方案详情
文
1.0 SCOPE AND APPLICATION
2.0 METHOD SUMMARY
3.0 SAMPLE PRESERVATION, CONTAINERS, HANDLING AND STORAGE
3.1 Sample Storage
3.2 Holding Times
4.0 INTERFERENCES AND POTENTIAL PROBLEMS
5.0 EQUIPMENT/APPARATUS
6.0 REAGENTS
7.0 PROCEDURES
方案详情

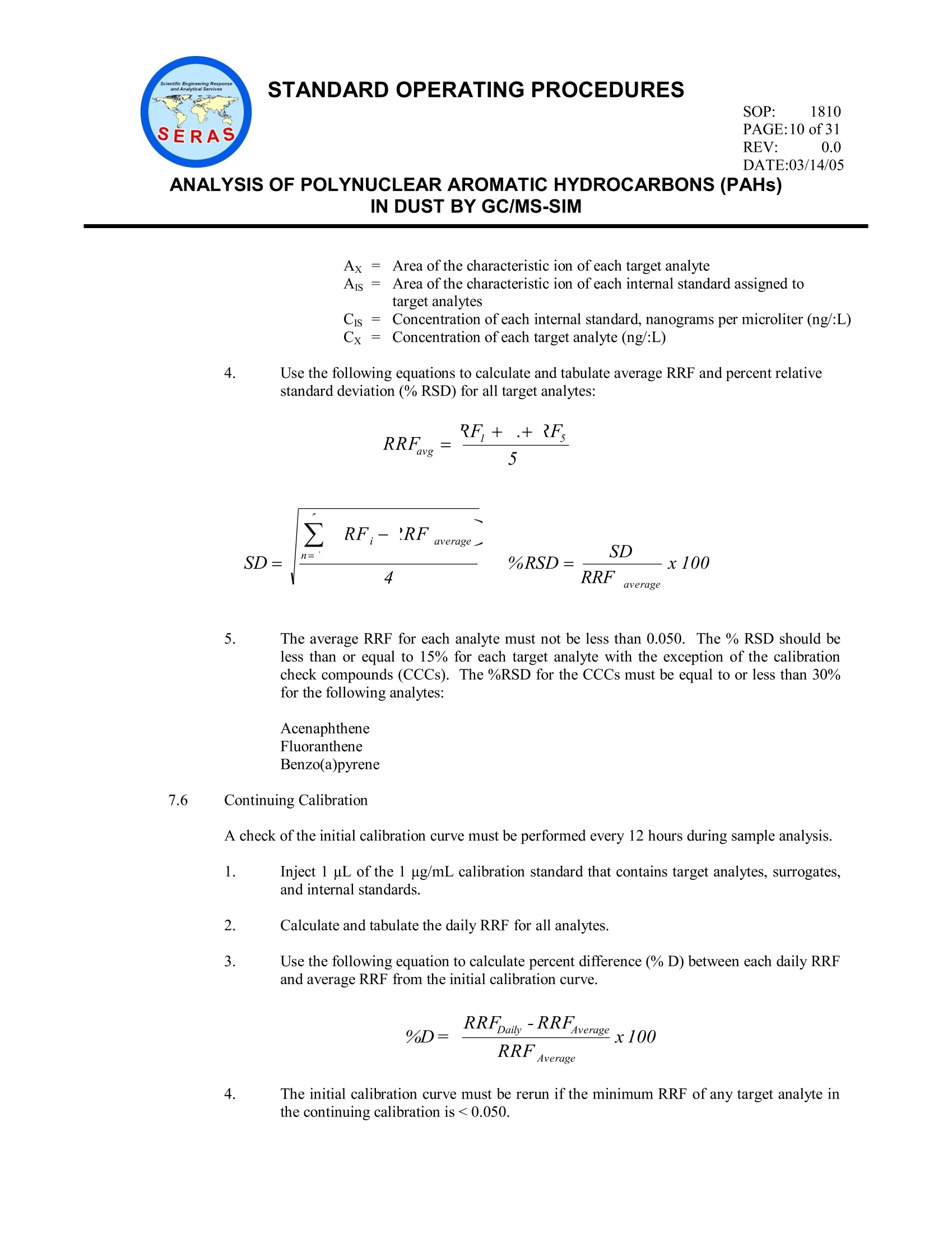
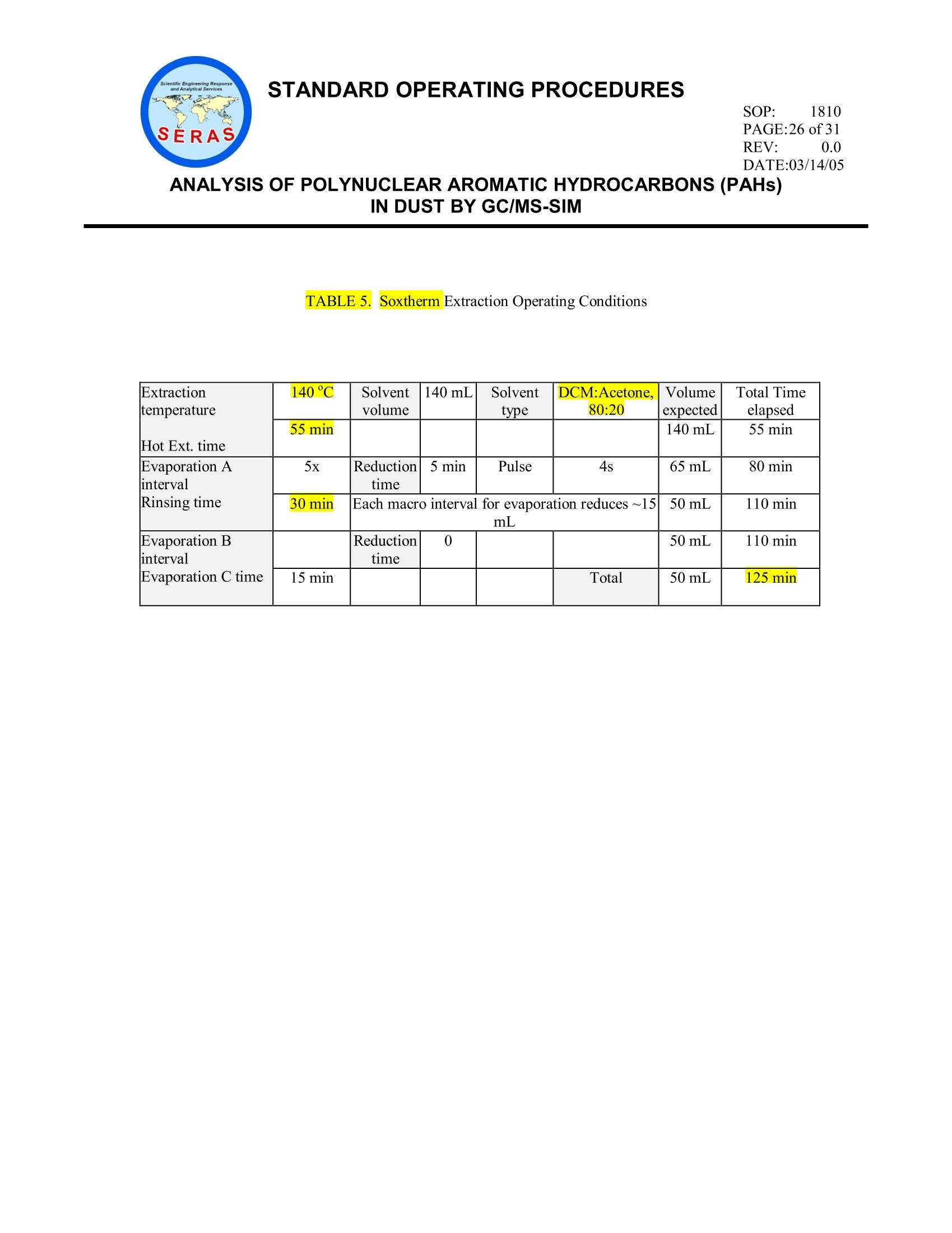
STANDARD OPERATING PROCEDURESSOP: 1810PAGE: 1 of31REV: 0.0DATE:03/14/05气相色谱/质谱-SIM法分析粉尘中的多环芳烃(PAHs) STANDARD OPERATING PROCEDURESREV: 0.0DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATICHYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM CONTENTS 1.0 SCOPE AND APPLICATION 2.0 METHOD SUMMARY 3.0 SAMPLE PRESERVATION, CONTAINERS, HANDLING AND STORAGE 3.1 Sample Storage 3.2 Holding Times 4.0 INTERFERENCES AND POTENTIAL PROBLEMS 5.0 EQUIPMENT/APPARATUS 6.0 REAGENTS 7.0 PROCEDURES 7.1 Sample Preparation 7.1.1 Sample Sieving Procedure 7.1.2 Soxtherm Extraction 7.1.3 TurboVap Concentration 7.2 Total Solids 7.3 GC/MS Operating Conditions 7.4 DFTPP Tune 7.5 Initial Calibration 7.6 Continuing Calibration 7.7 Sample Analysis 7.8 Identification of Target Analytes) 8.0 CALCULATIONS 8.1 Target Compounds 8.2 Surrogate Spike Recoveries 8.3 Matrix Spike Recoveries 8.4 Laboratory Control Sample SOP: 1810 PAGE: 2 of31 ANALYSIS OF POLYNUCLEAR AROMATICHYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM CONTENTS (cont) 9.0 QUALITY ASSURANCE/ QUALITY CONTROL 9.1 DFTPP Tune 9.2 Initial Calibration for Target Compounds and Surrogates 9.3 Continuing Calibration for Target Compounds and Surrogates 9.4 Internal Standard Responses and Retention Times 9.5 Method Blank Analysis 9.6 Solvent Blank 9.7 Surrogate Recoveries 9.8 Matrix Spike and Matrix Spike Duplicate Analysis 9.9 Laboratory Control Sample Analysis 9.10 Dilution Analysis 9.11 Manual Integrations 9.12 Initial Demonstration of Capability 9.13 Method Detection Limit Studies 10.0 DATA VALIDATION 11.0 HEALTH AND SAFETY 12.0 REFERENCES 13.0 APPENDICES A -Tables B-Sieve Cleaning Procedure SOP: 1810 PAGE: 3 of 31 REV: 0.0 DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATIC HYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM 1.0 SCOPE AND APPLICATION This Standard Operating Procedure (SOP) outlines the preparation and analysis of polynuclear aromatichydrocarbons (PAHs) in dust matrices using gas chromatography/mass spectrometry (GC/MS) in the selection monitoring (SIM) mode to achieve lower method detection limits (MDLs). The compounds of interestanalyzed and the corresponding reporting limits (RLs) are included in Table 1, Appendix A. 2.0 METHOD SUMMARY The dust sample is passed through a 100-mesh sieve prior to analysis. Approximately 0.5 gram (g) of thesieved sample is extracted with 140 milliliters (mL) of methylene chloride/acetone (80:20) in a Soxthermextractor. The extracted solution is subsequently concentrated to 0.5 mL. The final extract is spiked with an internal standard mix and analyzed using GC/MS in the SIM mode.Target analytes are identified by comparing the measured mass spectra and retention times with thoseobtained from calibration standards acquired under the same operating conditions used for the samples.Quantitation of each identified target compound is calculated based on the internal standard method. Table2, Appendix A lists the characteristic ions of each target analyte and Table 3, Appendix A lists the internalstandards with the corresponding target analytes assigned for quantitation. 3.0 SAMPLE PRESERVATION. CONTAINERS, HANDLING AND STORAGE 3. Sample Storage Samples may be collected in plastic ziplock bags or in wide mouth glass containers with a Teflon-lined cap. From the time of collection until after analysis, extracts and unused samples must beprotected from light. They must be refrigerated at 4±2 degrees Centigrade (℃) for the periodsspecified by Task Leader and/or Work Assignment Manager (WAM) of the project. Samples, sample extracts, and standards must be stored separately in an atmosphere free of allpotential contaminants. 