方案详情
文
罗马尼亚 中国林业大学 常用非甾体抗炎药人工暴露的根际微生物群的结构和代谢谱Structural and Metabolic Profiling of Lycopersicon esculentum Rhizosphere Microbiota Artificially Exposed at Commonly Used Non-Steroidal Anti-Inflammatory Drugs
使用格哈特公司振荡器LaboShake振荡2克根际土壤30分钟。
LaboShake最高可承载30千克的负载。
方案详情

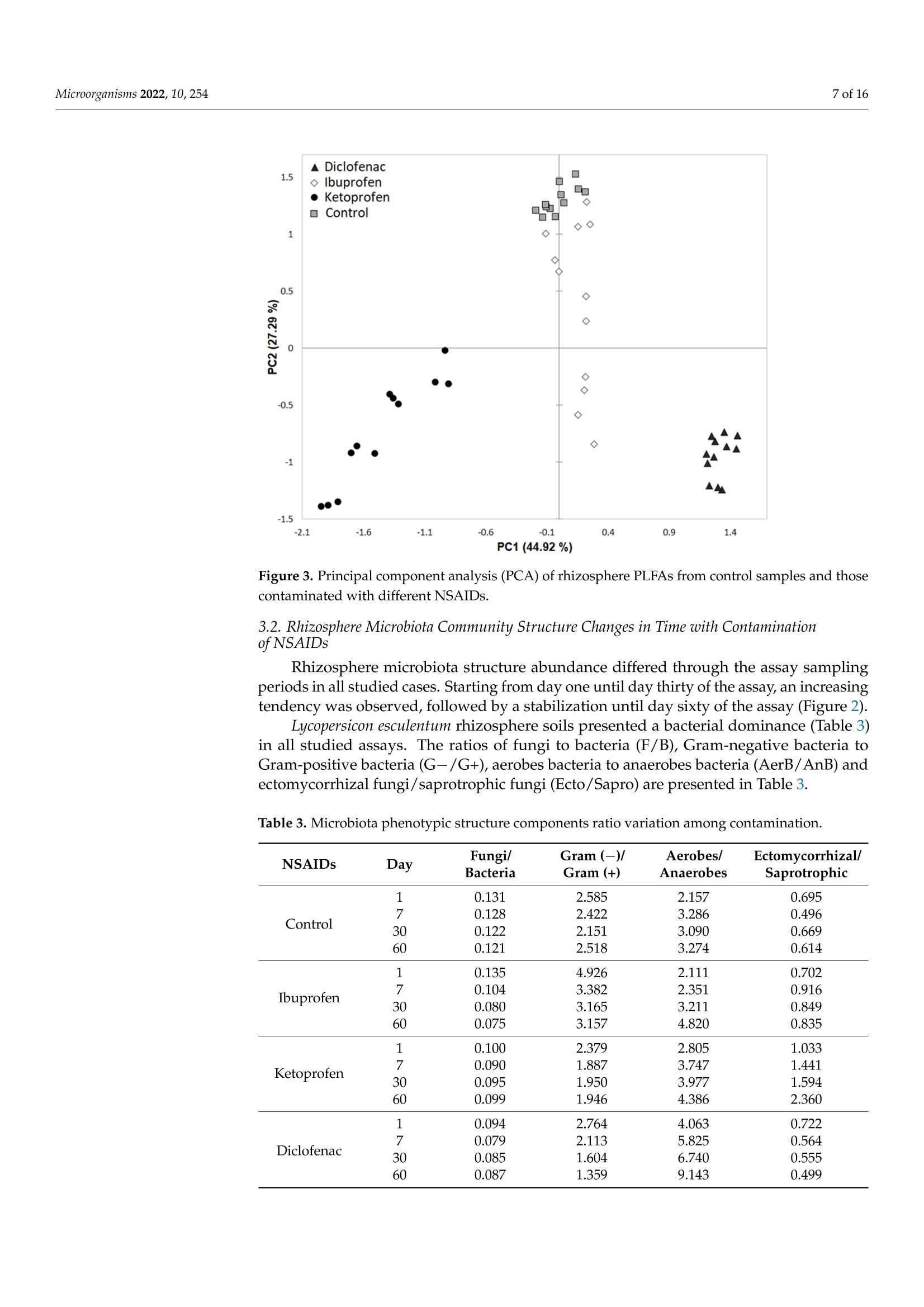
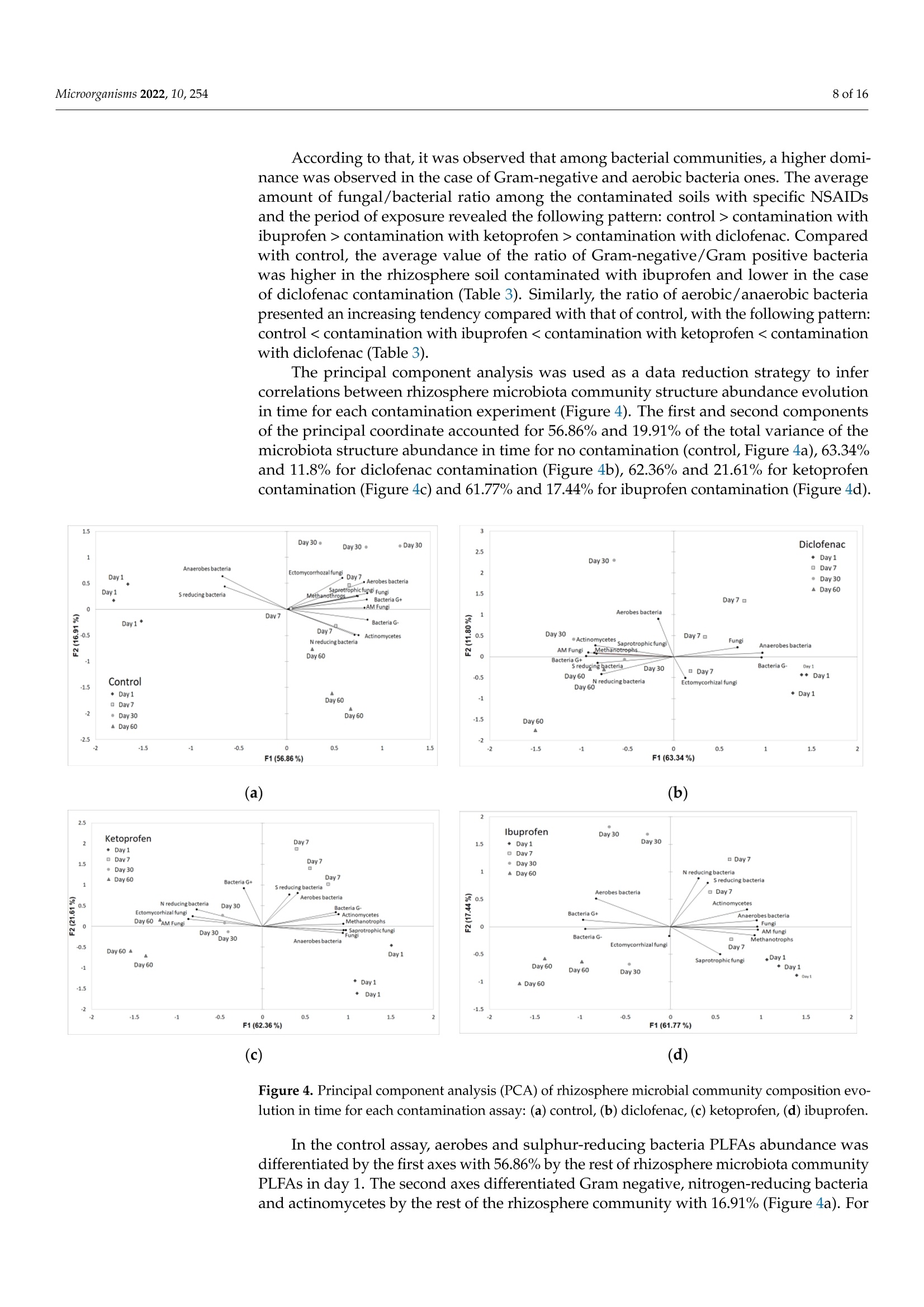
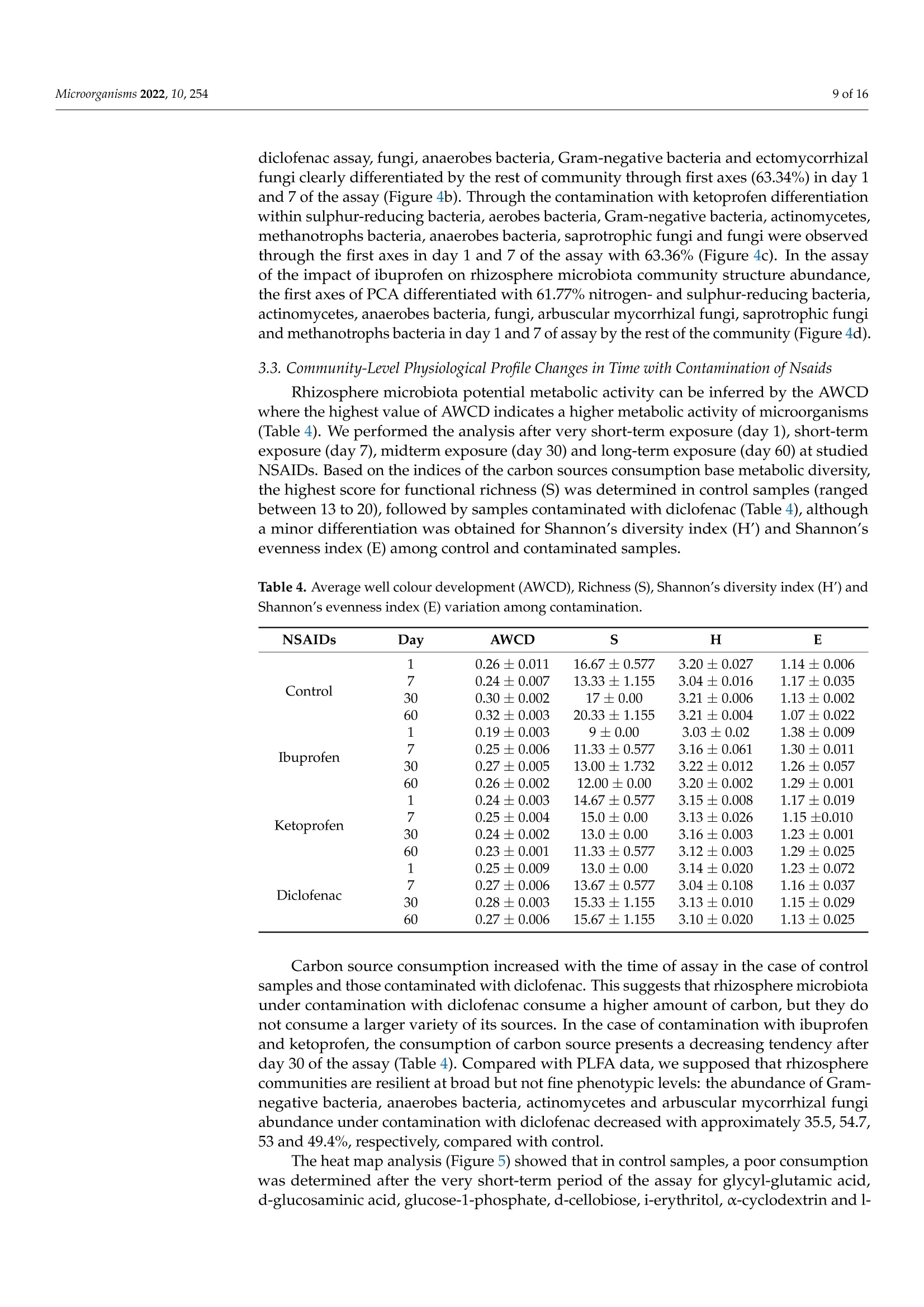
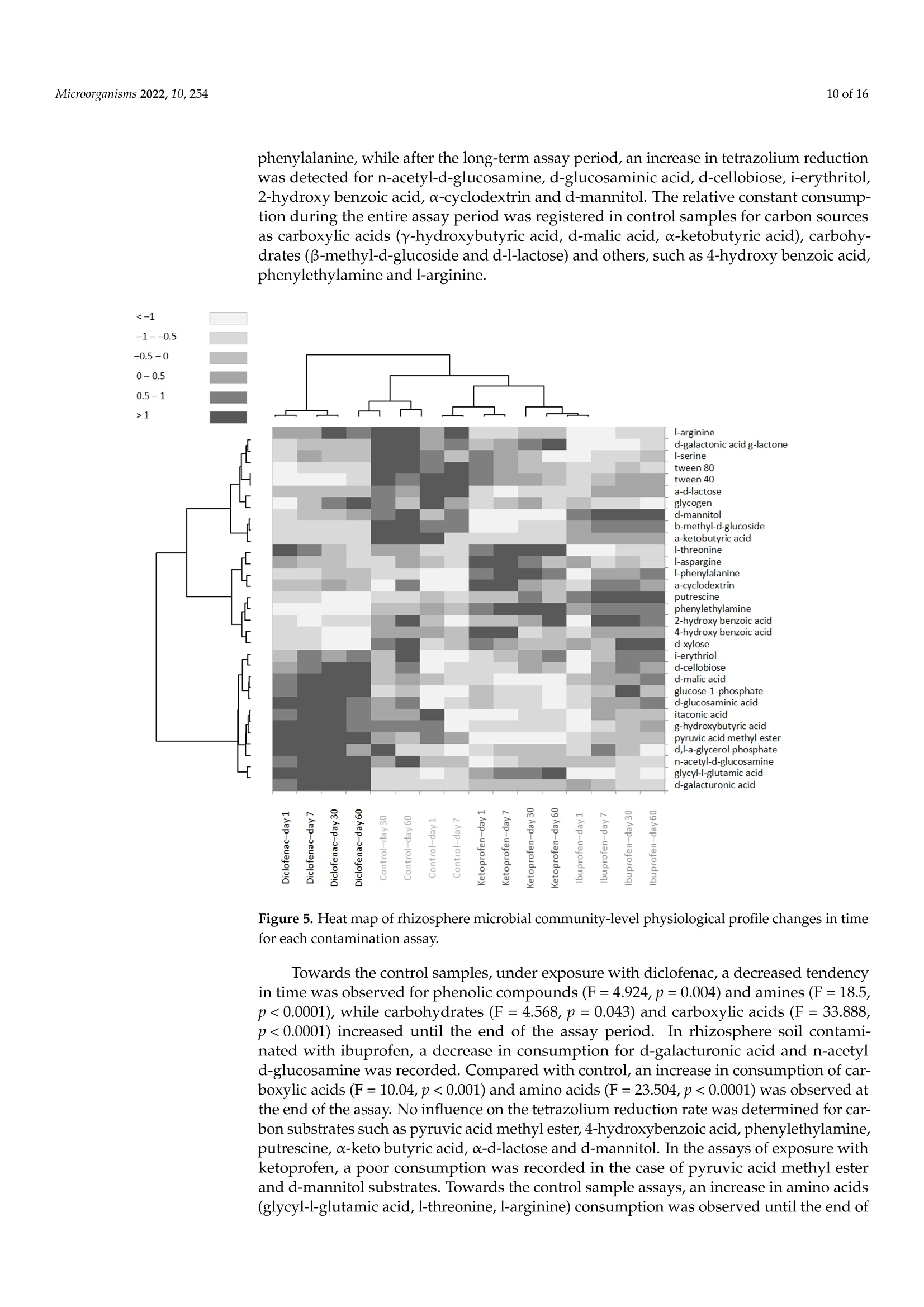
罗马尼亚 中国林业大学 常用非甾体抗炎药人工暴露的根际微生物群的结构和代谢谱Structural and Metabolic Profiling of Lycopersicon esculentum Rhizosphere Microbiota Artificially Exposed at Commonly Used Non-Steroidal Anti-Inflammatory DrugsAt each established sampling period, 2 g of rhizosphere soil samples were used to extract the microbiota with a 10 mL PBS solution. The extraction was allowed for 30 min through continuous mechanical shaking (LaboShake, Gerhardt Analytical System, Konigswinter, Germany), followed by 1 h of rest.使用格哈特公司振荡器LaboShake振荡2克根际土壤30分钟。LaboShake最高可承载30千克的负载。MDPImicroorganisms 2 of 16Microorganisms 2022, 10, 254 check forupdates Citation: Kovacs,E.D.; Silaghi-Dumitrescu,L.; Roman, C.;Tian, D. Structural and MetabolicProfiling of Lycopersicon esculentumRhizosphere Microbiota ArtificiallyExposed at Commonly UsedNon-Steroidal Anti-InflammatoryDrugs. Microorganisms 2022,10,254.https://doi.org/10.3390/ microorganisms10020254 Academic Editor: Daolin Du Received: 8 December 2021 Accepted: 18 January 2022 Published: 24 January 2022 Publisher's Note: MDPI stays neutralwith regard to jurisdictional claims inpublished maps and institutional affil-iations. Copyright: @ 2022 by the authors.Licensee MDPI, Basel, Switzerland.This article is an open access articledistributed under the terms andconditions of the Creative CommonsAttribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/) Emoke Dalma Kovacs 1,2*, Luminita Silaghi-Dumitrescu2, Cecilia Romanl and Di Tian 3D Research Institute for Analytical Instrumentation, INCDO-INOE 2000, 400293 Cluj-Napoca, Romania;cici_roman@yahoo.com 2 Faculty of Chemistry and Chemical Engineering, Babes-Bolyai University, 400028 Cluj-Napoca, Romania;luminita.silaghi@gmail.com 3 Research Center of Forest Management Engineering of State Forestry and Grassland Administration,College of Forestry, Beijing Forestry University, Beijing 100083, China; tiandi@bjfu.edu.cn Correspondence: dalmaemokekovacs@gmail.com Abstract: In this study, the effect of common non-steroidal anti-inflammatory drugs on Lycopersiconesculentum rhizosphere microbiota was monitored. The experiments were performed with artifi-cially contaminated soil with ibuprofen (0.5 mgkg-), ketoprofen (0.2 mgkg-1) and diclofenac(0.7 mgkg-). The results evidenced that the rhizosphere microbiota abundance decreased especiallyunder exposure to diclofenac (187-201 nmolg- dry weight soil) and ibuprofen (166-183 nmolg-dryweight soil) if compared with control (185-240 nmolg-1 dry weight soil), while the fungal/bacteriaratio changed significantly with exposure to diclofenac (<27%) and ketoprofen (<18%). Comparedwith control samples, the average amount of the ratio of Gram-negative/Gram-positive bacteria washigher in rhizosphere soil contaminated with ibuprofen (>25%) and lower in the case of diclofenac(<46%) contamination. Carbon source consumption increased with the time of assay in case of thecontrol samples (23%) and those contaminated with diclofenac (8%). This suggests that rhizospheremicrobiota under contamination with diclofenac consume a higher amount of carbon, but they donot consume a larger variety of its sources. In the case of contamination with ibuprofen and keto-profen, the consumption of carbon source presents a decreasing tendency after day 30 of the assay.Rhizosphere microbiota emitting volatile organic compounds were also monitored. Volatile com-pounds belonging to alcohol, aromatic compounds, ketone, terpene, organic acids, aldehyde, sulphurcompounds, esters, alkane, nitrogen compounds, alkene and furans were detected in rhizospheresoil samples. Among these, terpene, ketone, alcohol, aromatic compounds, organic acids and alkanewere the most abundant compound classes (>75%), but their percentage changed with exposureto diclofenac, ketoprofen and ibuprofen. Such changes in abundance, structure and the metabolicactivity of Lycopersicon esculentum rhizosphere microbiota under exposure to common non-steroidalanti-inflammatory drugs suggest that there is a probability to also change the ecosystem servicesprovided by rhizosphere microbiota. Keywords: microorganisms; pharmaceuticals; exposure; functioning 1. Introduction Diclofenac, ibuprofen and ketoprofen are common non-steroidal anti-inflammatorydrugs (NSAIDs) that are often reported in environmental assessment studies This is becauseof their high consumption rate [1,2] and improper removal during wastewater treatmentprocesses [3,4]. They reach the soil system either through the reuse of treated municipalwastewater in irrigation purposes [5,6], or from the resulting sludge and biosolids asfertilizers [7,8]. Their common occurrence in soil environments raised high concernsbecause of the continuous input [9], subsequent accumulation potential [5] and their potential ecotoxicological effects on nontargeted living organisms at different trophiclevels [10,11]. Soil microbiota resistance to pharmaceuticals is a global issue and understanding itsfunctional and molecular basis is essential. Bacterial resistance