方案详情
文
通过微波照射,设计并制备了一种新型的类固醇衍生的席夫碱化学传感器,即N-(2-羟基亚苄基)−?-羟基-胆烷酰肼(LA)。该传感器在乙醇/水(1:2, v/v)溶液中,在6.05至9.32的pH值范围内,对Al3+,显示出高选择性的荧光感应,检测限为34 nM。通过Job's plot确定LA和Al3+ 之间的结合化学计量为1:1,并通过1 HNMR研究进一步验证。在紫外灯下,荧光颜色的变化很容易被肉眼发现。此外,该传感器LA已被应用于检测实际水样中的Al3+ 。
方案详情

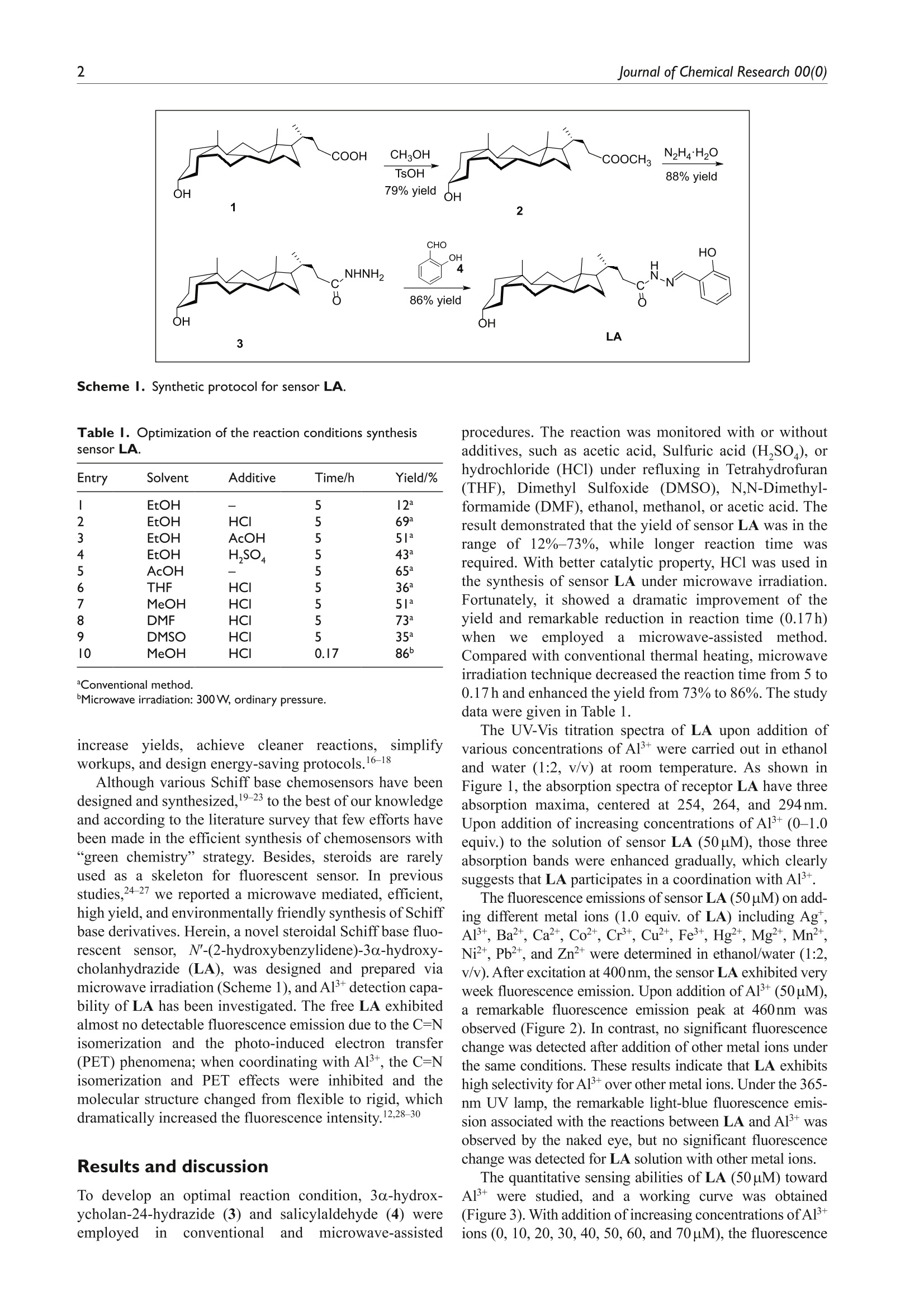
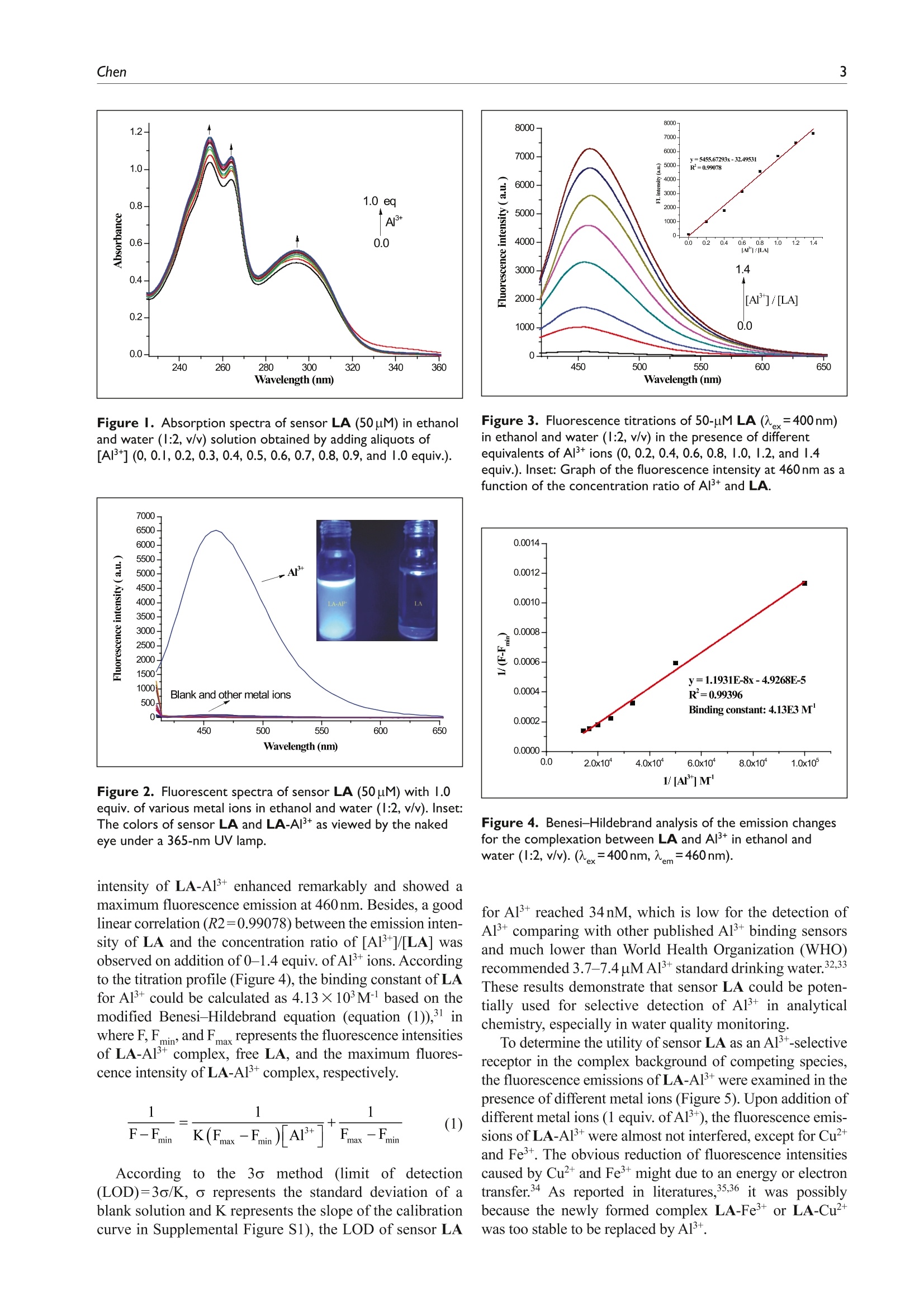
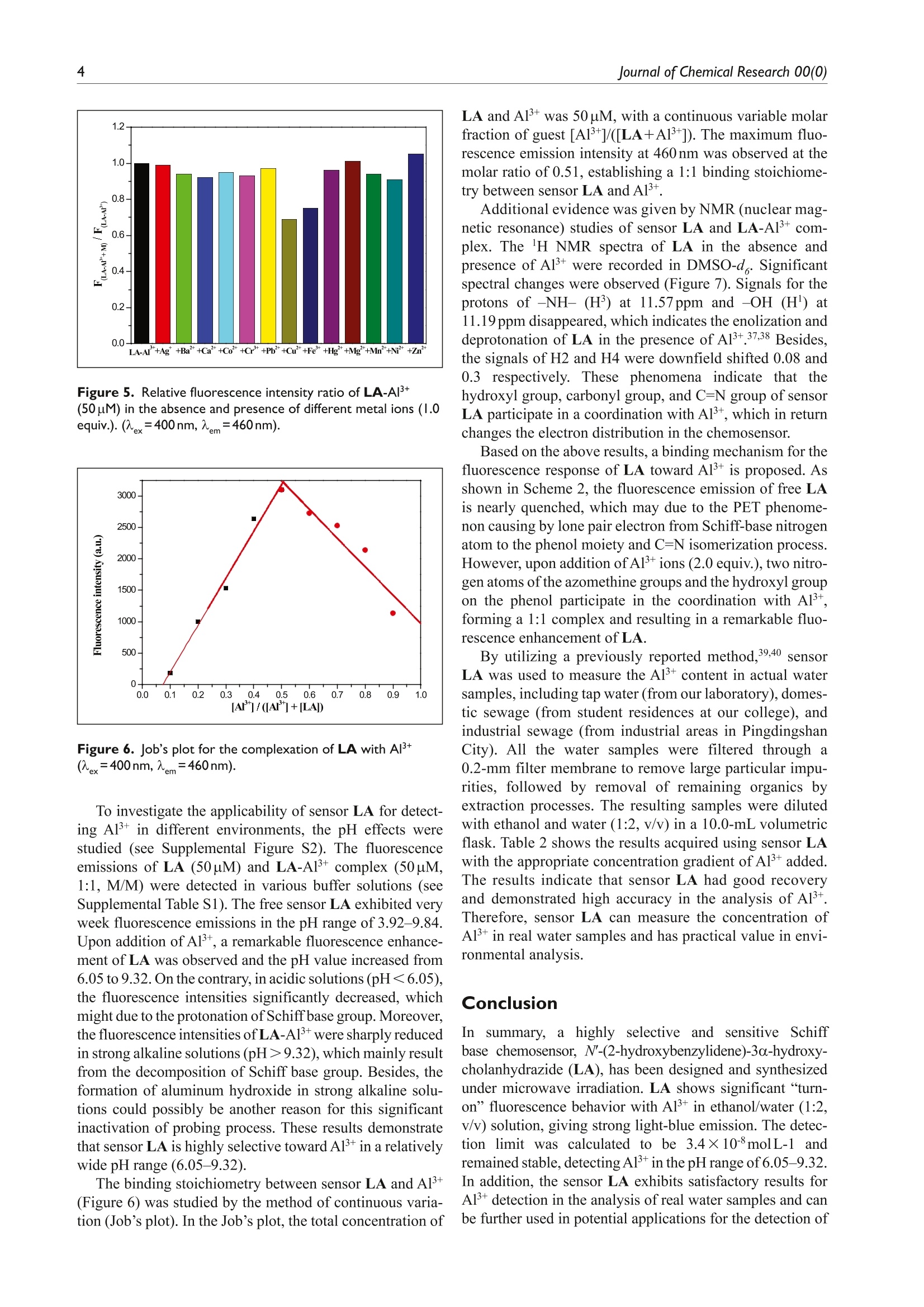
(Check for updateslournal ofChemicalResearchResearch Paper 2Journal of Chemical Research 00(0) lournal of Chemical Research Microwave-assisted synthesis of a novelsteroid-derived Schiff base chemosensorfor detection of Al’+in aqueous media 1-6@ The Author(s) 2020Article reuse guidelines:sagepub.com/journals-permissionsDOI: 10.1177/1747519820914827 journals.sagepub.com/home/chl OSAGE Yu CheniD Abstract A novel steroid-derived Schiff base chemosensor, N'-(2-hydroxybenzylidene)-3o-hydroxy-cholanhydrazide (LA), hasbeen designed and prepared via microwave irradiation. The sensor LA showed highly selective fluorescent sensing forAl+with a low detection limit of 34nM in the pH range from 6.05 to 9.32 in ethanol/water (1:2, v/v) solution. Thebinding stoichiometry between LA and Al’+ was determined as l:1 by Job’s plot and further verified with 'HNMRstudies. Under a UV lamp, the fluorescence color changes could be easily detected by the naked eye. In addition, thesensor LA has been applied in detection of Al’+ in real water samples. Keywords aluminum(III) ions, chemosensor, microwave irradiation, Schiff base, steroid Date received:25 November 2019;accepted: 3 March 2020 RESEARCHPAPER The C=N isomerization and PET inhibited Aluminum is the most abundant metal in the earth’s crust.forms approximately 8% of earth mass. Aluminum hasbeen widely used in modern life, such as water treatmentplants, food additives, textiles, and pharmaceuticals.However, with the rapid development of aluminum indus-try, the content of aluminum in natural water and soil isgradually increasing. As we all know, aluminum is not abiologically essential element, and excessive accumulationof Alwould be toxic and cause many serious diseases,such as neuronal disorder, loss of memory, listlessness,and Alzheimer’s disease.34Therefore, the design and syn-thesis of chemosensors with high selectivity and excellentsensitivity in detecting aluminum is of great significance. Schiff bases are good ligands for metal ions, with whichthey can form strong coordinate bonds. This characteristichas resulted in their extensive use in the design and synthe-sis of chemosensors.5-9 Two possible mechanisms that havebeen reported, the C=N isomerization and the suppression of this isomerization, are responsible for fluorescence emis-sion behavior of the Schiff bases.10-12 Binding with metalions, the C=N isomerization would be inhibited and pro-vides a