3.2 Holding Times Extraction of samples shall be completed within 14 days from date of collection, and analysiscompleted within 40 days after sample extraction. 4.0 INTERFERENCES AND POTENTIAL PROBLEMS Method interferences may be caused by contaminants in solvents, reagents, glassware and other sampleprocessing hardware that lead to discrete artifacts and/or elevated baselines in the total ion current profiles.All of these materials must be demonstrated to be free from interferences under the conditions of theanalysis by running laboratory reagent blanks on a routine basis. Matrix interferences may be caused bycontaminants that are co-extracted from the sample. The extent of matrix interferences will varyconsiderably from source to source. 5.0 EQUIPMENT/APPARATUS The following equipment/apparatus is required: SOP: 1810 PAGE: 4 of31 ANALYSIS OF POLYNUCLEAR AROMATIC HYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM Soxthermextractor, including extraction flask, sample holding vessel, chiller, manufactured byGerhardt or equivalent Cellulose thimbles, commercially available Analytical balance, capable of accurately weighing "0.001 g Class "S" weights for calibrating balance Spatula, stainless steel or Teflon Clear sampling jar, 4 and 8 ounce (oz) Pyrex glass wool, baked at 400EC for at least 4 hours .Test tube rack Teflon boiling chips, approximately 10/40 mesh. lHeat to 400EC for 30 minutes or Soxhlet extractwith methylene chloride Volumetric flasks, Class A, 50 mL, 25 mL, 10 mL Desiccator with indicating desiccant Disposable glass Pasteur pipettes TurboVap concentrator, with six positions for concentrating extracts. TurboVap concentrator cells, 200 mL TurboVap concentrator cell rack Clean Bath solution, for use in TurboVap concentrator GC autosampler glass vials with crimp caps, 2 mL Agilent Technologies 6890 gas chromatograph (GC) and 5972/5973 mass selective detector (MSD) orequivalent, equipped with an autosampler and controlled by Enviroquant (or equivalent) software Restek Rtx-5 fused silica capillary column, 30 meter (m) x 0.25 millimeter (mm) inner diameter (ID),0.5 micron (um) film thickness (or equivalent) Syringes, 500 microliters (uL) 100-Mesh Sieve, 150 um, brass or stainless steel, meeting American Society for Testing and Materials(ASTM) specifications E11 6.0 REAGENTS SOP: 1810 PAGE: 5 of31 REV: 0.0 DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATIC HYDROCARBONS(PAHs)IN DUST BY GC/MS-SIM Sodium Sulfate, anhydrous powder, reagent grade, heated at 400C for four hours, cooled in adesiccator and stored in a glass bottle Methylene Chloride, pesticide residue analysis grade or equivalent Acetone-pesticide residue analysis grade or equivalent PAH Stock Calibration Standard, custom mix, consisting of each of the target analytes in 50:50benzene/methylene chloride at 1000 micrograms per milliliter (ug/mL) (Table 1, Appendix A) PAH Intermediate Calibration Standard, 10 ug/mL- Add 100 uL of the 1000 ug/mL to methylenechloride in a 10 mL Class A volumetric flask. Dilute to the mark with methylene chloride. PAH Working Calibration Standards - Using a serial dilution technique, prepare the other fourstandards from the 10 pg/mL intermediate standard at concentrations of 0.1, 0.5, 1.0 and 5.0 pg/mL inmethylene chloride. Alternatively, calibration standards at the five concentration levels may be purchased from an outsidevendor, if available. Surrogate Stock Solution, 1000 pg/mL, containing nitrobenzene-ds, 2-fluorobiphenyl and terphenyl-d14in methylene chloride, commercially available (Supelco #4-8925 or equivalent) Surrogate Working Solution, 2 ug/mL - Add 50 pL of the 1000 ug/mL surrogate stock solution to a25-mL