方案详情
文
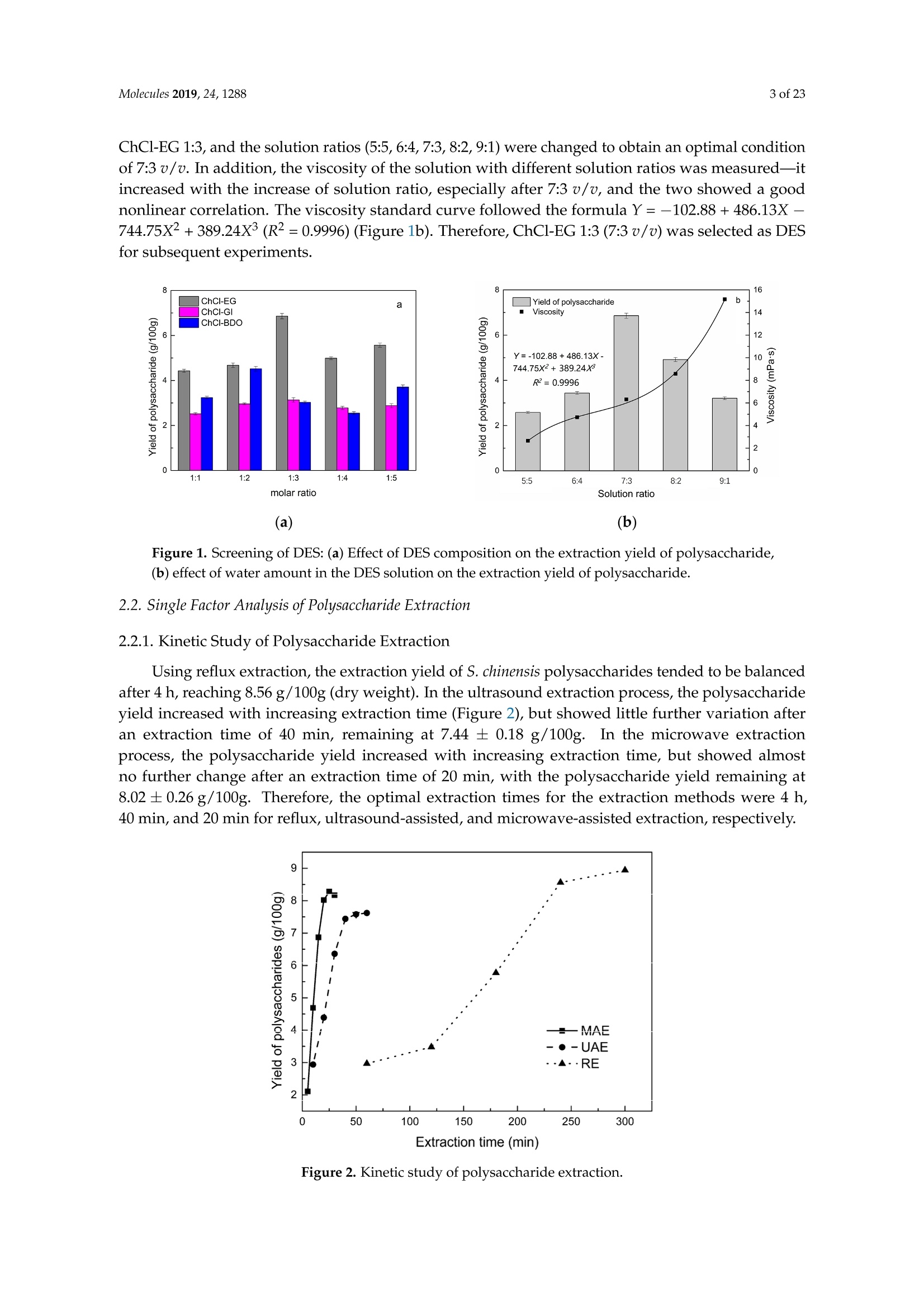
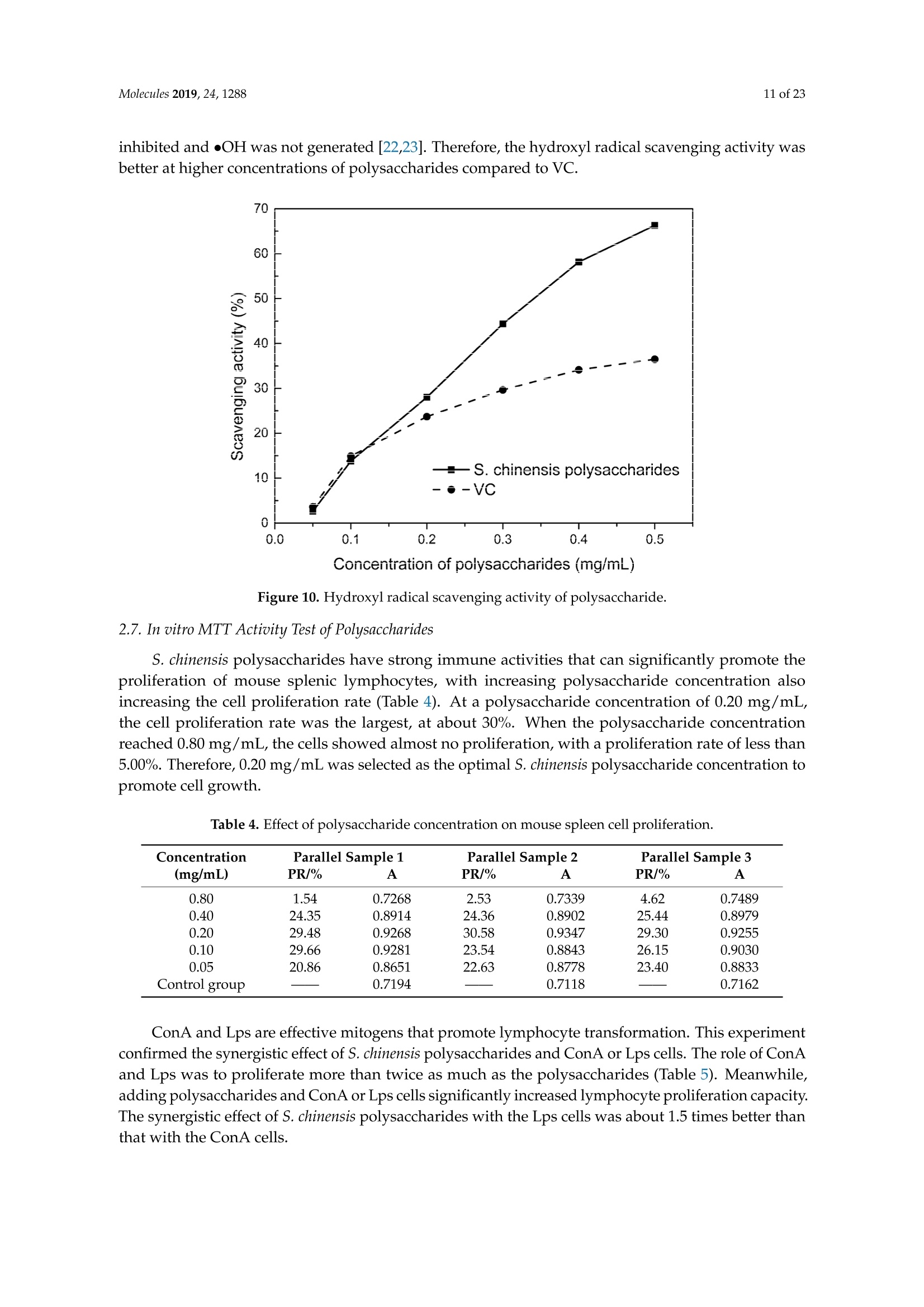
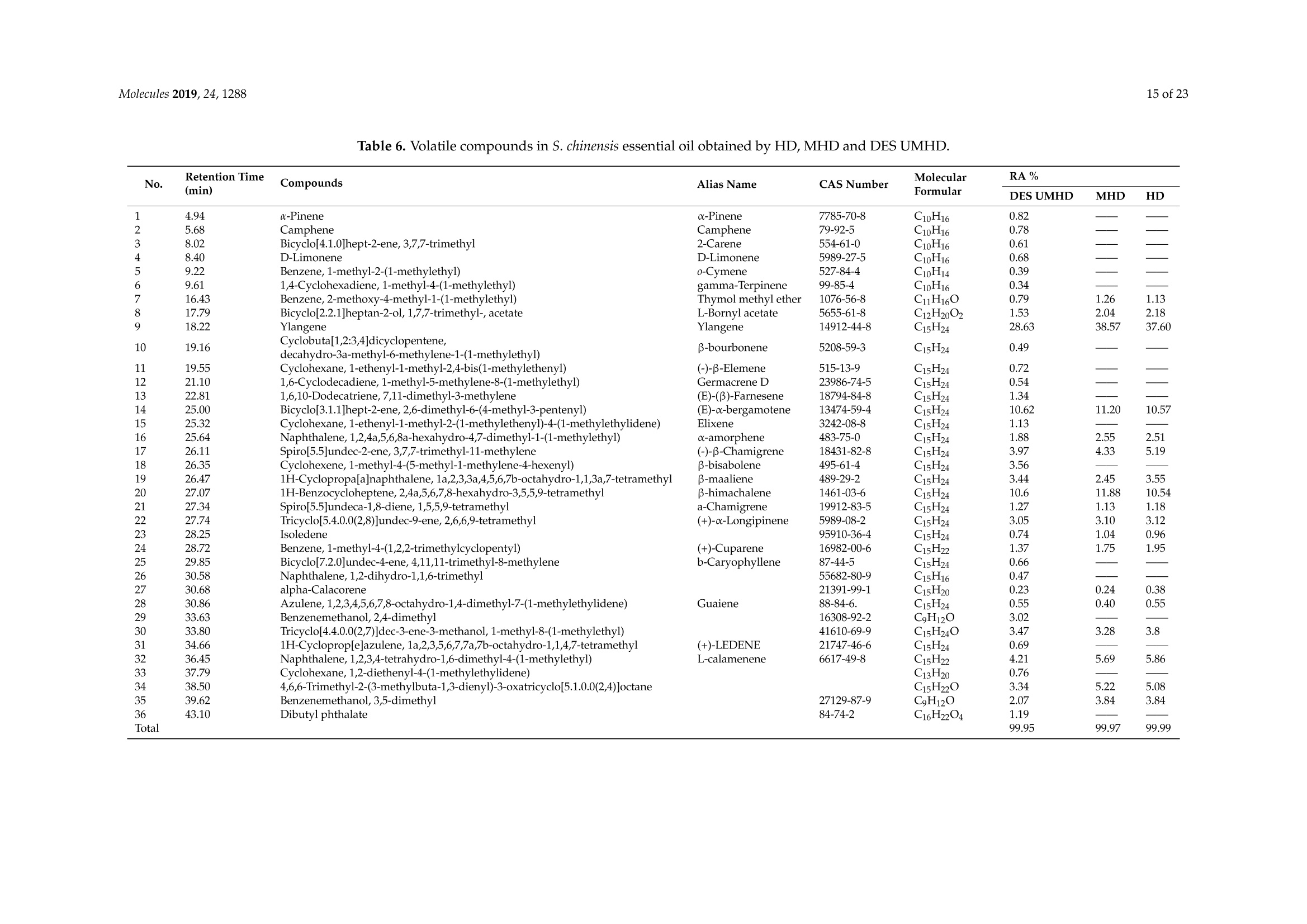
采用超声/微波交替协同的方法从五味子中同时提取精油和多糖。用深层共晶溶剂(DES)从五味子中提取精油和多糖。在最佳条件下,在选定的氯化胆碱-乙二醇1:3溶剂中提取,分别得到12.2毫升/千克和8.56克/100克的精油和多糖。还研究了多糖的自由基清除和免疫活性以及精油的抗氧化活性。淋巴细胞的增殖能力通过在多糖中加入协和素A或脂多糖而得到大幅提高。多糖(0.20毫克/毫升)精油清除DPPH的IC50值为水力蒸馏法和超声/微波辅助水力蒸馏法(DES UMHD)获得的精油清除DPPH的IC50值为52.34微克/毫升和29.82微克/毫升。通过DES UMHD获得的精油具有在150克/毫升时具有最高的还原力(856.05(TE/克),具有最强的抑制能力(SC%= 18.12%). S. chinensis具有作为天然抗氧化剂开发的潜力。
方案详情

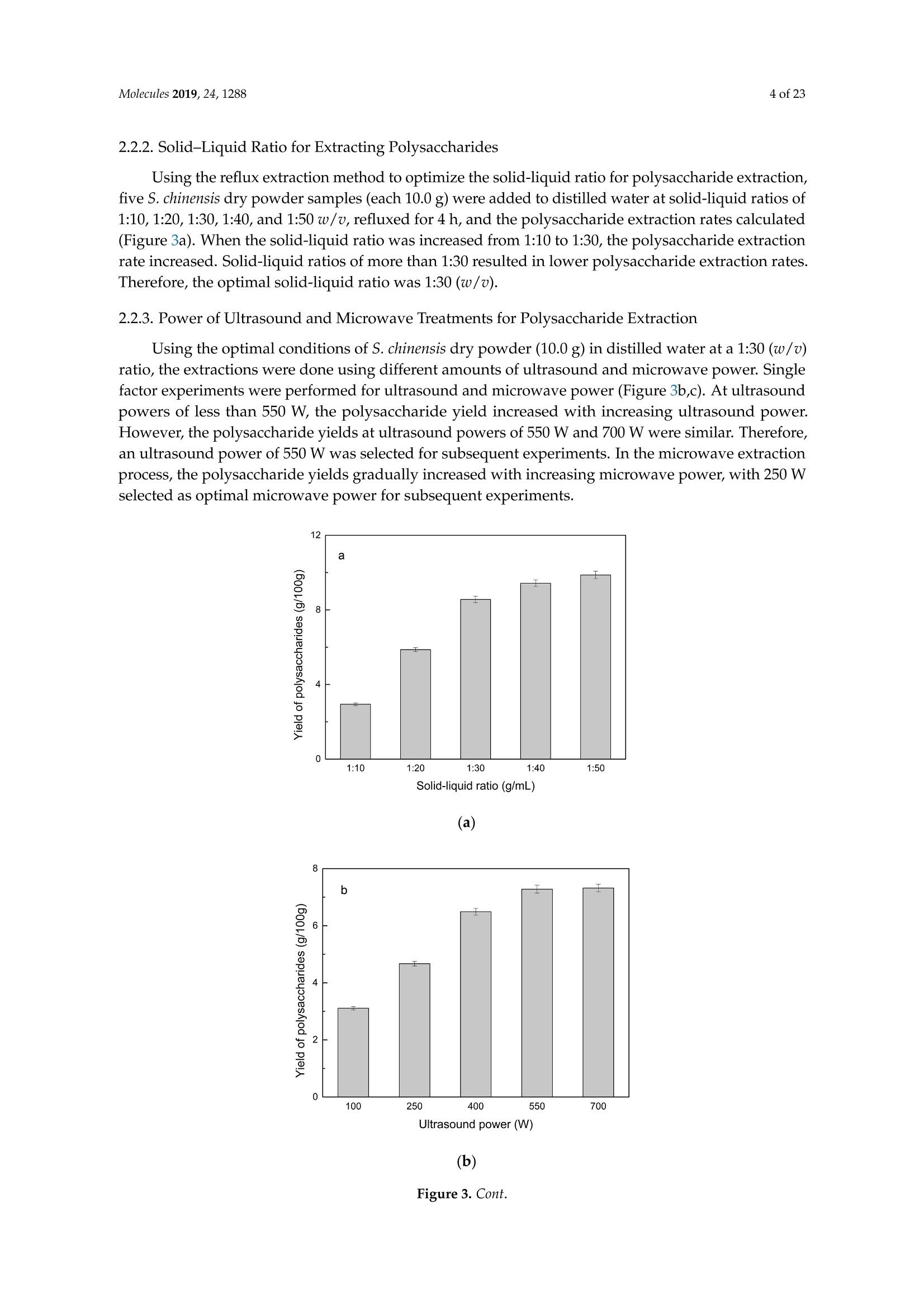
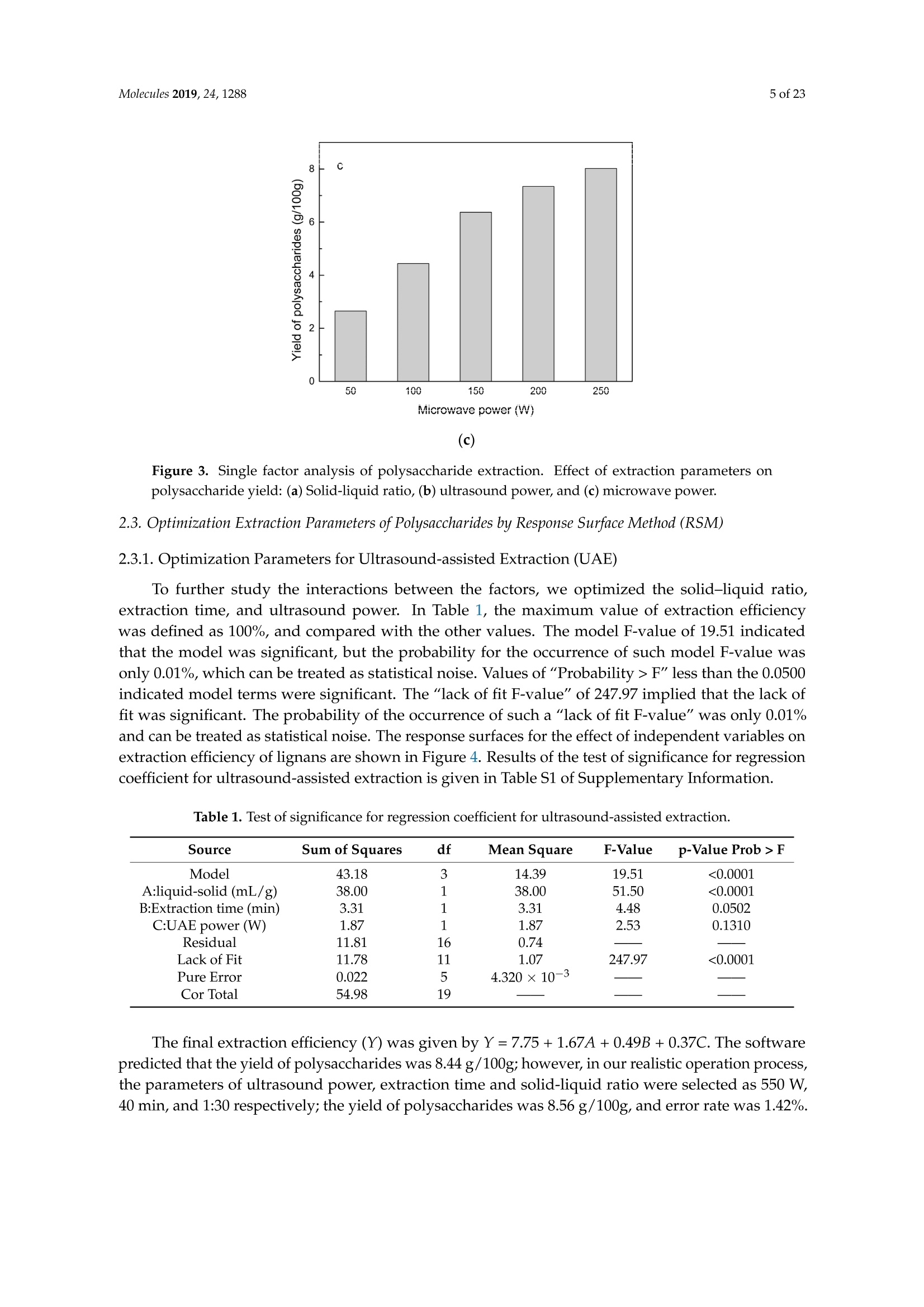
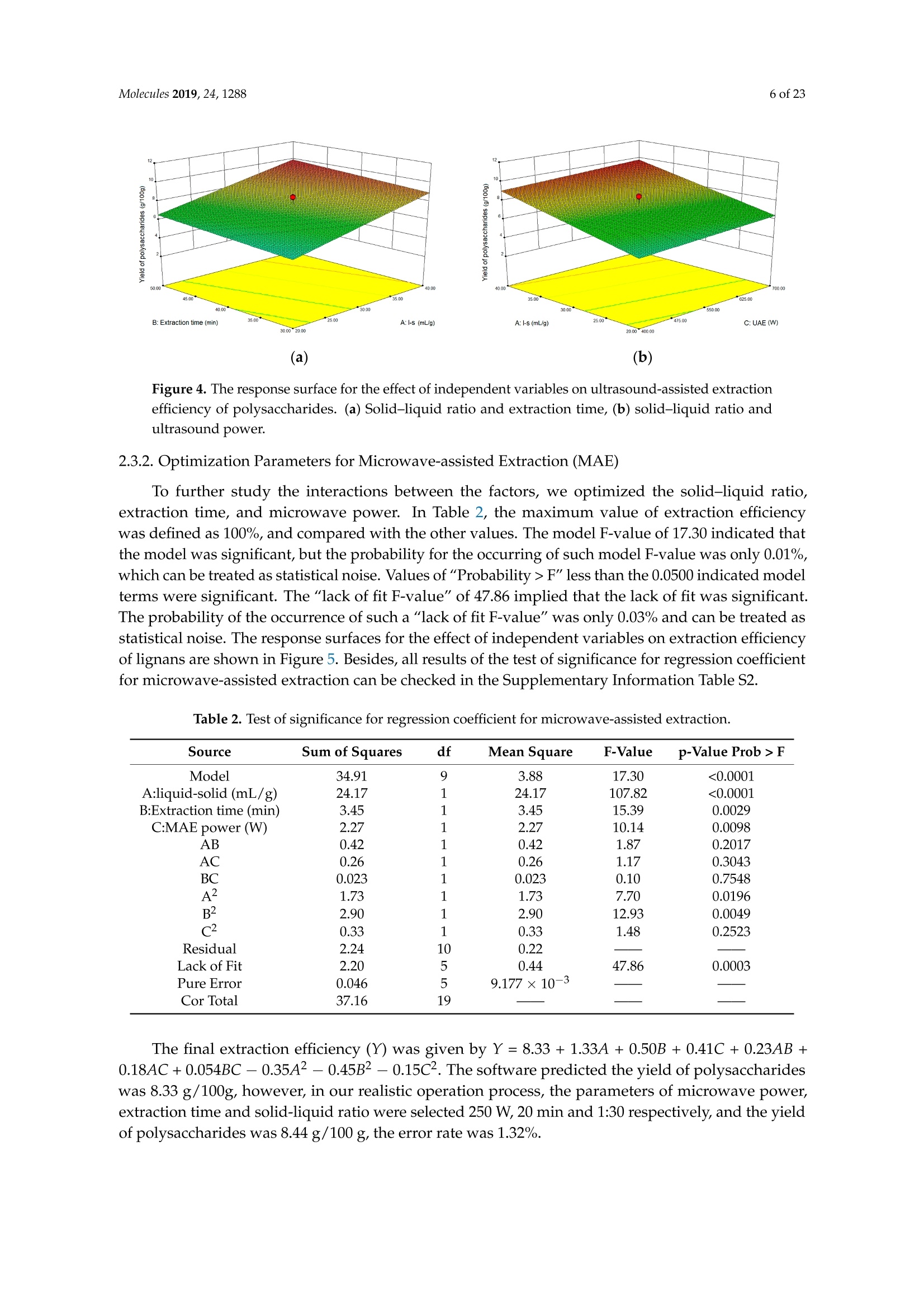
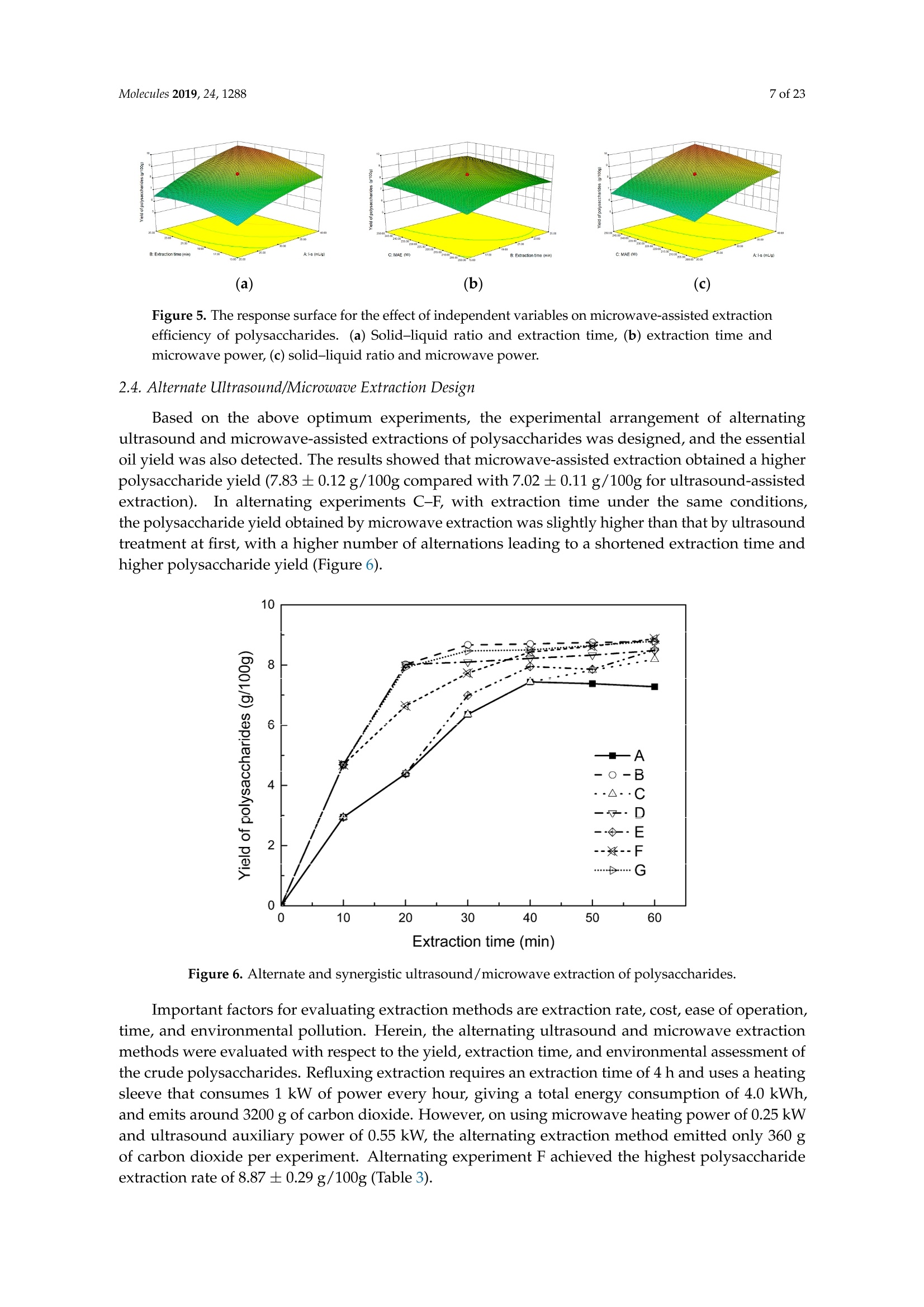
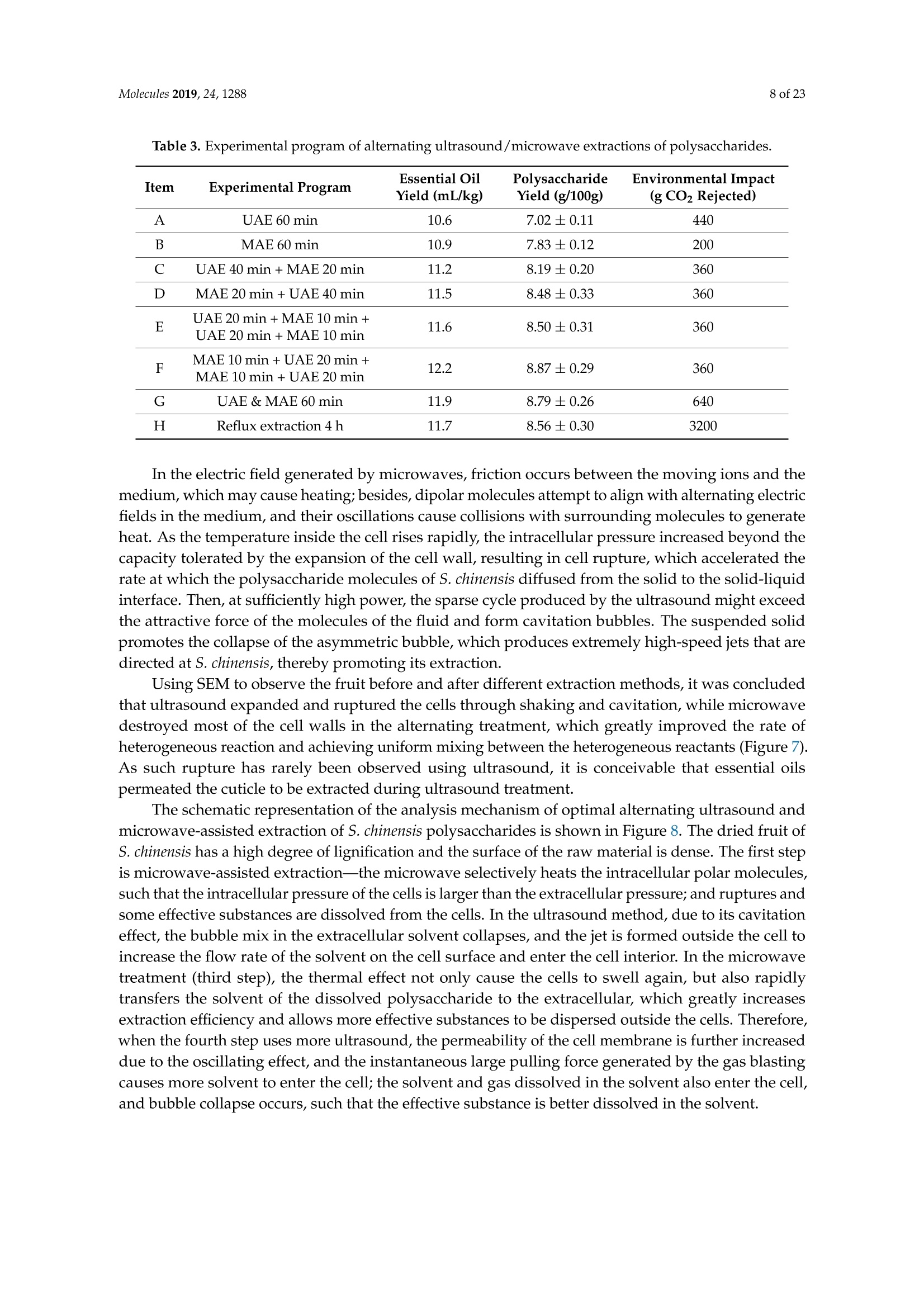
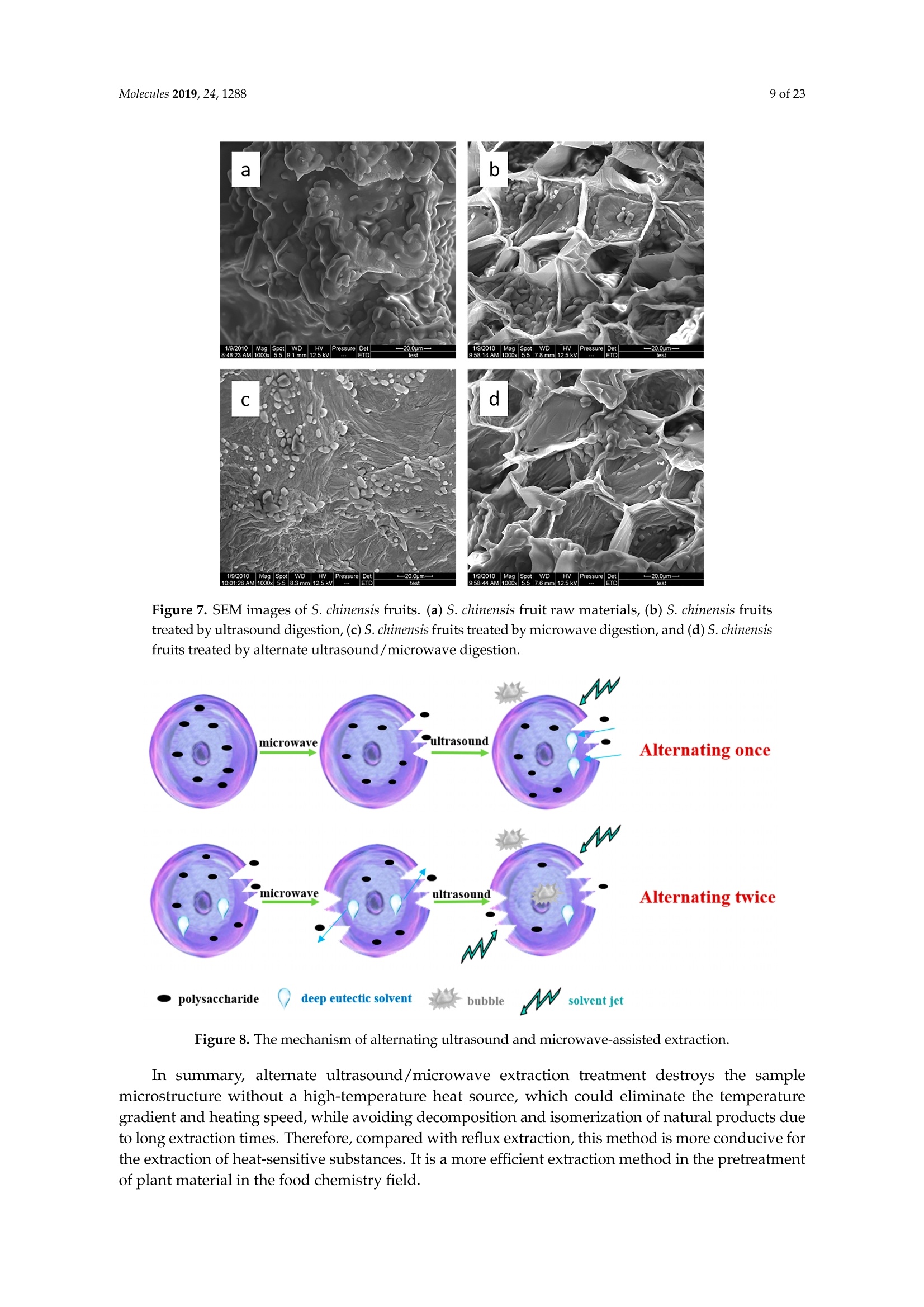
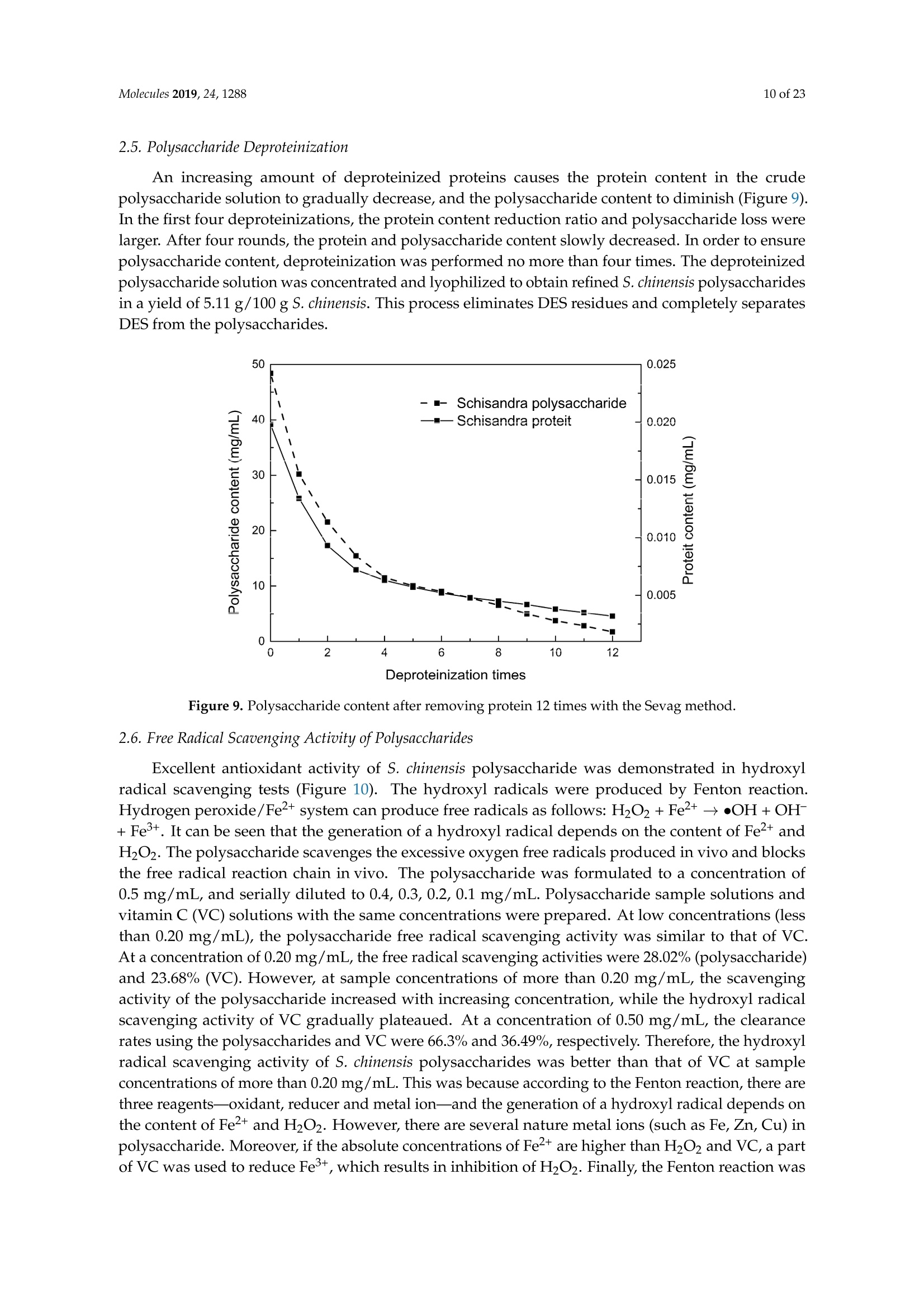
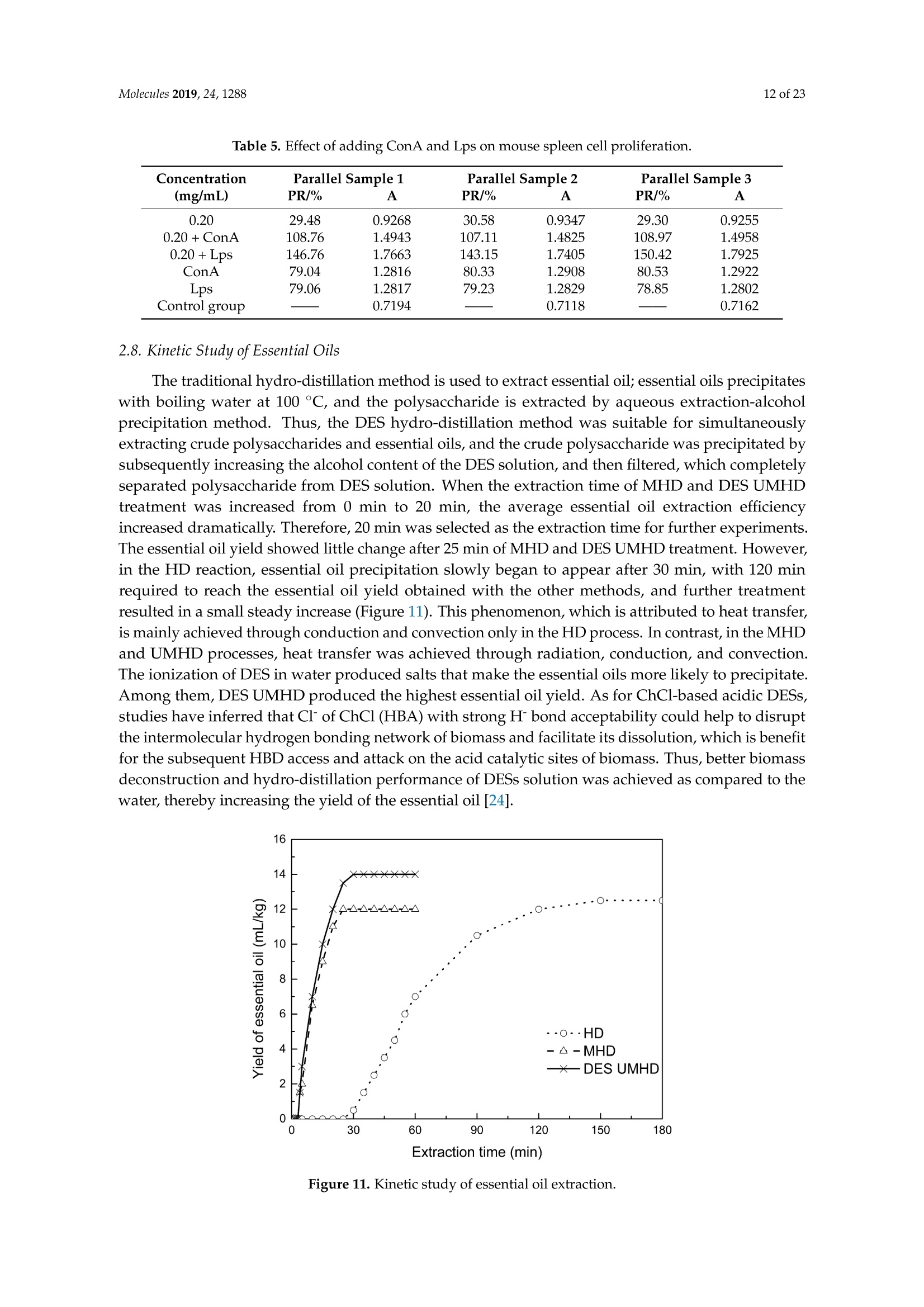
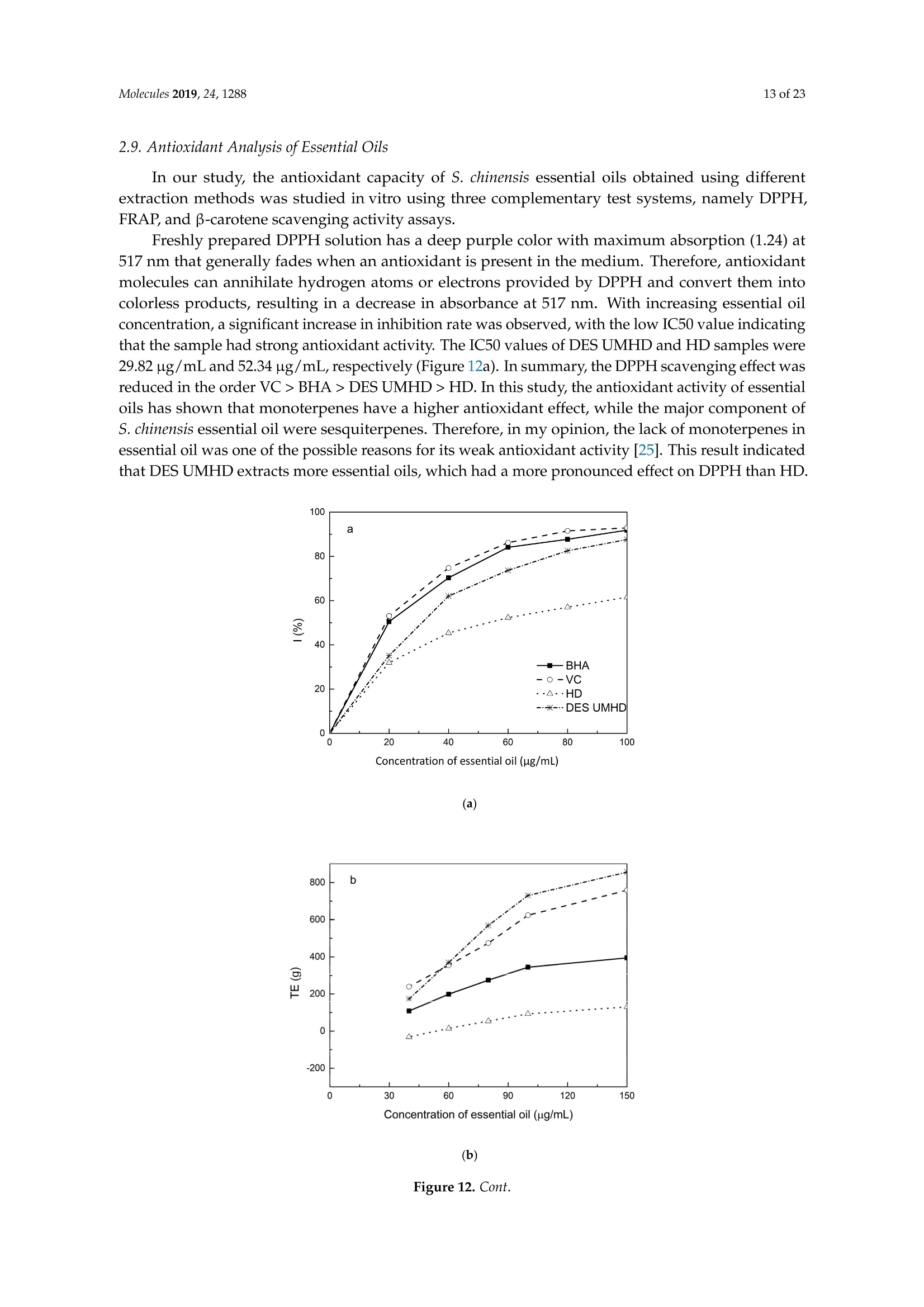
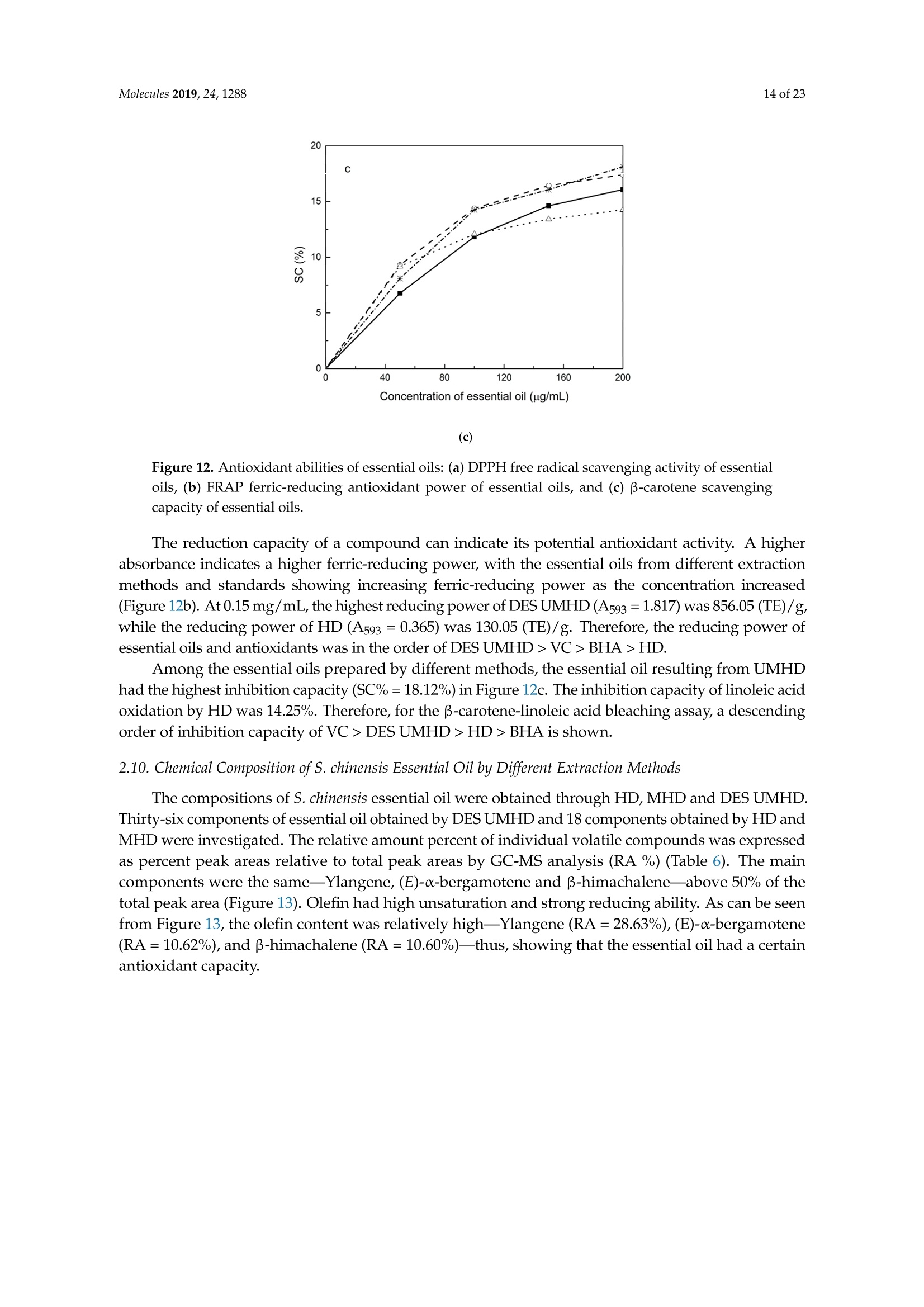
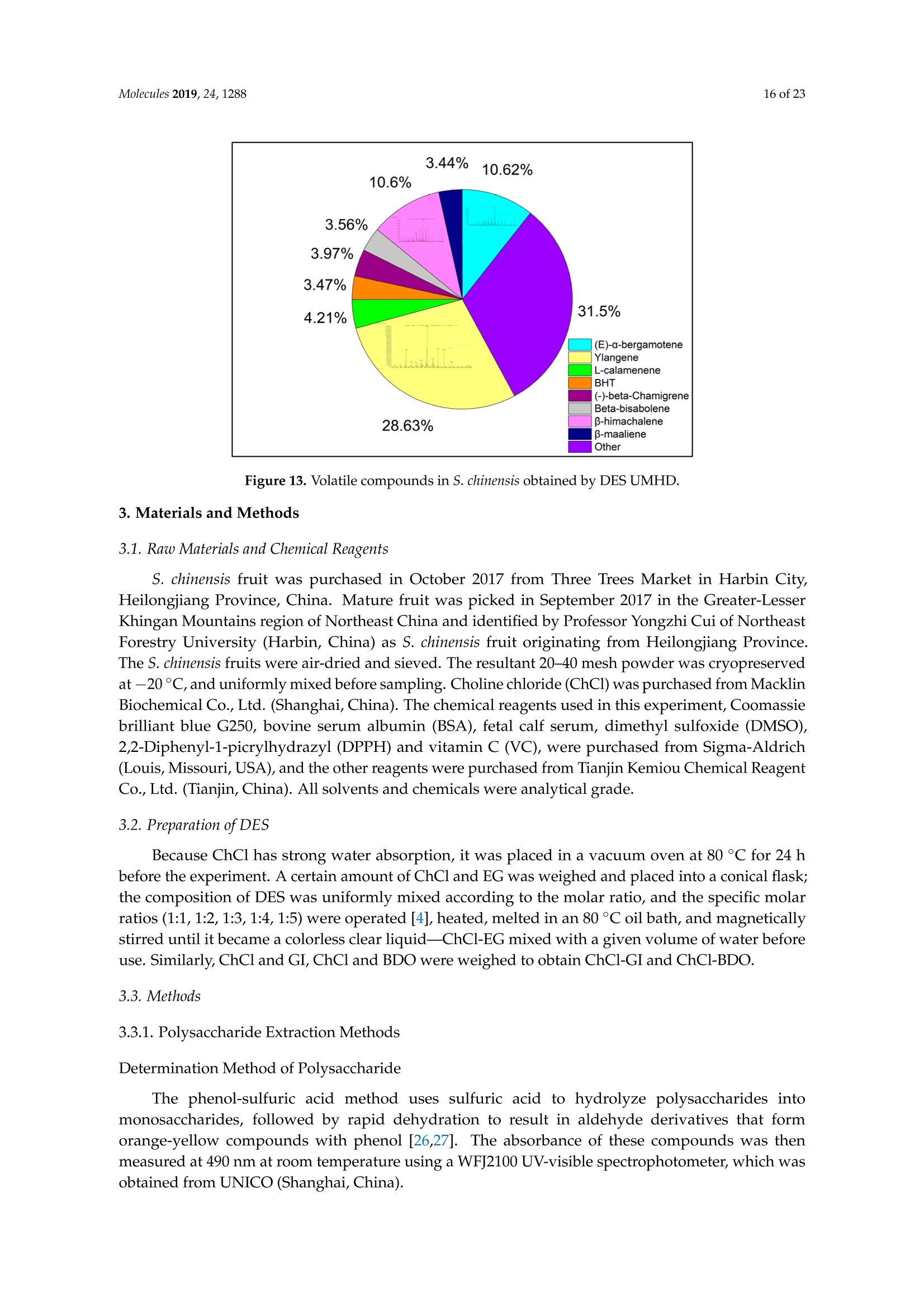
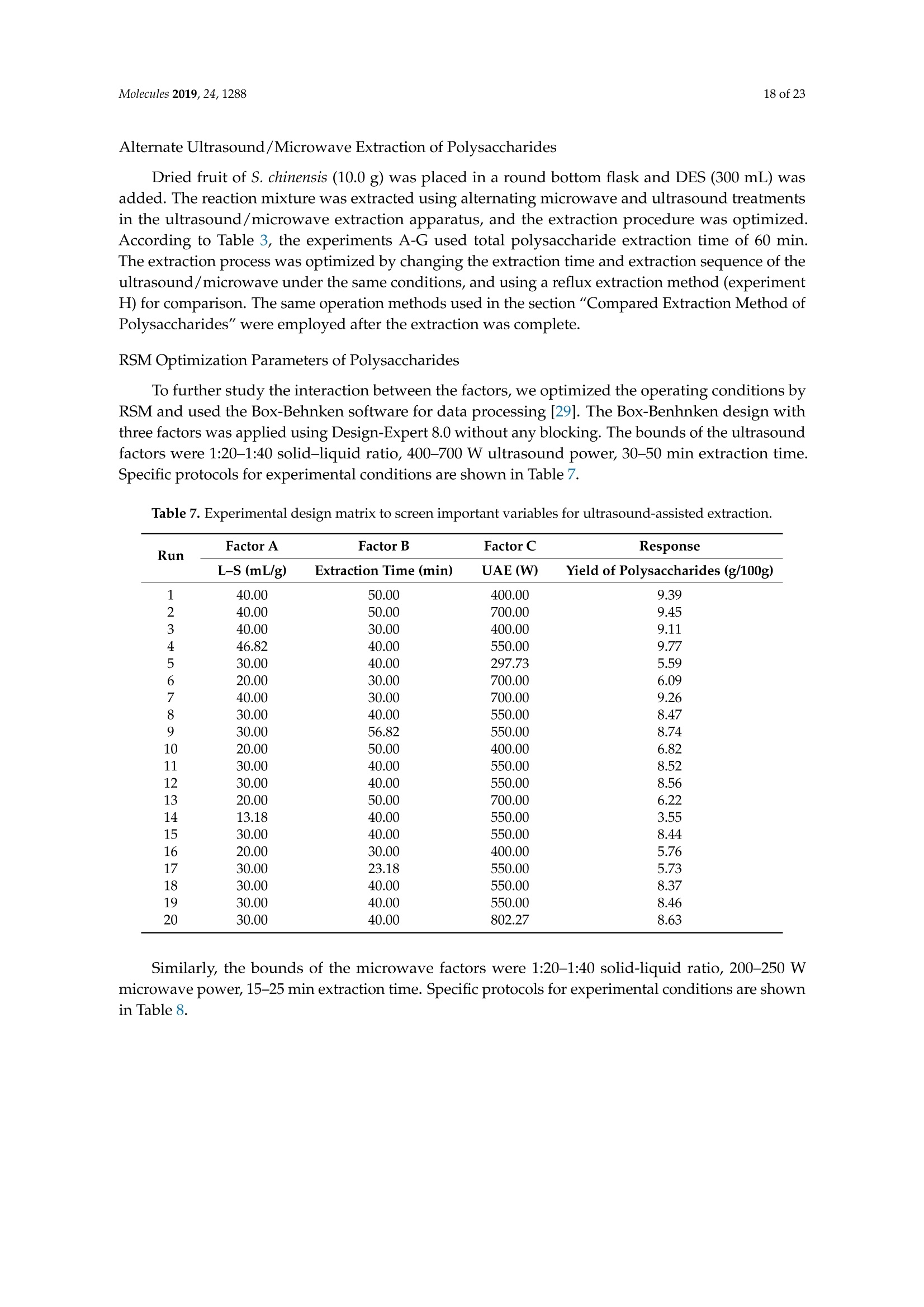
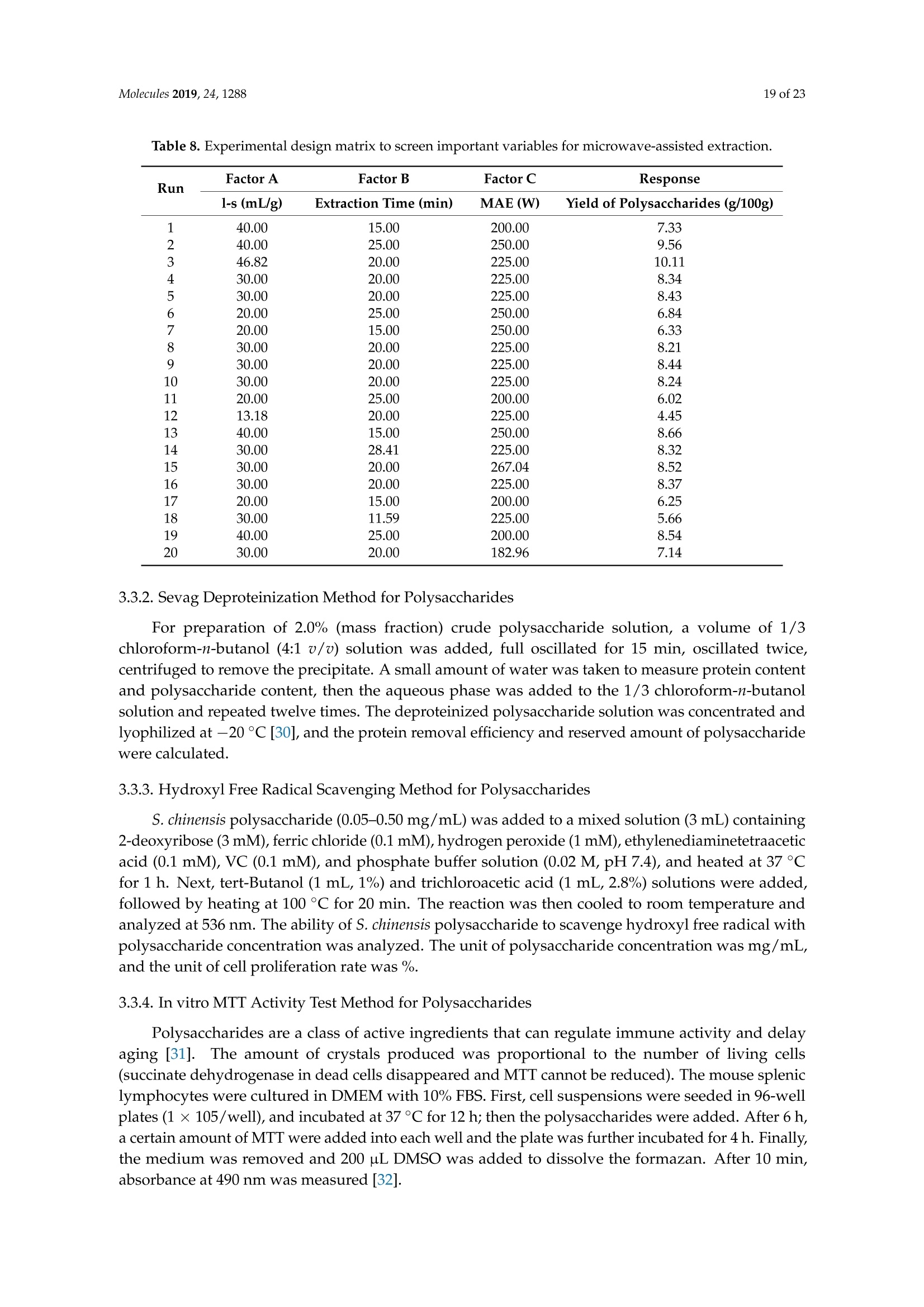
Molecules 2019, 24, 12882 of 23 Molecules 2019,24,1288; doi:10.3390/molecules24071288www.mdpi.com/journal/molecules Article Alternate Ultrasound/Microwave Digestion for DeepEutectic Hvdro-distillation Extraction of EssentialOil and Polysaccharide from Schisandra chinensis(Turcz.) Baill Jun-Han Li, Wei LiD, Sha Luo, Chun-Hui Ma*HiDand Shou-Xin Liu* College of Material Science and Engineering, Northeast Forestry University, Harbin 150040, China;nefulijunhan@163.com(J.-H.L.); liwei19820927@126.com (W.L.);luo.sha.85@163.com (S.L.) * Correspondence: mchmchmchmch@163.com(C.-H.M.);liushouxin@126.com (S.-X.L.);Tel.: +86-451-8219-1204 (C.-H.M.); +86-451-8219-1502 (S.