significant change in the fluorescence emission,which is a crucial advantage in practical applications. “Green chemistry"is the frontier of international chem-ical science research today and has received widespreadconcern and attention from governments, business, andchemical circles around the world. As a new technology inline with the concept of “green chemistry,”microwaveirradiation has attracted considerable interest in recentyears.14,15 Microwave-assisted organic synthesis5 Iis avaluable technology that helps reduce reaction times, Pingdingshan Polytechnic College, Pingdingshan, P.R. China ( Corresponding author: ) ( Y u Chen, Pingdingshan Polytechnic College, Pingdingshan 467001, P.R. China. ) ( Email: pdschenyu@163.com ) Scheme l. Synthetic protocol for sensor LA. Table 1.Optimization of the reaction conditions synthesissensor LA. Entry Solvent Additive Time/h Yield/% EtOH 5 12 2 EtOH HCI 5 69 3 EtOH AcOH 5 51 EtOH H,SO, AcOH THF HCI 7 MeOH HCI DMF HCI DMSO HCI 5 lo MeOH HCI 0.17 86 aConventional method. Microwave irradiation: 300 W, ordinary pressure. increase yields, achieve cleaner reactions, simplifyworkups, and design energy-saving protocols.16-18 Although various Schiff base chemosensors have beendesigned and synthesized,19-23 to the best of our knowledgeand according to the literature survey that few efforts havebeen made in the efficient synthesis of chemosensors with6"6green chemistry” strategy. Besides, steroids are rarelyused as a skeleton for fluorescent sensor. In previousstudies,24-27 we reported a microwave mediated, efficient,high yield, and environmentally friendly synthesis of Schiffbase derivatives. Herein, a novel steroidal Schiff base fluo-rescent sensor, N'-(2-hydroxybenzylidene)-3a-hydroxy-cholanhydrazide (LA), was designed and prepared viamicrowave irradiation (Scheme 1), and Aldetection capa-bility of LA has been investigated. The free LA exhibitedalmost no detectable fluorescence emission due to the C=Nisomerization and the photo-induced electron transfer(PET) phenomena; when coordinating with Al, the C=Nisomerization and PET effects were inhibited and themolecular structure changed from flexible to rigid, whichdramatically increased the fluorescence intensity.12,28-30 Results and discussion To develop an optimal reaction condition, 3o-hydrox-ycholan-24-hydrazide (3) and salicylaldehyde (4) wereemployedin conventionalandmicrowave-assisted procedures. The reaction was monitored with or withoutadditives, such as acetic acid, Sulfuric acid (H,SO ), orhydrochloride (HCl) under refluxing in Tetrahydrofuran(THF), Dimethyl Sulfoxide (DMSO), N,N-Dimethyl-formamide (DMF), ethanol, methanol, or acetic acid. Theresult demonstrated that the yield of sensor LA was in therange of 12%-73%, while longer reaction time wasrequired. With better