to pharmaceuticals could beeither natural or acquired [12]. Although a natural resistance could be not considered aserious clinical issue, the acquired resistance is a much more severe case when we considerthe high ability of bacteria to capture fragments of DNA and genes of resistance to manycommonly used pharmaceuticals, even from phylogenetically distinct organisms. Throughhorizontal gene transfer and cross-resistance development, bacteria could become resistantto multiple drugs [13]. Soil microbiota are key actors in soil processes, contributing significantly to numerousecosystem services provided by soil. They are involved in the processes of nutrient cyclingand organic matter degradation [14]. Microorganisms are also able to synthesize volatileorganic compounds, such as alcohols, terpenes, ketones, alkanes, etc. [15]. These are sec-ondary metabolites with multiple ecological roles and mechanisms of action. Reports havestarted to highlight that those volatile organic compounds emitted by soil microbiota can actas signalling molecules assuring distance communication between various organisms [16],can induce inhibitory activity against fungal spore germination [17], those changing mi-crobiota structure, and can modulate enzyme activity [18]. In this way, they can directlyinfluence the aboveground biodiversity and productivity. Through the chemicals that themicroorganism cycles or releases, they can stimulate or inhibit plant development [16].Rhizosphere, the interface between plant roots and soil is one of the most abundant anddynamic system inhabited by microorganism [19]. Rhizosphere microbiomes differ fromsoil microbiomes. Although it is well acknowledged that the microbiota of rhizospherehave positive effects on plant development and heath [20,21], there is less knowledge ontheir structure, abundance and function under challenging conditions. Considering thefrequency of NSAIDs' presence in soil environment [1,7,8], studies are essential to unravelthe functions of rhizosphere microbiota under exposure to frequently detected NSAIDs. A major goal in ecology is to assure the development of more stable agrosystemsthat can face current challenges. This could be achieved through the achievement of adeeper knowledge on rhizosphere ecology and the identification of rhizobiome chemicaland biological diagnostics and signatures for identified issues. Microbiota are characterizedas small organisms; therefore, compared to other organisms, they present a high surfacearea-to-volume ratio, which provides a large contact interface that interacts with theirsurrounding environment, respectively, with surrounding contaminants [22]. Under theexposure to pharmaceuticals, soil microbiome biological parameters such as structure,abundance and metabolic activity could suffer changes. Wang et al. [23] assessed the toxiceffects of enrofloxacin on soil enzymatic activities and showed that the activities of sucrasewas inhibited significantly at all incubation periods. In studies on microbial utilisation inthe Biolog plates, they reported that the utilisation of metabolites was inhibited severelyand reached essentially zero after 21 days, although they gradually decreased with theincreasing of time (until day 21 of the assay). In studies reported by Liu et al., [24] it wasfound that soil microbial functional diversity and the capacity of soil microbial communitiesto utilise substrates were sensitive to sulfamethoxazole and chlortetracycline. Similarly,in the triclosan ecotoxicity assessment of soil microbiota, Ramires et al. [25] state that thisantimicrobial agent inhibited the consumption pattern of carboxylic acids. In the studyof the influence of tetracycline presence from cow manure on soil microbiota, Chessaet al. [26] evidenced that tetracycline only transiently influenced the microbiota abundanceand functions. As soil microbiome could be changed in structure, abundance, or metabolic activityonce exposed to different pharmaceuticals, this could result in changes in key ecologicalprocesses of soil. At the best of our knowledge, there is at present minor information onhow the presence of NSAIDs could shape rhizosphere microbiome structure, abundanceand metabolic activity. Moreover, there is no information on how the presence of certain NSAIDs influence or not the secondary metabolites profile produced by rhizosphere mi-crobiome. The main aim of this study was to identify if the presence of common NSAIDssuch as ibuprofen, diclofenac and ketoprofen could shape Lycopersicon esculentum rhizo-sphere microbiota (i) abundance and phenotypic structure, (ii) metabolic activity, and (iii)secondary metabolites profile. 