Class A volumetric flask containing methylene chloride. Dilute to the mark with methylenechloride. Matrix Spike (MS) Stock Solution, containing acenaphthene and pyrene each at 1000 ug/mL inmethylene chloride, commercially available (Supelco #4-8869 or equivalent). This solution must be adifferent source from that used for calibration. MS Working Solution, 2 ug/mL-Add 50 pL of the 1000 :g/mL stock MS solution to methylenechloride in a25-mL Class A volumetric flask. Dilute to the mark with methylene chloride.Alternatively, a MS stockssolution containing acenaphthene and pyrene at 100 pg/mL may bepurchased (AccuStandard or Ultra Scientific) and diluted accordingly. Internal Standard Stock Mix, 2000 pg/mL, consisting of six internal standards in methylene chloride(1,4-dichlorobenzene-d4, naphthalene-dg,, acenaphthene-d10, phenanthrene-d1o,,chrysene-d12dandperylene-d12),commercially available (Supelco #4-8902 or equivalent). Internal Standard Working Solution, 1000 pg/mL-Make a 1:1 mixture of the internal stock standardmix in methylene chloride. Add oneuL of this solution to each 0.5-mL sample extract. The amount ofeach internal standard in each 1 uL injection will be 2 nanograms (ng). Decafluorotriphenylphosphine (DFTPP), 50 pg/mL, commercially available in methylene chloride(Supelco #4-7387 or equivalent). The amount of DFTPP in a 1-uL injection is 50 ng. NOTE: All calibration standard, internal standard, surrogate and spiking solution preparation will SOP: 1810 PAGE: 6of31 REV: 0.0 DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATIC HYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM be documented in accordance with Scientific, Engineering, Response and Analytical Services(SERAS) SOP #1012, Preparation of Standard Solutions and Reagents. NOTE: Store the working solutions at 4℃ (±2℃) in Teflon-sealed containers. 'The solutionsshould be checked frequently for stability. These solutions must be replaced every 6 months orsooner, if comparison with quality control (QC)check samples indicates a problem. Premixed pre-certified stock standards will be stored according to the manufacturer’s documented storagerequirements. These standards may be kept in storage up to the manufacturer’s stated expirationdate. Once the stock surrogate solutions are opened, the standards will be stored for a period notto exceed six months from the date opened or the original expiration date, whichever is less. 7.0 PROCEDURES 7.1 Sample Preparation 7.1.1 Sample Sieving Procedure If the sample has not been sieved, use the following procedure. If the sample is alreadysieved, proceed to step 7.1.2. Clean the sieve using the procedure in Appendix B. Select a clean working area in a facility equipped with a fume hood (a 4-foot by 4-foot area is sufficient). Weigh the receiver pan on an analytical balance and recordthe weight. Empty the entire contents of the sample into the 100-mesh sieve with the receiverpan attached. Place the cover on the sieve and manually or mechanically shake the sieve for aminimum of 5 minutes and a maximum of 10 minutes until all the fine dust particlesare collected in the bottom receiver pan. If manual shaking is performed, follow theinstructions given in ASTM D-422: “Conduct the sieving operation by means of alateral and vertical motion of the sieve, accompanied by a jarring action in order tokeep the sample moving continuously over the surface of the sieve. Continuesieving until not more than 1 mass percent of the residue on a sieve passes thatsieve during 1 minute of sieving. If mechanical shaking is performed, set up the recommended sieve shaker on aneven and stable surface. Proceed with the sieving operation following directions inthe manufacturer’s manual. Re-weigh the receiver pan using an analytical balance. The difference in weight isthe weight of the sieved sample. If