catalytic property, HCl was used inthe synthesis of sensor LA under microwave irradiation.Fortunately, it showed a dramatic improvement of theyield and remarkable reduction in reaction time (0.17h)when Weemployedamicrowave-assisted method.Compared with conventional thermal heating, microwaveirradiation technique decreased the reaction time from 5 to0.17 h and enhanced the yield from 73% to 86%. The studydata were given in Table 1. The UV-Vis titration spectra of LA upon addition ofvarious concentrations of A1+ were carried out in ethanoland water (1:2, v/v) at room temperature. As shown inFigure 1, the absorption spectra of receptor LA have threeabsorption maxima, centered at 254, 264, and 294nm.Upon addition of increasing concentrations of Al+(0-1.0equiv.) to the solution of sensor LA (50uM), those threeabsorption bands were enhanced gradually, which clearlysuggests that LA participates in a coordination with A13+. The fluorescence emissions of sensor LA (50uM) on add-ing different metal ions (1.0 equiv. of LA) including Ag,Al, Ba, Ca, Cot, Cr, Cu, Fe, Hg, Mg’,Mn,Ni, Pb, and Zn?t were determined in ethanol/water (1:2,v/v). After excitation at 400nm, the sensor LA exhibited veryweek fluorescence emission. Upon addition of A1+(50pM),a remarkable fluorescence emission peak at 460nm wasobserved (Figure 2). In contrast, no significant fluorescencechange was detected after addition of other metal ions underthe same conditions. These results indicate that LA exhibitshigh selectivity for Al+ over other metal ions. Under the 365-nm UV lamp, the remarkable light-blue fluorescence emis-sion associated with the reactions between LA and Al+ wasobserved by the naked eye, but no significant fluorescencechange was detected for LA solution with other metal ions. The quantitative sensing abilities of LA (50uM) towardAl were studied, and a working curve was obtained(Figure 3). With addition of increasing concentrations ofAl+ions (0, 10,20,30,40,50,60, and 70uM), the fluorescence Figure I. Absorption spectra of sensor LA (50puM) in ethanoland water (1:2, v/v) solution obtained by adding aliquots of[AI*](0,0.1,0.2,0.3,0.4,0.5,0.6,0.7, 0.8, 0.9, and 1.0 equiv.). Figure 2. Fluorescent spectra of sensor LA (50pM) with 1.0equiv. of various metal ions in ethanol and water (l:2,v/v). Inset:The colors of sensor LA and LA-AI’+ as viewed by the nakedeye under a 365-nm UV lamp. intensity of LA-Al+ enhanced remarkably and showed amaximum fluorescence emission at 460nm. Besides, a goodlinear correlation (R2=0.99078) between the emission inten-sity of LA and the concentration ratio of [Al]/[LA] wasobserved on addition of 0-1.4 equiv. of Al’ions. Accordingto the titration profile (Figure 4), the binding constant of LAfor A1+ could be calculated as 4.13×103Mbased on themodified