2. Materials and Methods 2.1. Experimental Set-Up Pharmaceuticals such as ibuprofen (C13H19O2), ketoprofen (C16H14O3) and diclofenac(C14H11Cl2NO2), belonging to the class of non-steroidal anti-inflammatory drugs, wereselected for our study. The selection of these NSAIDs was based on their increased con-sumption and widespread occurrence in environment. Argic phaeozem soil samples (0-40 cm), collected in April 2020 from Cojocna, ClujCounty was used in this study. The main physical chemical properties of the soil samplesare listed in Table 1. All soil samples were tested to be free of studied NSAIDs according to the method de-scribed by KoOVvaaccs et al. [27]. The samples were artificially contaminated individually witheach pharmaceutical in part (0.5 mgkg- ibuprofen, 0.2 mgkg-1 ketoprofen, 0.7 mg·kg-1diclofenac) as previously described [27]. For each rhizosphere soil sampling period, indi-vidual pots in triplicate were prepared for each soil type vs. pharmaceutical as presentedin the schematic diagram of the experiment set-up (Figure 1). Fourteen-days-old tomatoseeds (Lycopersicon esculentum) were planted in contaminated soil pots prepared one daybefore and allowed for development in a laboratory climate chamber with the followingday-night cycle conditions: day-14 h of light, 25°C; night-10 h of darkness, 18C. Thesoil water content was adjusted to assure a 58% water holding capacity (WHC) duringthe study. Figure 1. Schematic diagram of experiment set-up Table 1. Average values (n=12) of physical chemical properties of the studied soil samples. Soil Property Argic Phaeozem Clay 27.2±0.94 Sand 16.1±0.20 Silt 56.7±1.37 Texture Silty Clay Loam Moisture (cm/cm) 0.344±0.01 Soil temperature (C) 10.4±0.09 Organic carbon (%) 6.2±0.12 pH 5.9±0.09 2.2. Rhizosphere Microbiota Analysis through PLFA Approach The rhizosphere soil microbiota phenotypic structure and abundance assessment wasperformed considering the phospholipids-derived fatty acids (PLFA) gas chromatographicanalysis. The rhizosphere soils were sampled from pots contaminated with pharmaceuticals(Figure 1) after very short-term exposure (day 1), short-term exposure (day 7), mid-termexposure (day 30) and long-term exposure (day 60). The extraction of PLFA was performed on 1 g of freeze-dried (Labconco FreeZone6 freeze-dry system, Kansas, MO, USA) soil according to the method described by Blighand Dyer [28] and modified by Frostegard et al. [29]. The lipids were fractionated into phos-pholipids, glycolipids and neutral lipids using a silicic acid column (500 mg, Phenomenex,Torrance, CA, USA). After a mild alkaline methanolysis, 150 uL of extracts containingthe fatty acids methyl esters was injected into a gas chromatograph with flame ionizationdetector (7890A GC-FID, Agilent Technologies, Santa Clara, CA, USA). The fatty acidsmethyl esters separation was obtained using a 5% phenyl-methylpolysiloxane column(HP-Ultra 2, J&W Scientific, Folsom, CA, USA) with the following properties: 25 mm× 0.2 mm id., 0.33 um film thickness. The PLFAD1 method from the MIDI SherlockIMMicrobial Identification System (Microbial ID, Inc., Newark, DE, USA) was used for thephospholipids-derived fatty acids separation. The gas chromatograph operation parame-ters are listed in Table 2. Table 2. GC-FID operation parameters for phospholipids-derived fatty acids analysis from rhizo-sphere soils. Parameter Conditions Inlet temperature 280°C Split mode 40:1 170°C, increase with 28°Cmin-until 288°℃,followed by a new increase with 60°Cmin-until 310 °C. This final temperature was maintained constant for 1.25 min1.2mL·min-1 Oven temperature program Detector temperature 300°C For the interpretation of the phospholipids-derived fatty acids data, bacterial fatty acidstandards and software from MIDI SherlockM Microbial Identification System (MicrobialID, Inc., Newark, DE, USA) was used. Saprotrophic fungi were identified using 18:2w6cPLFA biomarker [29], and ectomycorrhizal fungi with 18:2w9c PLFA biomarker [30]. PLFAbiomarkers such as 18:2w6c and 18:3w3 were used for nitrogen-reducing bacteria iden-tification [31], and 17:1w7c,10Me16:0, 17:1w6, 15:1, i17:1w7c,cy18:0w7.8, i15:1w7c andi19:1w7c markers were used for sulphur-reducing bacteria identification [32,33]. 2.3. Rhizosphere Microbiota Responses Evaluation The rhizosphere microbiota response to ibuprofen, diclofenac and ketoprofen wasevaluated considering community-level physiological profile (CLPP) and emitted volatile organic compounds (VOCs). The sampling of the rhizosphere soil samples from the con-taminated pots was performed according to the schematic diagram presented in Figure 1. 2.3.1. Community-Level Physiological Profile (CLPP) The assessment of the metabolic activity of rhizosphere soil microbiota after exposureto NSAIDs was performed using Biolog EcoPlateM containing 31 different carbon sources.At each established sampling period, 2 g of rhizosphere soil samples were used to extractthe microbiota with a 10 mL PBS solution. The extraction was allowed for 30 min throughcontinuous mechanical shaking (LaboShake, Gerhardt Analytical System, Konigswinter,Germany), followed by 1 h of rest. The mixture of soil suspension and supernatant wassubjected to sonication and centrifugation as described by Lindahl and Bakken [34] until weobtained the final soil lixiviate. The soil particles from the obtained lixiviates were removedthrough low-speed centrifugation (1000 rpm for 1 min, LMC-3000 centrifuge, GrantBio,Riga, Latvia). From this final solution, 150 uL was added to each well of EcoPlate andincubated under dark conditions at 25 °C for 3 days (LabCompanion, Billerica, MA, USA).The optical density (OD) of each well was measured at 入= 590 nm using an SpectraMaxiD3 Microplate Reader (Molecular Devices, San Jose, CA, USA) and SoftMax Pro7 software(Molecular Devices) just after inoculation and once a day during the incubation period. 