total weight of material is desired, the coarsematerial remaining on top of the sieve must be collected on a pre-weighed sheet ofaluminum foil, re-weighed and the weight added to the weight of the sieved sample. Transfer the sieved sample from the receiver pan to an 8-oz wide-mouth glass jar.Use a camel hair brush to ensure complete transfer of the sample. Cap the glass jar SOP: 1810 PAGE: 7 of31 REV: 0.0 DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATICHYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM securely. Before processing the next sample, thoroughly wipe clean the cover, sieve andreceiver pan using a Kimwipe and deionized/distilled water.r.Let dry prior tosieving additional samples. 7.1.2. Soxtherm FExtraction ● Calibrate the balance with class "S" weights prior to weighing samples or blanks.The balance should be calibrated with a weight that is similar to the weight used toextract the sample (i.e., 0.5 gram). 1W eWiegih approximately 0.5 g of each sieved sample to the nearest 0.001 g into a 4-ozsampling jar. The sample is then thoroughly mixed with 20 g of anhydrous granularsodium sulfate. The sample should have a sandy texture at this point. . A method blank is prepared using 20 g of baked sodium sulfate at the frequency ofone for every 20 samples using the same preparation technique as for the samples. Prepare a Laboratory Control Sample (LCS) by adding 0.25 mL of the MS workingsolution to 20 g of anhydrous sodium sulfate at the frequency of one for every 20samples using the same preparation technique as for the samples. Select a sample to be used for the matrix spike/matrix spike duplicate (MS/MSD).Weigh two additional 0.5 g portions of this sample to the nearest 0.001 g.TheMS/MSD is done with the frequency of one per 10 samples or ten percent of each.batch. NOTE: The sample to be used for the MS/MSD may be specified on the Chain ofCustody record. . Place the blended sample and sodium sulfate in each prewashed cellulose thimbleand insert the thimble into a Soxthermextraction flask containing one or two cleanboiling chips ● Add 0.25 mL of the surrogate working solution to method blanks, MS/MSD, and allsamples Add 0.25 mL of the MS working solution to each of the MS/MSD samples. In a fume hood, place 140 mL of 80:20 methylene chloride/acetone into the sampleholding vessel. ● Connect the cooling hose from the Soxtherm to the chiller. Care must be taken toensure that cooling is sufficient (e.g., the temperature of the chiller should not behigher than 6EC). iAttach each extraction flask to theSoxtherm extractor and extract the sample(s) forapproximately 2 hours. The extraction conditions are listed in Table 5, Appendix A. ANALYSIS OF POLYNUCLEAR AROMATIC HYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM Allow the extract to cool after the extraction is completed. 1Transfer the extract from Soxtherm to TurboVap tube. 7.1.3. TurboVap Concentration Fill the TurboVap water bath with approximately one gallon of deionized watermixed with 10-15 drops of Clean Bath solution.Set the water bath temperatureat 45℃. Transfer the extract to a 200-mL TurboVap tube in a fume hood outside theconcentrator. Rinse the Soxtherm extraction flask several times with methylenechloride and add solvent rinses to the Turbovap tube. Start concentrating theextract by blowing a gentle stream of nitrogen into the tube(s). Carefully adjustthe nitrogen stream so that solvent does not splash out of the tube. Periodically,rinse the inner wall of the tube with methylene chloride. Concentrate the extract until the solvent level drops to the stem of the tube. Thisis visible by looking straight down into the tube and the solvent remains in theinner circle of the tube. At this point, the extract volume is approaching 0.5 mLand should be monitored carefully until it reaches 0.5 mL so the extract does notevaporate to dryness. When removing a tube from the water bath, ensure thatwater droplets do not fall into other extract tubes. Transfer the extract into a 2-mL GC autosampler vial. The extract is now readyfor analysis. If the analysis is not performed immediately, the extract should beprotected from light and refrigerated at4℃ (±2℃). 