement of vegetable frying oils with stable solid-l i ke oleogels will offer consumers the opportunity to consume low-calorie and nu-tritionally enhanced fried foods. Overall, oleogels c a n po-tentially replace liquid vegetable oils used to fry food items to achieve reduced oil uptake and improve d sensory quality. Further , long-term repeated frying experiments with various oleogels were suggested a s research needs. Table 7Sensory properties o f the fried onion ring samples (6*). Fried onion Sensory Attributes Color Aroma Crispness Taste Overall acceptability samples (p=0.187) (p=0.081) (p=0.235) (p=0.003) (p=0.674) Control 8.30±0.65 7.95±0.60 7.95±0.83 8.50±0.51° 8.15±0.66 PGSO-3 8.00±0.74 8.05±0.39 8.30±0.66 8.35±0.49° 8.40±0.50 PGSO-8 7.90±0.72 7.70±0.47 8.30±0.73 7.75±0.97° 8.30±0.44 a-b means followed by different lowercase letters i n each column represent significant differences among frying oil samples (p<0.05). Informed Consent Consent form was obtained from al l individual panellist included in the sensory analysis i n the study. Author Contributions Buket Aydeniz Guneser conducted frying process,meth-odology , formal analyses and writing and editing of the manuscr i pt as a corresponding author. Emin Yilmaz designed the experiments and discussed the results and also guided the writ i ng of the manuscript and improved the qual i ty of this manuscript . References 1) Hosseini , H.; Ghorbani, M.; Meshginfar , N.; Mahoonak,A .S. A review on frying: procedure, fat , deter i oration progress and health hazards. J. A m. Oil Chem. Soc .93, 445-466(2016). 2)I L i m, J .; Jeong, S.; Lee, S. Evaluation of soybean oil-carnauba w a x oleogels as an alternative to high satu-rated fat frying me d ia for instant fried noodles. LWT-Food Sci . Technol . 84, 788-794(2017). 3))Y u , X.; Li , L.; Xue , J ., Wang, J.; Song, G. et al. Effect of air -frying conditions on the quality attributes and lipi -domic characteristics of sur i mi during processing. In-nov . Food Sc i. Emerg. Technol . 60,02305(2020). 4)Martins, A .J.; Vicente, A .A.; Cunha , R.L.; Cerqueira ,M.A. Edible oleogels: An opportunity for fat replace-ment in foods. Food Funct. 9,758-773(2018). 5))I Puscas, A.; Muresan, V .; Socaciu, C.; Muste, S . Oleo-gels in food: A review of current and potential applica -tions. Foods 9, 70(2020). 6)N Marangoni , A.G.; van Duynhoven, J.P.M.; Acevedo,N.C.; Nicholson, R.A.; Patel , A.R. Advances in our un-derstanding of the structure and functionality of edi -ble fats and fat mimetics. Sof t Matter 16,289-306(2020). 7)I Hwang , H.S. A cr i tical review on structures, health ef-fects, oxidative stabi l ity, and sensory properties of oleogels. Biocatal. Agric. Biotechnol. 26, 101657(2020). 8))A nonymous _Internet_Emulsifiers - Finamu l PGE 2000-Polyglycerol Stearate. Retrieved from https://www.i n-diamart .com/proddetail/emulsifiers-finamul-pge-2000-polyglycerol-stearate-14300128191.html Ac-cessed 4 Apr i l 2021. 9)1Naka j ima , N .; Hamada, M. Synthesis of structurally well -defined polyglycelols and their fatty acid esters as new oi l gelators. J . S y nth. Org. Chem. Jpn. 70, 742-753(2012). 10) Keskin Us l u , E.; Yilmaz, E. Preparation and character- ization of glycerol monostearate and polyglycerol stea -rate oleogels with selected amphiphiles. Food Struct .28,100192(2021). 11)N Matthaus, B.; Schubert , M.; Er l enbusch, N .; Smit , I .;Weber , L.; Nikolay , S. Oleogels as alternatives for fry-ing fats and oils. Inform 7, 22-26(2020). 12)Y i lmaz, E.; Ogutcu, M.; Guneser, O. Infuence of stor-age on physico -chemical and vol a tile features of en-riched and aromatized wax organogels . J. Am. Oil Chem. Soc . 92,1429-1443(2015). 13))1Mezger, T.G. The Rheology Handbook. For users of ro-t a t i ona l and osc i llatory rheometers(4th ed.)A u s tria:Anton Paar GmbH(2014). 14))1Mendez-Cid,F.J.;Lorenzo,J.M.; Martinez, S.; Carballo,J . Oxidation of edible animal fats. Comparison of the performance of different quanti f ication methods and of a proposed new semi -objective colo u r scale-based method . Food Chem. 217, 743-749(2017). 