-X.L.) Received: 4March 2019; Accepted: 1 April 2019;Published: 2 April 2019 check forupdates Abstract: An alternating synergetic ultrasound/microwave method was applied to the simultaneousextraction of essential oils and polysaccharides with deep eutectic solvent (DES) from Schisandrachinensis. Under the optimal conditions, extract in the selected choline chloride-ethyleneglycol 1:3 solvent yielded 12.2 mL/kg and 8.56 g/100g of essential oils and polysaccharides,respectively. The free radical scavenging and immunological activities of the polysaccharidesand the antioxidant activity of the essential oils have also been investigated. The lymphocyteproliferation capacity was substantially improved by adding concanavalin A or lipopolysaccharidesto polysaccharides (0.20 mg/mL). The IC50 values of the essential oils for scavenging DPPH obtainedby hydro-distillation and DES ultrasound/microwave-assisted hydro-distillation (DES UMHD) were52.34 ug/mL and 29.82 ug/mL,respectively. The essential oil obtained by DES UMHD had thehighest reducing power (856.05 (TE)/g) at 150 g/mL and had the strongest inhibitory capacity (SC%=18.12%). S. chinensis has the potential to be developed as a natural antioxidant. Keywords: deep eutectic solvent; ultrasound/microwave digestion; Schisandra chinensis (Turcz.) Baill;essential oil; polysaccharide 1. Introduction Deep eutectic solvents (DESs) are a sustainable alternative to ionic liquids derived fromgreen natural and renewable components. Compared with the traditional extraction methods,DES pretreatment can enhance digestibility, reduce energy consumption, and simplify procedures forsolvent recovery. DES can be formed by mixing simple quaternary ammonium