2.3.2. Emitted VOCs The rhizosphere soil-emitted volatile organic compounds content was determinedthrough headspace-solid phase microextraction sampling using 85 um polyacrylate fibre(Supelco Inc., Bellefonte, PA, USA). An amount of 1 g of soil samples was diluted with2 mL of PBS solution in 20 mL headspace glass vials (Agilent Technologies). The headspacevials were tightly capped with Teflon-faced rubber liner caps and subjected for incubationfor 72 h in dark at 25 °C. After the incubation period, the vials were equilibrated for 30 minat 60 °C using a TriPlus RSH autosampler (Thermo Scientific, Austin, TX, USA). The SPMEfibre after activation in the SSL injector was exposed and maintained in the vial headspacesurface for 15 min. The volatile profile analysis was performed on GC-MS/MS (Trace1310, TSQ 9000, Thermo Scientific, Austin, TX, USA) with electron impact ionization (70 eVionization energy). The separation was performed on Agilent HP-5MS capillary column(30 m x 0.25 mm, 0.25 um) using helium as carrier gas with 1.2 mLmin-1 flow. SPME fibrewas injected into the GC injection port and adsorbed volatiles in the fibre were desorbedonto the column at 250 °C for 5 min in splitless mode. The volatile organic compoundswere identified by comparison of their mass spectra with compounds corresponding tomass spectra library (NIST/EPA/NIH, Chromeleon 7.2 CDS Software, Thermo Scientific,Austin, TX,USA).The identified volatile organic compounds were expressed in percentagesas a normalised amount of each volatile organic compound resulted after the division ofpeak areas of identified volatile organic compounds by total peak area of all identifiedvolatile organic compounds. 2.4. Statistical Interpretation of Data The differences in rhizosphere soil microbial community composition were investi-gated by principal component analysis (PCA) using Statistica 10 software (StatSoft, Ham-burg, Germany). For the statistical analysis, the OD of each well after inoculation wassubtracted from the OD after each measurement period during the incubation. The aver-ages and standard deviations corresponding to each carbon source were determined assamples were analysed in triplicate, since Biolog EcoPlates contain three replicates of eachcarbon source. The average well colour development (AWCD), Richness (S), Shannon’sdiversity index (H') and Shannon's evenness index (E) were determined according tothe formulas presented by Sofo and Ricciuti [35]. All these parameters were calculatedseparately for all incubation times. For Richness, a 0.25 value for optical density (OD) wasset as the threshold for a positive response [35]. ANOVA was conducted to assess the effectof the studied pharmaceuticals on the community-level utilisation of carbon sources. The assumption of the homogeneity of variance and the test for normality of distributions wereverified applying Levene’s test and Shapiro-Wilk's test using Statistica 10 software version(StatSoft, Germany). A level of p= 0.05 was considered to assume statistical significance.The UpSetR diagrams were performed according to Khan and Mathelier [36]. 3. Results and Discussions 3.1. Rhizosphere Microbiota Abundance Changes with Contamination of NSAIDs The structure and abundance of microbiota were monitored in Lycopersicon esculentumrhizosphere soils with and without the artificial contamination with commonly consumedNSAIDs during a 60-day assay period. The total microbial biomass was expressed asthe sum of PLFAs with concentrations in control samples (without contamination) andthose contaminated ranged between 165.6 and 240 nmolg-dry weight soil during theassay (Figure 2). The control rhizosphere soil recorded higher values of PLFA (p<0.05)with concentration ranges of 184.8-240 nmolg-1 dry weight soil. In soils contaminatedwith NSAIDs, the microbial biomass in Lycopersicon esculentum rhizosphere soils has thefollowing pattern: 187.9-215.4 (ketoprofen contamination)>186.7-201.2 (diclofenac con-tamination)> 165.6-182.7 (ibuprofen contamination) nmolg-dry weight soil, respectively(Figure 2). 250 Day1 Day 7 Day 30 Day 60 口 Total PLFA ■ Total Bacteria 圆Total Fungi Figure 2. Soil microbiota abundance variation in rhizosphere soil during assay period. The PCA of the 48 PLFAs data (Figure 3) indicated that rhizosphere soil microbialcommunity abundance was markedly affected by soil contamination with NSAIDs,butpoor differentiation between control and contamination with ibuprofen was observed,indicated by their closest scores along the first principal component (PC1) and secondprincipal component (PC2). The first two components, PC1 and PC2 explained 44.92% and27.29% of the total variance in PLFAs abundance. The PC1 axis differentiated contaminationwith diclofenac and ketoprofen but did not differentiate controls by contamination withibuprofen, whereas the PC2 axis did not differentiate well control samples by contaminationwith specific NSAIDs. Figure 3. Principal component analysis (PCA) of rhizosphere PLFAs from control samples and thosecontaminated with different NSAIDs. 