7.2 Total Solids (Optional) The total solids of the sample is prepared in conjunction with the sample extraction. The totalsolids for the MS/MSD samples is based on the corresponding sample. The total solids of theblank is treated as 100 percent(%). Weigh and record an empty aluminum dish to the nearest 0.01 g. Weigh at least 10 g of a dustsample into the aluminum dish. The sample dish is heated in an oven set at 103 to 105℃ inside afume hood overnight. Remove the sample dish from the oven the following day and place in adesiccator to cool before re-weighing.Weight the residue and calculate the percent total solidsbased on the following equation: 7.3 GC/MS Operating Conditions The typical operating conditions used for analysis of standards, samples and blanks on theGC/MSD are listed below. Other conditions may be used as long as quality assurance (QA)/QC SOP: 1810 PAGE: 9 of 31 REV: 0.0 DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATIC HYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM and peak identification criteria are met. Any deviations from this SOP must be documented in thecase narrative. * May be extended to reduce carryover from samples that contain a high concentration of target ornon-target compounds. MSD SIM conditions are as follows: Group Number** Monitoring ions Dwell time 1(4.5) 54,82,102,108,115,128,136 and 152 20 msec 2(7.5) 76,152,153,154,162,164,165,166,171 and 172 20 msec 3(10.0) 101,122,176,178,188,189,202, and 244 20 msec 4(13.5) 120,114,228, and240 20 msec 5 (16.0) 126,132,138,139,252,264,276, and 278 20 msec ** Numbers in parentheses represent approximate start time msec = milliseconds 7.4 DFTPP Tune The instrument must be tuned by injecting 50 ng of DFTPP to meet the ion abundance criterialisted in Table 4, Appendix A.. The tune is acquired using either the apex or ± one scan.TTheDFTPP tune criteria must be met every 12 hours during sample analysis. 7.5 Initial Calibration 1. Add 1 uL of the internal standard working solution to each 0.5 mL aliquot of the fivecalibration standards. 2. After DFTPP passed the criteria, set up the run using the five-level calibration standards. 3. Use the following equation to calculate and tabulate relative response factor (RRF) of alltarget compounds and surrogates in all five calibration standards. The primary ions ofthe internal standard must be used for calculation. where: soP: 1810 PAGE:10 of31 REV: 0.0 DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATICHYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM Ax= Area of the characteristic ion of each target analyte Ais =AArea of the characteristic ion of each internal standard assigned totarget analytes Cis = CConcentration of each internal standard, nanograms per microliter (ng/:L) Cx = Concentration of each target analyte (ng/:L) 4. Use the following equations to calculate and tabulate average RRF and percent relativestandard deviation (%RSD) for all target analytes: 5. The average RRF for each analyte must not be less than 0.050. The % RSD should beless than or equal to 15% for each target analyte with the exception of the calibrationcheck compounds (CCCs). The %RSD for the CCCs must be equal to or less than 30%for the following analytes: Acenaphthene Fluoranthene Benzo(a)pyrene 7.6 Continuing Calibration A check of the initial calibration curve must be performed every 12 hours during sample analysis. 1. Inject 1 uL of the1 ug/mL calibration standard that contains target analytes, surrogates,and internal standards. 