salt such as cholinechloride (ChCl) with appropriate Hydrogen Bond Donor (HBD) (such as metal halides, acids andalcohols) under heating [1]. These prepared DES are non-toxic, easily synthetic, and have low cost ofpreparation. There are reports on the applications of DESs for the extraction of phenolic compounds [2],polysaccharides [3], and proteins [4] from plants, and research continues to explore more applications. The study of different assisted methods, such as ultrasound-assisted,which use the mechanical,cavitation, and thermal effects of ultrasound to increase penetration of the medium by increasing thevelocity of its motion [5], has been conducted in recent years. The cavitation produced by ultrasoundgreatly improves the heterogeneous reaction rate, achieves uniform mixing between heterogeneousreactants, accelerates reactant and product diffusion, promotes the formation of new solid phases,and controls the size and distribution of particles [6,7]. In contrast, microwave irradiation is asan energy source to induce molecular movement and rotation of liquids with permanent dipoles [8]. Thus, the biomass material absorbs microwave energy and cell temperature increases rapidly, such thatthe internal pressure of the cell exceeds the expansion capacity of the cell wall, resulting in cellularbreakdown. This allows intracellular active ingredients to flow freely and be captured and dissolvedat lower temperatures. Thus, it has a wide application range [9,10]. Alternate ultrasound/microwave extraction technology, which directly combines the twoextraction modes to make full use of both ultrasound vibrational energy and thermal effect ofmicrowave irradiation [11]. This method has many