Benesi-Hildebrand equation (equation (1)), inwhere F, Fmin and Fmax represents the fluorescence intensitiesof LA-Al+complex, free LA, and the maximum fluores-cence intensity of LA-Alcomplex, respectively. According to t·thee 3o method (limit of detection(LOD)=3o/K, o represents the standard deviation of ablank solution and K represents the slope of the calibrationcurve in Supplemental Figure S1), the LOD ofsensor LA Figure 3. Fluorescence titrations of 50-uM LA(=400nm)in ethanol and water (1:2, v/v) in the presence of differentequivalents of Al+ ions (0, 0.2,0.4,0.6,0.8,1.0, 1.2, and 1.4equiv.).Inset: Graph of the fluorescence intensity at 460 nm as afunction of the concentration ratio of Al+ and LA. Figure 4. Benesi-Hildebrand analysis of the emission changesfor the complexation between LA and Al’+ in ethanol andwater (1:2, v/v).(x=400nm,m=460nm). for Al+ reached 34nM, which is low for the detection ofAl+ comparing with other published Al binding sensorsand much lower than World Health Organization (WHO)recommended 3.7-7.4 uM Al+ standard drinking water.32,33These results demonstrate that sensor LA could be poten-tially used for selective detection of Al in analyticalchemistry, especially in water quality monitoring. To determine the utility of sensor LA as an Al’-selectivereceptor in the complex background of competing species,the fluorescence emissions of LA-A1+were examined in thepresence of different metal ions (Figure 5). Upon addition ofdifferent metal ions (1 equiv. of Al+), the fluorescence emis-sions of LA-Al’+ were almost not interfered, except for Cu²+and Fe. The obvious reduction of fluorescence intensitiescaused by Cuand Femight due to an energy or electrontransfer.34 As reported in literatures,35,36 it was possiblybecause the newly formed complex LA-Fe+or LA-Cu²+was too stable to be replaced by Al. Figure 5. Relative fluorescence intensity ratio of LA-AI’+(50uM) in the absence and presence of different metal ions (1.0equiv.).(ex=400nm,em=460nm). Figure 6. Job’s plot for the complexation of LA with Al*(ex=400nm,em=460nm). To investigate the applicability of sensor LA for detect-ing Al in different environments, the pH effects werestudied (see Supplemental Figure S2). The fluorescenceemissions of LA (50uM) and LA-A1+ complex (50pM,1:1, M/M) were detected in various buffer solutions (seeSupplemental Table S1). The free sensor LA exhibited veryweek fluorescence emissions in the pH range of 3.92-9.84.Upon addition of Al, a remarkable fluorescence enhance-ment of LA was observed and the pH value increased from6.05 to 9.32. On the contrary, in acidic solutions (pH<6.05),the fluorescence intensities significantly decreased, whichmight due to the protonation of Schiff base group. Moreover,the fluorescence intensities of LA-Al+ were sharply reducedin