3.2. Rhizosphere Microbiota Community Structure Changes in Time with Contaminationof NSAIDs Rhizosphere microbiota structure abundance differed through the assay samplingperiods in all studied cases. Starting from day one until day thirty of the assay, an increasingtendency was observed, followed by a stabilization until day sixty of the assay (Figure 2). Lycopersicon esculentum rhizosphere soils presented a bacterial dominance (Table 3)in all studied assays. The ratios of fungi to bacteria (F/B), Gram-negative bacteria toGram-positive bacteria (G-/G+),aerobes bacteria to anaerobes bacteria (AerB/AnB) andectomycorrhizal fungi/saprotrophic fungi (Ecto/Sapro) are presented in Table 3. Table 3. Microbiota phenotypic structure components ratio variation among contamination. Day Fungi/ Gram (-)/ Aerobes/ Ectomycorrhizal/ NSAIDs Bacteria Gram (+) Anaerobes Saprotrophic 1 0.131 2.585 2.157 0.695 Control 7 0.128 2.422 3.286 0.496 30 0.122 2.151 3.090 0.669 60 0.121 2.518 3.274 0.614 1 0.135 4.926 2.111 0.702 Ibuprofen 7 0.104 3.382 2.351 0.916 30 0.080 3.165 3.211 0.849 60 0.075 3.157 4.820 0.835 1 0.100 2.379 2.805 1.033 Ketoprofen 7 0.090 1.887 3.747 1.441 30 0.095 1.950 3.977 1.594 60 0.099 1.946 4.386 2.360 1 0.094 2.764 4.063 0.722 Diclofenac 7 0.079 2.113 5.825 0.564 30 0.085 1.604 6.740 0.555 60 0.087 1.359 9.143 0.499 According to that, it was observed that among bacterial communities, a higher domi-nance was observed in the case of Gram-negative and aerobic bacteria ones. The averageamount of fungal/bacterial ratio among the contaminated soils with specific NSAIDsand the period of exposure revealed the following pattern: control > contamination withibuprofen > contamination with ketoprofen > contamination with diclofenac. Comparedwith control, the average value of the ratio of Gram-negative/Gram positive bacteriawas higher in the rhizosphere soil contaminated with ibuprofen and lower in the caseof diclofenac contamination (Table 3). Similarly, the ratio of aerobic/anaerobic bacteriapresented an increasing tendency compared with that of control, with the following pattern:control < contamination with ibuprofen 1 Figure 5. Heat map of rhizosphere microbial community-level physiological profile changes in timefor each contamination assay. Towards the control samples, under exposure with diclofenac, a decreased tendencyin time was observed for phenolic compounds (F=4.924, p=0.004) and amines (F=18.5,p<0.0001), while carbohydrates (F = 4.568, p=0.043) and carboxylic acids (F=33.888,p <0.0001) increased until the end of the assay period. In rhizosphere soil contami-nated with ibuprofen, a decrease in consumption for d-galacturonic acid and n-acetyld-glucosamine was recorded. Compared with control, an increase in consumption of car-boxylic acids (F=10.04,p<0.001) and amino acids (F=23.504,p<0.0001) was observed atthe end of the assay. No influence on the tetrazolium reduction rate was determined for car-bon substrates such as pyruvic acid methyl ester, 4-hydroxybenzoic acid, phenylethylamine,putrescine, a-keto butyric acid, α-d-lactose and d-mannitol. In the assays of exposure withketoprofen, a poor consumption was recorded in the case of pyruvic acid methyl esterand d-mannitol substrates. Towards the control sample assays, an increase in amino acids(glycyl-l-glutamic acid, l-threonine, l-arginine) consumption was observed until the end of the assay (F=13.009, p<0.0001). The polymer substrates consumption decreased in timecompared with control samples (F=20.547,p=0.012). Overall, the rhizosphere communities in the presence of different NSAIDs changedthe carbon consumption for each substrate classes. In soils exposed to ketoprofen anddiclofenac, a significant decrease in the utilisation of carbon substrates was observedcompared with soil exposed to ibuprofen or the control sample (Table 4, Figure 5). 3.4. Microbiota Emitted Volatile Organic Compounds Changes in Time with Contaminationof Nsaids We performed a VOCs analysis by SPME-GC-MS in all control and contaminatedrhizosphere soils during the exposure assay. Volatile compounds belonging to alcohol,aromatic compounds, ketone, terpene, organic acids, aldehyde, sulphur compounds,esters,alkane, nitrogen compounds, alkene and furans were detected in the rhizosphere soilsamples. Terpene, ketone, alcohol, aromatic compounds, organic acids and alkane werethe most abundant compound classes (Table 5). In the control samples, the highest alcoholcompound was hexan-1-ol followed by 1-octen-3-ol with the average amount during theassay periods of 2.87 and 2.64%, respectively. Benzaldehyde was the highest aromaticcompound identified in control samples and those contaminated with diclofenac (3.38%)and ibuprofen (6.61%). In the case of ketoprofen contamination, phenol was the highestaromatic compound released by rhizosphere microbiota (3.25%). Among ketones, theprevalent compounds were decane-2-one (3.41%, diclofenac contamination), octan-3-one(control and ketoprofen contamination, 2.84%) and decan-2-one (ibuprofen contamination,3.34%). Terpineol was determined in similar amounts in the case of the control sample, aswell in the case of contamination with ketoprofen (5.55%). Compounds such as geranylacetone (5.84%) and germacradien-1l-ol (4.89%) were the most abundantly released terpenesby rhizosphere microbiota under contamination with ibuprofen and diclofenac, respectively.Butanoic acid was the most prevalent organic acid in all studied rhizosphere soils, withthe highest amount identified in rhizosphere soils under contamination with diclofenac(4.32%),followed by control and ketoprofen-contaminated soil samples (3.44%) and thosecontaminated with ibuprofen (2.84%) Table 5. Distribution of volatile organic compounds (%) produced in rhizosphere soil samples (controland NSAIDs contaminated). Volatile Organic Compounds Control Ketoprofen Ibuprofen Diclofenac Alcohol 12 13 18 12 Aromatic compounds 11 11 15 11 Ketone 13 14 11 12 Terpene 18 18 14 12 Organic acids 12 12 9 15 Aldehyde 6 8 6 8 Sulphur compounds 2 1 6 5 Ester 4 4 5 5 Alkane 12 11 10 11 Nitrogen compounds 5 5 4 3 Alkene 4 3 2 6 Furans 