advantages, such as fast heating speed, low energyconsumption, low solvent requirements, and high recovery rate. Simultaneous ultrasound/microwaveextraction is also beneficial for the extraction of polar and thermally unstable components and avoidsthe decomposition that results from extraction or synthetic procedures with long processing times,high temperatures, and high pressures [12]. This technique has been applied to the extraction ofalkaloids [13], flavonoids [14], polysaccharides [15], and other active substances from plants. Schisandra chinensis (Turcz.) Baill (S. chinensis) polysaccharides have attracted the attentionof researchers with its liver protecting, kidney reinforcing, anti-fatigue, anti-oxidative, immunityenhancing, antiaging, tumor inhibition, and anti-diabetic effects [16,17]. S. chinensis essential oils areextracted from plant-specific aromatic substances and can prevent cough, promote DNA synthesis,and be an inhibitor of plasminogen activation [17,18]. The production of natural ingredients requiresa preliminary study of compound separation and for optimal extraction methods and conditionsto be established [19]. Although a variety of solid-liquid extraction methods can be used to obtainnatural ingredients, these procedures have shortcomings, including long extraction times, large solventrequirements, and partial loss of natural molecules, such as phenolic compounds. In the field of food chemistry, simultaneous ultrasound/microwave is often used to extractnatural products. In contrast, alternating ultrasound/microwave extraction technology has rarely beenreported [15]. However, the efficiency of synergetic ultrasound/microwave extraction is not necessarilybetter