2. Calculate and tabulate the daily RRF for all analytes. 3. Use the following equation to calculate percent difference (%D) between each daily RRFand average RRF from the initial calibration curve. 4. The initial calibration curve must be rerun if the minimum RRF of any target analyte inthe continuing calibration is <0.050. SOP: 1810 REV: 0.0 DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATIC HYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM 5. Rerun the initial calibration curve if the %D of any quantitated target compound or anyMS compound (acenaphthene and/or pyrene) exceed 20%. 7.7 Sample Analysis Sample extracts may be analyzed only after the GC/MS has met the requirements of DFTPP,initial calibration, and continuing calibration as described above. The operating conditions usedfor calibration standards must be employed for analysis of samples. 1. Add 1 pL of the internal standard working solution into each method blank, MS/MSD, and allsample extracts. 2. Inject 1 uL ofeach sample, method blank or MS/MSD extract into the GC/MS. 3. If the response of any analyte exceeds that of the highest calibration standard (i.e., 10ug/mL),the sample extract must be diluted so that the analyte response falls within the linear rangeestablished in the initial calibration. Ideally, the concentration of the analyte should fallbetween midrange and the upper portion of the curve after dilution. 4. If a dilution is prepared, the internal standard mix is added to the diluted sample to maintainthe required concentration of 2 ng/uL of each internal standard in the diluted extract. 7.8 Identification of Target Analytes The target analytes are identified by comparison of the SIM sample mass spectra with the SIMmass spectra of a calibration standard. Two criteria must be satisfied to verify the identifications: Elution ofthe sample component at the GC relative retention time (RRT) as the standardcomponent Correspondence of the sample component and standard component mass spectra 1. For establishing correspondence of the RRT, the sample component RRT must comparewithin ± 0.06 RRT units of the RRT of the standard component. For reference, the standardmust be run on the same 12-hour clock as the sample. If co-elution of interfering componentsprohibits; accurateaassignment of the sample componentRRT from the total]ionchromatogram, the RRT should be assigned by using extracted ion current profiles for ionsunique to the component of interest. 2. For comparison of standard and sample components, reference mass spectra must be obtainedfrom the 1 :g/mL calibration standard. The standard mass spectra may be obtained from therun used to obtain the reference RRTs. In the case of co-elution of standard components, theanalyst can use professional judgment to establish the presence of target analytes. Ifprofessional judgment is used, it will be documented in the case narrative. 3. The requirements for qualitative verification of mass spectra are as follows: a. All secondary ions present in the standard mass spectra (most abundant ion equals 100%) DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATIC HYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM must be present in the sample mass spectra b. The relative intensities of secondary ions specified in (a) must agree within "20%between the standard and sample spectra. For example, if an ion with an abundance of50% in the standard spectra, the corresponding sample ion abundance must be between30-70%. 4. If a analyte cannot be verified by all of the criteria in Step 3 but is identified by the technicaljudgment of the mass spectral interpretation specialist, the analyst will report thatidentification and proceed with the calculation described in Section 8.0. The analyst mustreport in the case narrative that the technical judgment was utilized. 