1 0 0 0 To identify volatile organic compound-specific or shared regulations, we comparedthe sets of upregulated volatile organic compounds obtained for each exposure case andtime of assays (Figure 6). Figure 6. UpSet plot of interactions and the amounts of similar and different volatile organiccompounds released by rhizosphere microbial community composition evolution in time for eachcontamination assay. (a) after one day assay; (b) after 7 day assay; (c) after 30 day assay; (d) after60 day assay. Between 16 and 11 upregulated volatile organic compounds responded specificallyto the presence of NSAIDs. Among the remaining volatile organic compounds that wereupregulated by at least two different pharmaceutical compounds, the highest intersectionsize was found to increase once with the duration of the assay (19 volatile organic com-pounds representing 37% of the total number of upregulated volatile organic compoundsin rhizosphere soils after 60 days of assay, Figure6d). Interactions varied in time with assayduration, volatile organic compounds that were upregulated by control and at least twodifferent NSAIDs. The highest size was found after midterm exposure (day 30, Figure 6c)and short-term exposure (day 7, Figure 6b) of the assay. The similarities of volatile organiccompounds in assays at very short-term exposure (day 1) were found for two upregulatedvolatile organic compounds when rhizosphere microbiota were exposed to ibuprofen andketoprofen and two other upregulated volatile organic compounds were found whenthe exposure was for ibuprofen and diclofenac (Figure 6a). In the case of the short-termexposure assay (day 7), similarities of the volatile organic compounds were found for sixupregulated volatile organic compounds when rhizosphere microbiota were exposed toibuprofen and diclofenac (Figure 6b), five upregulated volatile organic compounds whenrhizosphere microbiota were exposed to ibuprofen and diclofenac during the midterm expo-sure assay, and six upregulated volatile organic compounds when rhizosphere microbiotawere exposed to ibuprofen and ketoprofen (Figure 6a). In this study, it was observed that the Lycopersicon esculentum rhizosphere microbiomeabundance decreased especially under exposure to ibuprofen and diclofenac. Changesin the phenotypic structure was also observed in the experimental data, especially in thecase of fungal/bacteria, Gram-positive/Gram-negative bacteria and aerobic/anaerobicbacteria ratios. These data followed those reported by Cycon et al. [10], Paje et al. [41]and Dastidar et al. [43]. Considering the rhizosphere microbiome emitted volatile organiccompounds, a differentiated pattern was observed in time, depending also on the NSAIDsused in the exposure experiment. In the case of diclofenac exposure assessment, ourdata reveal an increasing tendency with time in the case of specific alcohol compounds(2-methyl-butan-1-ol, hexan-1-ol, and 1-octen-3-ol), aromatic compounds (benzaldehyde,1-methoxy-4-methylbenzene, and phenol) and germacradien-1l-ol. In the case of alcoholcompounds, a similar pattern was observed even when the contamination was performedwith ketoprofen. The remaining volatile organic compounds either decreased during theassay time or presented an increasing tendency during the first half period of the assayand decreased after that. Comparing these patterns with those reported in the literature,we found that Zhou et al.[44] in their study on extracellular polymeric substances onceobserved an increase with an exposure to ibuprofen. However, in all contaminationassessments, different patterns of volatile organic compounds emission were founded.Because volatile organic compounds are essential for above and belowground diversity,it becomes important to understand how these are affected by the presence of certainpharmaceuticals. Similarly, rhizosphere microbiome carbon consumption pattern differedalso once with contamination to studied NSAIDs. Towards the control samples, in soilsexposed to ketoprofen and diclofenac carbon substrates, the consumption was lower. Minorchanges were observed in the case of exposure to ibuprofen. Pharmaceuticals are essential for human and animal health treatment and mainte-nance. As soil microbiota abundance, structure and functioning could be modified in thepresence of pharmaceuticals, it becomes urgent to identify solutions to limit the spread ofpharmaceutical residues, antibiotic-resistant bacteria and genes in environmental compart-ments. One way is by improving wastewater treatment plant efficiency to remove suchmicropollutants. However, better knowledge is required on the fate of pharmaceuticalsin the environment and on the underlying processes, on interactions within biota andinduced changes. 4. Conclusions In this study, the impact of commonly reported NSAIDs in the environment on Ly-copersicon esculentum rhizosphere microbiota was investigated. The obtained resultsclearly illustrate that the presence of drugs could shape rhizosphere microbiota abundanceand phenotypic structure. Comparing the data with those of control, it was observed that especially under exposure to diclofenac and ibuprofen, the rhizosphere microbiotaabundance decreased with approximately 10-20%. Additionally, microbiota metabolicactivity modified in contact with NSAIDs (e.g., increase of carbon consumption rate with8% under exposure to diclofenac). Such changes make us suppose that there is a probabilityto also change ecosystem services provided by rhizosphere microbiota. Therefore, to protectsoil ecosystem services mediated by rhizosphere microbiome, the presence of potentialpharmaceuticals should be considered. On this basis, it will be possible not only to assessthe presence of NSAIDS or their impact on microorganisms, but also to look for linksbetween the changes of microbiota community functioning and soil ecosystem servicesthat they mediate. Author Contributions: Conceptualization, E.D.K.; methodology, E.D.K.; formal analysis, L.S.