strong alkaline solutions (pH>9.32), which mainly resultfrom the decomposition of Schiff base group. Besides, theformation of aluminum hydroxide in strong alkaline solu-tions could possibly be another reason for this significantinactivation of probing process. These results demonstratethat sensor LA is highly selective toward Al in a relativelywide pH range (6.05-9.32). The binding stoichiometry between sensor LA and Al+(Figure 6) was studied by the method of continuous varia-tion (Job’s plot). In the Job’s plot, the total concentration of LA and Al’+ was 50 uM, with a continuous variable molarfraction of guest [Al*]/([LA+Al+]). The maximum fluo-rescence emission intensity at 460 nm was observed at themolar ratio of 0.51, establishing a 1:1 binding stoichiome-try between sensor LA and Al’+. Additional evidence was given by NMR (nuclear mag-netic resonance) studies of sensor LA and LA-A1+ com-plex. The H NMR spectra of LA in the absence andpresence of Al’+ were recorded in DMSO-d. Significantspectral changes were observed (Figure7). Signals for theprotons of -NH- (H’) at 11.57ppm and -OH (H') at11.19 ppm disappeared, which indicates the enolization anddeprotonation of LA in the presence of A13+ 37,38 Besides,the signals of H2 and H4 were downfield shifted 0.08 and0.3 respectively. These phenomena indicate that thehydroxyl group, carbonyl group, and C=N group of sensorLA participate in a coordination with Al+, which in returnchanges the electron distribution in the chemosenSor. Based on the above results, a binding mechanism for thefluorescence response of LA toward Al+ is proposed. Asshown in Scheme 2, the fluorescence emission of free LAis nearly quenched, which may due to the PET phenome-non causing by lone pair electron from Schiff-base nitrogenatom to the phenol moiety and C=N isomerization process.However, upon addition of Alions (2.0 equiv.), two nitro-gen atoms ofthe azomethine groups and the hydroxyl groupon the phenol participate in the coordination with Al,forming a 1:1 complex and resulting in a remarkable fluo-rescence enhancement of LA. By utilizing a previously reported method,39,40 sensorLA was used to measure the Al+ content in actual watersamples, including tap water(from our laboratory), domes-tic sewage (from student residences at our college),andindustrial sewage (from industrial areas in PingdingshanCity). All the water samples were filtered through a0.2-mm filter membrane to remove large particular impu-rities, followed by removal of remaining organics byextraction processes. The resulting samples were dilutedwith ethanol and water (1:2, v/v) in a 10.0-mL volumetricflask. Table 2 shows the results acquired using sensor LAwith the appropriate concentration gradient of Al’+ added.The results indicate that sensor LA had good recoveryand