8.0 CALCULATIONS 8.1 Target Compounds Identified target compounds must be quantitated by the internal standard method. The internalstandard used must be the one nearest the retention time to that of the given analyte listed in Table3, Appendix A.. The area of the characteristic ion of each target analyte listed in Table 2,Appendix A is used for quantitation. Use the following equation to calculate the concentration of the identified analytes using the dailyRRF obtained from the continuing calibration curve in Section 7.6. If samples are analyzed afterthe initial calibration, the average RRF must be used. where: A=Area of the characteristic ion of each target analyteA FAmount of each internal standard injected (ng) Volume of the concentrated extract (mL) Dilution factor Area of the characteristic ion of each internal standard RRF=Relative response factor Weight of dust extracted, kilograms (kg) Decimal percent solid V; Injection volume (usually 1pL) When the concentration of any identified target analyte is below the reporting limit but the massspectrum meets the identification criteria, report the concentration by flagging the results with "J".All target analyte concentrations are reported to three significant figures. 8.2 Surrogate Spike Recoveries Calculate surrogate standard recovery on all samples, blanks, and spikes by the followingequation: ANALYSIS OF POLYNUCLEAR AROMATICHYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM Percent Recovery(%R)=2Dx100 where: Qp = Quantity determined by analysis QAA= Quantity added to sample 8.3 Matrix Spike Recoveries Matrix spike recoveries and the relative percent difference (RPD) between the recoveries of thetwo analytes in MS/MSD will be calculated by the following equations: where: SSR =Spike sample result SR =Sample result SA =Spike added and where: RPD Relative percent difference MSR Matrix spike recovery MSDR = Matrix spike duplicate recovery Note: RPD is always expressed as a positive value. 8.4 Laboratory Control Sample SSR- R\ Laboratory Control Sample Re coveryR=- 1x100SA where: SSR = Spike sampleresult = Sample result Spike added 9.0 QUALITY ASSURANCE/ QUALITY CONTROL 9.1 DFTPP Tune REV: 0.0 DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATIC HYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM The GC/MS must meet the ion abundance tune criteria specified in Table 4, Appendix A, beforeinitiating acquisition activities involving samples, blanks, or standards. The tune ensures correctmass calibration, mass resolution, and mass transmission. It must be performed every 12 hoursduring analysis. 9.2 Initial Calibration for Target Compounds and Surrogates Once the tune criteria has been met, the GC/MS system must be initially calibrated using aminimum of five concentrations (0.1, 0.5, 1.0,5.0, and 10ug/mL) to determine the linear responseof target compounds and surrogates. The initial calibration of the GC/MS is evaluated according to the magnitude and stability of therelative response factors of (RRF) of each target analyte and surrogate. The minimum RRF ofeach compound at all five levels must be equal to or greater than 0.050. The %RSD should be lessthan or equal to 15% for each target compound with the exception of the CCCs. The %RSD forthe CCCs must be equal to or less than 30% for the following compounds: Acenaphthene Fluoranthene Benzo(a)pyrene If the RSDs exceed criteria, then linearity through the origin cannot be assumed..A linearregression analysis, plot not forced through“zero”, may be used to calculate concentrations usingarea counts on the “y”axis as the dependent variable versus concentrations on the“x”axis as theindependent variable. The correlation coefficient (r) must be $0.99. NOTE: All initial calibration standards must be analyzed prior to the analysis of any methodblanks, QC samples or environmental samples. 