-D.;investigation, E.D.K. and C.R.; writing-original draft preparation, E.D.K.; writing-review andediting, E.D.K.,D.T. and C.R.; visualization, D.T.; supervision, C.R. and L.S.-D. All authors have readand agreed to the published version of the manuscript. Funding: This research was funded by ANCS, Program NUCLEU, grant number PN 19-18.01.01-18N/08.02.2019 and Programul Operational Competitivitate 2014-2020, grant number PREPARESMIS 107874; Ctr. 253/2.06.2020. Institutional Review Board Statement: Not applicable Informed Consent Statement: Not applicable. Data Availability Statement: The data that support the fundings of this study are available onrequest from the corresponding author (KED). Conflicts of Interest: The authors declare no conflict of interest. References 1. Kamat, S.; Marathe, P.; Tripathi, R.; Raut, S.; Khatri, N.Over-the-counter medicines: Global perspective and Indian scenario.J.Postgrad. Med. 2020,66,28-34. [CrossRef] [PubMed] 2. Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact ofpharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res.2021,194,110664. [CrossRef] [PubMed] 3. Praveenkumarreddy, Y.; Vimalkumar, K.; Ramaswamy, B.R.; Kumar, V.; Singhai, R.K.; Basu, H.; Gopal, C.M.; Vandana, K.E.; Bhat,K.; Udayashankar, H.K.; et al. Assessment of non-steroidal anti-inflammatory drugs from selected wastewater treatment plants ofSouthwestern India. Emerg. Contam. 2021,7,43-51. [CrossRef] 4. Alenzi, A.; Hunter, C.; Spencer, J.; Roberts, J.; Craft, J; Pahl, O.; Escudero, A. Pharmaceuticals effect and removal, at environmen-tally relevant concentrations, from sewage sludge during anaerobic digestion. Bioresour. Technol. 2021, 319,124102.[CrossRef][PubMed1 5. Mordechaay, E.B.; Mordehay, V.; Tachitzky, J.; Chefetz, B. Pharmaceuticals in edible crops irrigated with reclaimed wastewater:Evidence from large survey in Israel. J. Hazard. Mater. 2021, 416,126184. [CrossRef] 6. Grabicova, K.;Grabic, R.; Fedorova,G.; Stanova,A.V.;Blaha, M.;Randak, T.; Brooks, B.W.;Zlabek, V. Water reuse and aquaculture:Pharmaceutical bioaccumulation by fish during tertiary treatment in wastewater stabilization pond. Environ. Pollut. 2020, 267,115593.[CrossRef 7 Mejias, C.; Santos, L.J.; Aparicio, I.; Alonso, E. Occurrence of pharmaceuticals and their metabolites in sewage sludge and soil: Areview on their distribution and environmental risk assessment. Trends Environ. Anal. Chem. 2021,30,e00125.[CrossRef] 8. Xu, J.; Wu, L.; Chen, W.;Chang, C.A. Leaching potential of nonsteroidal anti-inflammatory drugs in soils. Environ. Toxicol. Chem.2010,29,800-807.[CrossRef] 9 Duan, Y.P.; Meng,X.Z.; Wen, Z.H.; Chen, L. Acidic pharmaceuticals in domestic wastewater and receiving water from hyper-urbanization city of China (Shanghai): Environmental release and ecological risk. Environ. Sci. Pollut. Res. 2013, 20,108-116.[CrossRef] [PubMed] 10. Cycon, M.; Borymski, S.; Zolnierczyk, B.; Piotrowska-Seget, Z. Variable effects of Non-steroidal Anti-inflammatory Drugs(NSAIDs) on selected biochemical processes mediated by soil microorganisms. Front. Microbiol. 2016,7,1969. [CrossRef] 11. Papaioannou,D.; Koukoulakis, P.H.; Papageorgiou, M.; Lambropoulou,D.A.; Kalavrouziotis, I.K. Investigation of pharmaceutical and personal care product interactions of soil and beets (Beta vulgaris L.) under the effect of wastewater reuse. Chemosphere 2020,236,124553. [CrossRef] [PubMed] 12. Wolny-Koladka, K.; Lenart-Boron, A. Phenotypic and molecular assessment of drug resistance profile and genetic diversity ofwaterborne Escherichia coli. Water Air Soil Pollut. 2016, 227,146.[CrossRef] [PubMed] 13. Wolny-Koladka, K.;Zdaniewicz, M. Antibiotic resistance of Escherichia coli isolated from processing of brewery waste with theaddition of bulking agents. Sustainability 2021, 13,10174. [CrossRef] 14. Gama-Rodrigues, A.C. Soil organic matter, nutrient cycling, and biological dinitrogen-fixation in agroforestry systems. Agrofor.Syst. 2011, 81,191-193. [CrossRef] 15. Ramirez-Guizar, S.; Sykes, H.;Perry, J.D.; Schwalbe,E.C.; Stanforth, S.P.; Perez-Perez, C.I.; Dean, J.R. A chromatographic approachto distinguish Gram-positive from Gram-negative bacteria using exogenous volatile organic compound metabolites. J. Chromatogr.A 2017, 1501,79-88.[CrossRef] 16. Brown, R.W.; Bull, I.D.; Journeaux, T.; Chadwick, D.R.; Jones, D.L. Volatile organic compounds (VOCs) allow sensitive differentia-tion of biological soil quality. Soil Biol. Biochem. 2021,156,108187. [CrossRef] 17. Orlandini, V.; Maida, I.; Fondi, M.; Perrin, E.; Papaleo, M.C.; Bosi, E.; Pascale, D.; Tutino, M.L.;Michaud, L.; Giudice, A.L.; et al.Genomic analysis of three sponge associated Arthrobacter Antactic strains, inhibiting the growth of Burkholderia cepacian complexbacteria by synthesizing volatile organic compounds. Microbiol. Res. 2014, 169,593-601. [CrossRef] 18. Lopez, S.M.Y.; Pastorino, G.N.; Balatti, P.A. Volatile organic compounds profile synthesized and released by endophytes of tomato(Solanum lycopersici L.) and their antagonistic role. Arch. Microbiol. 