than that of alternating ultrasound/microwave digestion, and the extraction efficiency ofnatural products is greatly influenced by combining extraction modes in an alternating fashion [14].Therefore, this work aimed to develop an alternating ultrasound/microwave extraction method withDES that simultaneously extracts higher yields of polysaccharides and essential oils to study theoptimal combination of alternating ultrasound/microwave extraction methods, and analyzed itsmechanism. The extraction mechanisms of the alternating ultrasound/microwave methods are alsoexplained in detail. After deproteinization, free radical scavenging activity and cell proliferationof the polysaccharides, the antioxidant activities of the essential oils were investigated [20,21].This also demonstrates that S. chinensis polysaccharides and essential oils might be used as potentialnatural antioxidants and immunostimulants that conform to the principles of sustainability andgreen chemistry. 2. Results and Discussion 2.1. Screening of DES In a typical reaction, ChCl (hydrogen bond acceptor) and ethylene glycol (EG), glycerol (GI),or 1,4-butanediol (BDO) (hydrogen bond donors) were mixed. A greater number of OH is provided byGI when the ratios of polyol are the same; however, better water absorbent performance of GI mayform intermolecular hydrogen bonds, resulting in a decrease in the number of hydrogen bonds withChCl. Compared with BDO,steric effected the formation of intermolecular hydrogen bonds withsmaller ChCl. Therefore, the screening optimization of DES HBA and HBD is of great significance.In our study, ChCl and EG, GI or BDO were mixed separately in different molar ratios (1:1, 1:2, 1:3,1:4, 1:5), and