15))/AOCS. Offici a l method Ca 5a -40: Free fatty acids. Off i-cial method s an d recommended practices of the American Oil Chemi s ts ’ Society (4t h ed.). A merican Oil Chemists Society Press, Champaign, Il, USA (1997). 16))Tan, C.; Man, Y .C.; Se l amat , J.; Yusoff, M. Comparative studies of oxidative stability of edible oils by differen-t i al scanning calor i metry and oxidative st a bility index methods . Food Chem. 76,385-389(2002). 17)AOAC . Official methods of analysis of Association of Offic i a l Analytical Chemists (AOAC). AOAC Interna -tiona l International, Maryland , USA(2005). 18))1Meilgaard, M.C.; Carr , B.T.; Civille, G.V . Sensory evalu-ation techniques , 4th edn. Boca Raton: CRC Press,USA, pp. 307-343(2015). 19)1Minitab. Minit a b, version 16.1.0. Ic . Minit a b Inc ., State College, Pennsylvania, USA(2010). 20))(Co, E.D.; Marangoni, A .G . Organogels: An alternative edible oi l -structuring method. J. A m. Oil Chem. Soc.89,749-780(2012). 21)I Patel, A.R. Oi l structuring: concepts, overview and fu-ture perspect i ves. in Edible Oil Structuring: Con-cept, Methods and Applications (Patel , A.R. ed.).Roya l Soc . Chem., Chambr i dge, UK, pp.3-24(2018) 22)N Mattice, K.D.; Marangoni, A.G. New insights into wax crystal networks in oleogels . in Edible Oil Stru c tur -ing: Concept, Methods and A pplications (Pate l, A .R.ed.). Roya l Soc. Chem., Chambr i dge, UK, pp. 71-94(2018) 23))Alizadeh, L.; Abdolmaleki, K.; Nayebzadeh, K.; Hossei-ni, S.M. Oleoge l fabrication based on sodium caseinate,hydroxypropy l methylcellulose, a nd beeswax: Effect of concentration, oleogelation method, and their opt i-mi z ation.J. Am. Oil Chem. Soc . 97, 485-496 (2020). 24))Toro-V a zquez,J.F ;Morales-Rueda , J .; Dibil d ox -Alvara -d o,E.; Charo-Alonso, M.; Alonzo-Maci a s, M.; Gonzalez - Chavez, M.M. Thermal and textural properties of or -ganogels developed by candelilla wax in safflower oil .J. Am. Oil Chem. Soc. 84,989-1000(2007). 25))/Ahmadi, E.; Mosaferi, M.; Nikniaz, L.; Tabrizi , J.S.; Ja-farabadi, M.A. et al. Frying oils quality control: Neces-sity for new approach of supervision. Br . Food J. 120,490-498(2018). 26)2Zhang,Z.S.; Li ,D.; Zhang, L.X.; Liu, Y.L . Heat i ng effect on the DSC melting curve of flaxseed oil. J. Th e rm.A nal . Calorim . 115,2129-2135(2014). 27)I Krokida, M.K.; Oreopoulou, V.; Maroulis, Z.B.; Marinos Kour i s , D. Colour changes during deep fat frying . J.Food Eng. 48, 219-225(2001). 28))IDana, D.; Saguy, I.S. Mechanism of oil uptake during deep-fat frying an d the surfactant effect -theory and myth . Adu . Colloid Interf a ce Sci . 128,267-272(2006). 29) Szczesniak, A.S. Classification of textural characteris-tics.J. Food Sci . 28,385-389(1963). 30) Mudgil, D.; Barak, S.; Khatkar, B.S. Texture profile a n a lysis of yogurt as influenced by partially hydrolyzed guar gum and process variables.J . Food Sci. Technol.54,3810-3817(2017). 31) Radoěaj , O.; Vujasinovic, V.; Dimic, E. Secondary TPA characteristics of a spread containing hull-less pump-kin(Cucurbita pepo l.)seed oil press-cake and cold-pressed hemp(Cannabis sativa l .)oil . Uljarst u o 43,33-41(2012). 32)Yang, S.; Li, G.; Saleh, A.S.M.; Yang, H.; Wang, N. et al .Functional characteristics of oleogel prepared from sunflower oil with B-sitosterol and stearic acid. J . A m.Oil Chem. Soc. 94,1153-1164(2017). CC ①BY CC BY 4.0(Attr i bution 4.0 Int e rnat i onal ). This license a llow s u sers to s h are an d a dapt a n a rt i cle,even commerci a l l y , as lon g as appropri a te credit is given. That is , this license lets others c opy, distrib-ute, r e m i x, and build upon t h e A rt i c l e, eve n com-mercially , prov i ded the orig i nal source and Authors are credited.
确定







还剩10页未读,是否继续阅读?
产品配置单
中国格哈特为您提供《炸洋葱圈吸油量的检测》,该方案主要用于其他蔬菜制品中营养成分检测,参考标准《GB 5009.6 食品中脂肪的测定》,《炸洋葱圈吸油量的检测》用到的仪器有格哈特全自动超级总脂肪测定系统、德国加液器MM、滤纸筒
该厂商其他方案
更多