2021, 203, 1383-1397. [CrossRef] 19. Sanon, A.; Andrianjaka, Z.N.; Prin, Y.; Thioulouse, B.J.; Comte, G.; Duponnois, R. Rhizosphere microbiota interferes withplant-plant interactions. Plant Soil 2009,321,259-278. [CrossRef] 20. Bhattacharyya, D.; Lee, Y.H. The bacterial community in the rhizosphere of Kimchi cabbage restructured by volatile compoundsemitted from rhizobacterium Proteus vulgaris JBLS202. Appl. Soil Ecol. 2016,105,48-56.[CrossRef] 21. Fincheira, P.;Quiroz, A.; Tortella, G.; Diez, M.C.; Rubilar, O. Current advances in plant-microbe communication via volatileorganic compounds as an innovative strategy to improve plant growth. Microbiol. Res. 2021, 247, 126726.[CrossRef] [PubMed] 22. Pino-Otin, R.M.; Muniz, S.; Val, J; Navarro, E. Effects of 18 pharmaceuticals on the physiological diversity of edaphic microorgan-isms. Sci. Total Environ. 2017, 595,441-450. [CrossRef] [PubMed] 23. Wang, L.; Zhang, W.; Wang, J; Zhu, L.; Wang, J; Yan, S.; Ahmad, Z. Toxicity of enrofloxacin and cadmium alone and incombination to enzymatic activities and microbial community structure in soil. Environ. Geochem. Health 2019,41,2593-2606.[CrossRef] [PubMed] 24. Liu, F; Wu, J.; Ying, G.G.; Luo, Z.; Feng, H. Changes in functional diversity of soil microbial community with addition ofantibiotics sulfamethoxazole and chlortetracycline. Appl. Microbiol. Biotechnol. 2012,95,1615-1623.[CrossRef][PubMed] 25. Ramires, P.F.; Tavella, A.R.; Escarrone, L.M.; Volcao, L.M.; Honscha, L.C.; Brum, R.L.; Silva, A.B.; Silva, F.M.R.,Jr. Ecotoxicity oftriclosan in soil: An approach using different species. Environ. Sci. Pollut. Res. 2021, 28, 41233-41241.[CrossRef] 26. Chessa, L.; Pusino, A.; Garau, G.; Mangia, N.P.; Pinna, M.V. Soil microbial response to tetracycline in two different soils amendedwith cow manure. Environ. Sci. Pollut. Res. 2016,23,5807-5817. [CrossRef] 27. Kovacs, E.D.; Silaghi-Dumitrescu, L.; Kovacs, M.H.; Roman, C. Determination of the uptake of ibuprofen, ketoprofen, anddiclofenac by tomatoes, radishes, and lettuce by gas chromatography-mass spectrometry (GC-MS). Anal. Lett. 2021,54,314-330.ICrossRef 28. Blight, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37,911-917.ICrossRef 29. Frostegard, A.; Tunlid, A.; Baath, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621-1625.ICrossRefl 30. Joergensen, R.G.; Wichern, F. Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biol. Biochem.2008,40,2977-2991.[CrossRef] 31. Veuger, B.; Pitcher, A.; Schouten, S.; Sinninghe Damste,J.S.; Middelburg,J.J. Nitrification and growth of autotrophic nitrifyingbacteria and Thaumarchaeota in the costal North Sea. Biogeoscience 2013,10,1775-1785. [CrossRef] 32. Moulineux, C.J.; Gange, A.C.; Connop, S.P.;Newport, D.J. Are microbial communities in green roof substrates comparable tothose in post-industrial sites?-A preliminary study. Urban Ecosyst. 2015,18,1245-1260.[CrossRef] 33. Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil.Soils 1996, 22,59-65. [CrossRef 34. Lindahl, V.; Bakken, L.R. Evaluation of methods for extraction of bacteria from soil. FEMS Microbiol. Ecol. 1995, 16,135-142.[CrossRef| 35. Sofo, A.; Ricciuti, P. A standardized method for estimating the functional diversity of soil bacterial community by BiologEcoPlatesTM assay-The case study of a sustainable olive orchard. Appl. Sci. 2019,9,4035. [CrossRef] 36. Khan, A.; Mathelier, A. Intervene: A tool for intersection and visualization of multiple gene genomic region sets. BMC Bioinform.2017,18,287. [CrossRef][PubMed] 37. Aguilar-Romero,I.; Romero, E.; Wittich, R.M.; Dillewijn, P. Bacterial ecotoxicity and shifts in bacterial communities associatedwith the removal of ibuprofen, diclofenac and triclosan in biopurification systems. Sci. Total Environ. 2020, 741, 140461. [CrossRef] 38. Frkova, Z.; Vystavna, Y.; Koubova, A.;Grabicova, K.; Grabic,R.; Kodesova,R.; Chronakova, A. Microbial response to selectedpharmaceuticals in agricultural soils: Microcosm study on the roles of soil, treatment, and time. Soil Biol. Biochem. 2020, 149,107924. [CrossRef] 39. Dai,L.; Wu, T.; Xiong, Y.;Ni, H.; Ding, Y.; Zhang, W.; Chu, S.; Ju, S.; Yu, J. Ibuprofen-mediated potential inhibition of biofilmdevelopment and quorum sensing in Pseudomonas aeruginosa. Life Sci. 2019,237,116947.[CrossRef] 40. Oliviera, I.M.; Borges, A.; Borges,F; Simoes, M. Repurposing ibuprofen to control Staphylococcus aureus biofilm. Eur. J. Med. Chem.2019,166,197-205. [CrossRef] 41. Paje, M.L.F.; Kuhlicke, U.; Winkler, M.; Neu, T.R. Inhibition of lotic biofilms by diclofenac. Appl. Microbiol. Biotechnol. 2002,59,488-492. [PubMed] 42. Aleanizy, F.S.; Alqahtani,F.Y.; Eltayb,E.K.; Alrumikan,N.; Almebki,R.; Alhossan, A.; Almangour, T.A.; AlQahtani, H. Evaluatingthe effects of antibiotics sub-inhibitory dose on Pseudomonas aeruginosa quorum sensing dependent virulence and its phenotypes.Saudi J. Biol. Sci. 2021,28,550-559. [CrossRef] [PubMed] 43. Dastidar, S.G.; Ganguly, K.; Chaudhuri, K.; Chakrabarty, A.N. The anti-bacterial action of diclofenac shown by inhibition of DNAsynthesis. Int. J. Antimicrob. Agents 2000, 14, 249-251.[CrossRef]
确定












还剩14页未读,是否继续阅读?
产品配置单
中国格哈特为您提供《根际土壤的振荡提取》,该方案主要用于土壤中生物检测,参考标准--,《根际土壤的振荡提取》用到的仪器有格哈特强力高重现振荡器LS500/RO500、德国加液器MM
相关方案
更多
该厂商其他方案
更多