heated at 80 °C oil bath until a transparent solution was obtained. Then, water wasadded to maintain a solution ratio (DES:H2O) of 7:3 v/v. The polysaccharide extracted by usingvarious DESs is presented in Figure 1a. The results show that the DES solvent (ChCl-EG 1:3 molarratio) extracted chitin showed a high yield of 6.86 ±0.10 g/100g. After that, the DES was selected as ChCl-EG 1:3, and the solution ratios (5:5, 6:4, 7:3, 8:2, 9:1) were changed to obtain an optimal conditionof 7:3 v/v. In addition, the viscosity of the solution with different solution ratios was measured-itincreased with the increase of solution ratio, especially after 7:3 v/v, and the two showed a goodnonlinear correlation. The viscosity standard curve followed the formulaY=-102.88+486.13X-744.75X²+389.24X3(R²=0.9996) (Figure 1b). Therefore, ChCl-EG 1:3 (7:3 v/v) was selected as DESfor subsequent experiments. Figure 1. Screening of DES: (a) Effect of DES composition on the extraction yield of polysaccharide,(b) effect of water amount in the DES solution on the extraction yield of polysaccharide. 2.2. Single Factor Analysis of Polysaccharide Extraction 2.2.1. Kinetic Study of Polysaccharide Extraction Using reflux extraction, the extraction yield of S. chinensis polysaccharides tended to be balancedafter 4 h, reaching 8.56 g/100g (dry weight). In the ultrasound extraction process, the polysaccharideyield increased with increasing extraction time (Figure 2), but showed little further variation afteran extraction time of 40 min, remaining at 7.44±0.18 g/100g.In the microwave extractionprocess, the polysaccharide yield increased with increasing extraction time, but showed almostno further change after an extraction