9.3 Continuing Calibration for Target Compounds and Surrogates After 12 hours of sample acquisition have passed, the GC/MS must be re-tuned using DFTPP andthe initial calibration curve verified by the mid-level calibration standard. 1. The DFTPP tune must pass the criteria in Table 4, Appendix A. 2. The 1 pg/mL calibration standard must be used for the continuing calibration. 3. The continuing calibration of the GC/MS is evaluated based on the magnitude of RRFand %D between the average RRF of each compound from the initial calibration and theRRF of that compound in the continuing calibration standard. The minimum RRF of eachtarget analytes in the continuing calibration must be equal to or greater than 0.050. The%D for each CCC must not exceed 20%. For any target compounds present in the sampleat a concentration greater than RL, those compounds must meet the minimum RRF of0.050 and the %D criteria of #20%. 4. The quant ion area for each internal standard in the continuing calibration must bebetween 50% and 200% of the respective internal standard quant ion area in the mid- SOP: 1810 PAGE:15 of31 REV: 0.0 DATE:03/14/05 ANALYSIS OF POLYNUCLEAR AROMATICHYDROCARBONS (PAHs)IN DUST BY GC/MS-SIM point standard of the current initial calibration. If this criterion is not met, reanalysis isrequired. 5. A maximum of two continuing calibrations may be run to meet the requirements in item3 above. A new calibration curve must be reanalyzed if both continuing calibrations areunacceptable. NOTE: It is not acceptable to use the first continuing calibration if the second continuingcalibration is out. 6. If any of the requirements listed in Step 3 are not met, notify the Organic Group Leaderand/or Analytical Section Leader. 9.4 Internal Standard Responses and Retention Times The response of each of internal standard in all calibration standards, samples, and blanks iscrucial for obtaining reliable analytical results because the quantitative determination of targetanalytes is based on the area of each internal standard. 1. The amount of each internal standard in a 1 pL injection of sample extract must be 2 ng. 2. The response and the retention time of each internal standard are evaluated for stability.The area of each internal standard in a sample must not vary by more than a factor of 2(i.e., -50% to +100%) from the area of the same internal standard in the continuingcalibration standard.In addition, the retention time of each internal standard must bewithin "0.50 minutes (30 seconds) of its retention time in the continuing calibrationstandard. 3. The area of each internal standard at 1 :g/mL calibration standard must be used forevaluation. 4. The response of each internal standard in all samples, blanks and spikes must betabulated. If an internal standard area is outside the QC limits, the extract must bereanalyzed to confirm a matrix effect or to determine if it was within the laboratory’scontrol. If the reanalysis is within QC limits, report only the reanalysis if within the 40-day analysis holding time. If reanalysis confirms matrix effects, submit both sets of databut report the initial run. 9.5 Method Blank Analysis A method blank is a known weight of sodium sulfate that is carried through the entire analyticalprocedure. The weight of the sodium sulfate is approximately equal to that added to the dustsamples. The purpose of a method blank is to determine the level of contaminations associatedwith preparation and analysis of samples. 1. One method blank must be prepared for each batch of 20 samples. 2. A method blank must not contain target compounds at concentrations
确定



























还剩27页未读,是否继续阅读?
中国格哈特为您提供《粉尘中多环芳烃(PAHs)检测方案(快速溶剂萃取)》,该方案主要用于其他中有机污染物检测,参考标准--,《粉尘中多环芳烃(PAHs)检测方案(快速溶剂萃取)》用到的仪器有格哈特全自动快速溶剂萃取仪Sox416、德国移液器MM
该厂商其他方案
更多