demonstrated high accuracy in the analysis of A1+.Therefore, sensor LA can measure the concentration ofAl+in real water samples and has practical value in envi-ronmental analysis. Conclusion In summary, aahighly selective and sensitive Schiffbase chemosensor, N'-(2-hydroxybenzylidene)-3a-hydroxy-cholanhydrazide (LA), has been designed and synthesizedunder microwave irradiation. LA shows significant “turn-on” fluorescence behavior with Al’ in ethanol/water (1:2,v/v) solution, giving strong light-blue emission. The detec-tion limit was calculated to be 3.4×10molL-1 andremained stable, detecting Al’ in the pH range of 6.05-9.32.In addition, the sensor LA exhibits satisfactory results forAl+ detection in the analysis of real water samples and canbe further used in potential applications for the detection of Figure 7. 'H NMR spectra of sensor LA and LA-AI’+ in DMSO-d. Scheme 2. Proposed sensing mechanism between sensor LA and AI. Table 2. Determination of Al’+ in real water samples with sensor LA. Sample pH Added AI+ (nM) Found Al+ (nM) Recovery (%) RSD(%) lap water 6.71 40 39.18 97.95 1.25 60 60.51 100.85 80 79.76 99.70 100 100.25 100.25 Domestic sewage 7.96 40 39.66 99.15 0.60 60 60.33 100.55 80 80.20 100.25 100 99.89 99.89 Industrial sewage 8.43 40 39.41 98.53 0.98 60 60.39 100.65 80 79.67 99.59 100 100.52 100.52 RSD: relative standard deviation. Determined with a pH meter before treating with Al’. bResults are based on three measurements. Experimental All chemicals and solvents were obtained from commercialsources and used without further purification, except whenspecified. Solvents used in fluorescent experiments were ofspectroscopic grade. The 'HNMR and 13c NMR spectra ofthe ligand were recorded on an Agilent 400-NMR DDR2spectrometer. The chemical shifts (8) are recorded in ppm,relative to tetramethylsilane (SiMe ). The Fourier transform infrared spectrum was recorded on a Perkin-Elmer 1700FTIR spectrophotometer. Mass spectra were obtained usinga Finnigan LCQ DECA mass spectrometer in electrosprayionization (ESI) positive mode. A Mapada UV-6100 UV-Visspectrophotometer was used for recording the UV-Visspectra. Fluorescence emission spectra were recorded on aDual-FL fluorescence spectrophotometer. Elemental analy-sis (C, H, and N) was performed with an Elementar VarioMICRO cube,and the results are within ±0.4% of thecalculated values. All the reactions were performed in acommercial microwave apparatus (XH-100A, 100-1000W;Beijing Xianghu Science and Technology Development Co. Ltd, Beijing, China). All the solvents were purified beforeuse. Compounds 2 and 3 (Scheme 1) were prepared startingfrom lithocholic acid according to a literature procedure.4 Preparation ofN'-(2-hydroxybenzylidene)-3o-hydroxy-cholanhydrazide (sensorLA) To a solution of compound 3 (1 mmol) in methanol (5mL),salicylaldehyde (0.95mmol) and acetic acid (2 drops) wereadded successively. The mixture was heated to reflux in themicrowave oven at 300 W for 10 min. After completion, themixture was cooled and filtered to afford crude product.The crude product was recrystallized from ethanol to givethe desired product LA (428mg) as yellow solid. Yield86%; m.p. 254.5-254.9℃ (EtOH). IR (KBr, cm):3453,3221, 3070,2941,2872,1687,1624,855,750,627. HNMR (400MHz,DMSO-d):8 11.57 (s, 1H, NH), 11.19 (s,1H, Ar-OH), 8.33 (s, 1H,=CH), 7.48 (d,J=8.0Hz, 1H,ArH), 7.25-7.29 (m, 1H, ArH), 6.87-6.91 (m, 2H, ArH),4.45 (d,J=1.2Hz, 1H, 3a-OH), 0.92 (t, J=8.0Hz, 3H,21-CH,), 0.87 (s, 3H, 19-CH,), 0.61 (s,3H, 18-CH,). 13CNMR (100MHz, DMSO-d):8 173.98, 162.46, 151.48,134.61,131.73,125.24,123.78,121.45,75.04,61.26,60.71, 60.54, 47.46,46.70,41.48,40.56,40.33,40.13,39.39,36.34,36.08,35.56,34.18,32.92,32.07, 31.34,29.03,28.46,25.59,23.45,17.07. ESI-MS, m/z: 495.86[M+1]*. Anal. calcd for C,H4NO: C, 75.26; H, 9.37; N,5.66; found: C, 75.06; H, 9.34; N, 5.68%. Declaration of conflicting interests The author(s) declared no potential conflicts of interest withrespect to the research, authorship, and/or publication of thisarticle. Funding The author(s) received no financial support for the research,authorship, and/or publication of this article. ORCID iD Yu CheniDhttps://orcid.org/0000-0002-2711-6535 Supplemental material Supplemental material for this article is available online. References ( 1. He T, Xin Q, Zhen L Z , e t al. Spectro Chim Acta A 2 019;207: 31. ) 2.(Campbell A, Becaria A, Lahiri DK, et al. J Neurosci Res2004;75:565. ( 3. ) . F laten TP. Brain Res Bull 2001;55: 1 87. ) ( 4. . G C ood PF, O lanow CW and Perl D P . B r ain Res 1992;5 9 3:343. ) ( 5. Wang R, Jiang GQ and Li XH. I norg Chim A cta 2017; 455: 247. ) 6Supriti S, Titas M, Basab C, et al. Analyst 2012;137:3975.78 ( U padhyay KK and Ajit K. Org Biomol Chem 2010;8: 4892. ) ( Wei HD, Dan W, Xiang JZ, et al . S e ns Actuators B 2015; 209:359. ) ( 9.(Onder A and Serkan E. Sens Actuators B 2015;208:159. ) ( 10. 2 Zhao C X, Ju Y Y a n d D a vid R S . C h em So c Re v 20 1 0; 39: 1996. ) ( 11. L I ei L, Yong QD, Hong WL,et a l . T etrahedron Lett 2010;51: 618. ) ( 12. J J un Y , L ong F , Jing CQ, et al. T etrahedron Lett 2 016; 57: 2910. ) ( 13.E Ece E , U mit E a nd O zgiir M uhammed iF K . Sp e ctrochimActa A 2018;203:273. ) ( 14. Li MX, Chen C L , Ling CS, et a l . B ioorg Med Chem Lett 2009: 1 9:2704. ) ( Kappe CO. Angew Chem Int Ed 2004;43:6250. ) ( Polshettiwar V and Varma RS. Acc Chem Res 2008;41:629. ) ( 17. Katrizky AR and Angrish P. S t eroids 2006; 71:6 6 0. ) ( 18. Ramesh E and Raghumathan R. Tetrahedron L e tt 2 008; 49:1812. ) ( 19. Yu LX, S h an SM, Hong PP, et al. J Lumin 2017; 192: 56. ) ( 