time of 20 min, with the polysaccharide yield remaining at8.02±0.26 g/100g. Therefore, the optimal extraction times for the extraction methods were 4 h,40 min, and 20 min for reflux, ultrasound-assisted, and microwave-assisted extraction, respectively. Figure 2. Kinetic study of polysaccharide extraction. 2.2.2. Solid-Liquid Ratio for Extracting Polysaccharides Using the reflux extraction method to optimize the solid-liquid ratio for polysaccharide extraction,five S. chinensis dry powder samples (each 10.0 g) were added to distilled water at solid-liquid ratios of1:10, 1:20,1:30,1:40, and 1:50 w/v, refluxed for 4 h, and the polysaccharide extraction rates calculated(Figure 3a). When the solid-liquid ratio was increased from 1:10 to 1:30, the polysaccharide extractionrate increased. Solid-liquid ratios of more than 1:30 resulted in lower polysaccharide extraction rates.Therefore, the optimal solid-liquid ratio was 1:30 (w/v). 2.2.3. Power of Ultrasound and Microwave Treatments for Polysaccharide Extraction Using the optimal conditions of S. chinensis dry powder (10.0 g) in distilled water at a1:30 (w/v)ratio, the extractions were done using different amounts of ultrasound and microwave power. Singlefactor experiments were performed for ultrasound and microwave power (Figure 3b,c). At ultrasoundpowers of less than 550 W, the polysaccharide yield increased with increasing ultrasound power.However, the polysaccharide yields at ultrasound powers of 550 W and 700 W were similar. Therefore,an ultrasound power of 550 W was selected for subsequent experiments. In the microwave extractionprocess, the polysaccharide yields gradually increased with increasing microwave power, with 250 