20. K alyani R, Amit K M, Meman S, et al. Inorg Chim A c ta 2019:492:156. ) ( 21. Xin S, Y a WW and Yu P. Org Lett 2012; 1 4 :3420. ) ( 22 . Zohreh S. Mehdi A and Soraia M . J Lumin 2019: 207: 78. ) ( 23. DIas B , J ana A, M ahapatra A D, et a l. Spectrochim Acta A 2019;212:222. ) ( 24. .SShi ZC, Zhao ZG, L iu M, et al. C R Chim 2013 ; 16:977. ) ( 25. S S hi ZC, Zhao ZG, Liu XL, et al. Chin Chem Lett 2011;22: 405. ) ( 26. SShi ZC, Zhao ZG, L iu XL, et al. J Chem Res 2011;35:198. ) ( 27. LIi GH, Shi ZC, Li XR, et al. J Chem Res 2011; 35:278. ) ( 28.Tang KC, Chang MJ, Lin TY, et al.J Am Chem Soc 2011;133:17738. ) ( 29. . W V u JS, Liu WM, Zhuang XQ, et al. Org Lett 2007;9: 33. ) ( 30. F F ujikawa Y, Urano Y, Komatsu T , et al . J Am Chem Soc 2008;130: 1 4533. ) ( 31. Liu SR and Wu SP. Sens Actuators B 2012; 1 71:1110. ) ( 32. S inha S,Koner RR, Kumar S, et al.RSC Adv 2013;3:345. ) ( 33. S Sen B , M ukherjee M, Banerjee S, et al. Dalton T rans 2 015;44:8708. ) ( 34. Martinez R and Molina P . Org Lett 2 005;7 : 5869. ) ( 35.Burdette SC, Frederickson CJ, Bu W, et al. J Am Chem Soc 2003;125:1778. ) ( 36. Liu W, Xu L, Sheng R, et a l. Org Lett 2007;9: 3829. ) ( 37. M N onfared HH, Falakian H, Bikas R, et a l . Inorg Chim Acta 2013;394:526. ) ( 38. X iao W, Lu ZL, Wang XJ, et a l . Polyhedron 2000; 19:1 2 95. ) ( 39. . Qu SZ, Zheng CH, Liao GM, et al. RSC Adv 2017;7:9833. ) ( 40. . W V u X, Chen J and Zhao JX. Analyst 2013; 1 38: 5281. ) ( 41. Zhou CE, Zhao ZG a n d T a ng XL. C h in J Org Chem 2007;27: 513. ) (微波辅助合成的新型类固醇希夫碱化学传感器用于检测水介质中的Al3+。)关键词:铝(III)离子,化学传感器,微波照射,席夫碱,类固醇摘要:通过微波照射,设计并制备了一种新型的类固醇衍生的席夫碱化学传感器,即N-(2-羟基亚苄基)−-羟基-胆烷酰肼(LA)。该传感器在乙醇/水(1:2, v/v)溶液中,在6.05至9.32的pH值范围内,对Al3+,显示出高选择性的荧光感应,检测限为34 nM。通过Job's plot确定LA和Al3+ 之间的结合化学计量为1:1,并通过1 HNMR研究进一步验证。在紫外灯下,荧光颜色的变化很容易被肉眼发现。此外,该传感器LA已被应用于检测实际水样中的Al3+ 。简介:铝是地壳中最丰富的金属,约占地球质量的8%。铝已被广泛用于现代生活,如水处理厂、食品添加剂、纺织品和药品。然而,随着铝工业的快速发展,天然水和土壤中的铝含量逐渐增加。1众所周知,铝不是生物必需的元素,铝的过量积累3+,会产生毒性,引起许多严重的疾病,如神经元紊乱、丧失记忆、无精打采、阿尔茨海默病等。因此,设计和合成具有高选择性和优异灵敏度的检测铝的化学传感器具有重要意义。希夫碱是金属离子的良好配体,它们可以与金属离子形成强烈的配位键。这一特点导致它们被广泛用于化学传感器的设计和合成。已经报道的两种可能的机制,C=N异构化和抑制这种异构化的结果是导致希夫碱的荧光发射行为。与金属离子结合,C=N异构化将被抑制,并使荧光发射发生重大变化,这在实际应用中是一个重要的优势。绿色化学是当今国际化学科学研究的前沿领域,得到了世界各国政府、企业和化学界的广泛关注和重视。作为一种符合 "绿色化学"概念的新技术,微波辐照在近年来引起了人们的极大兴趣。微波辅助有机合成是一项宝贵的技术,有助于缩短反应时间。祥鹄仪器的使用过程:所有反应均在商用微波设备(XH-100A,100–1000W;北京祥鹄科技发展有限公司,中国北京)。结论:综上所述,我们在微波照射下设计并合成了一种高选择性和高灵敏度的席夫碱化学传感器,即N′-(2-羟基苯亚甲基)−-羟基-胆烷酰肼(LA)。3+在乙醇/水(1:2,v/v)溶液中,LA显示出明显的 "开启"荧光行为,给出了强烈的浅蓝色发射。计算出的检测极限为3.4 × 10-8 mol L-1,并保持稳定,在6.05-9.32的pH范围内检测Al3+。此外,该传感器LA在实际水样分析中表现出令人满意的Al3+检测结果,并可进一步用于检测以下物质的潜在应用在化学和环境系统中,纳摩尔浓度的Al 。3+。
确定



还剩4页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《新型类固醇希夫碱化学传感器中微波辅助合成检测方案(微波萃取仪)》,该方案主要用于其他中其他检测,参考标准--,《新型类固醇希夫碱化学传感器中微波辅助合成检测方案(微波萃取仪)》用到的仪器有电脑微波催化合成萃取仪 祥鹄XH-100A
相关方案
更多
该厂商其他方案
更多