Wselected as optimal microwave power for subsequent experiments. (b) Figure 3. Cont. (c) Figure 3. Single factor analysis of polysaccharide extraction. Effect of extraction parameters onpolysaccharide yield:(a) Solid-liquid ratio, (b) ultrasound power, and (c) microwave power. 2.3. Optimization Extraction Parameters of Polysaccharides by Response Surface Method (RSM) 2.3.1. Optimization Parameters for Ultrasound-assisted Extraction (UAE) To further study the interactions between the factors, we optimized the solid-liquid ratio,extraction time, and ultrasound power. In Table 1, the maximum value of extraction efficiencywas defined as 100%, and compared with the other values. The model F-value of 19.51 indicatedthat the model was significant, but the probability for the occurrence of such model F-value wasonly 0.01%, which can be treated as statistical noise. Values of "Probability>F" less than the 0.0500indicated model terms were significant. The "lack of fit F-value" of 247.97 implied that the lack offit was significant. The probability of the occurrence of such a "lack of fit F-value" was only 0.01%and can be treated as statistical noise. The response surfaces for the effect of independent variables onextraction efficiency of lignans are shown in Figure 4. Results of the test of significance for regressioncoefficient for ultrasound-assisted extraction is given in Table S1 of Supplementary Information. Table 1. Test of significance for regression coefficient for ultrasound-assisted extraction. Source Sum of Squares df Mean Square F-Value p-Value Prob>F Model 43.18 14.39 19.51 <0.0001 A:liquid-solid (mL/g) 38.00 38.00 51.50 <0.0001 B:Extraction time (min) 3.31 3.31 4.48 0.0502 C:UAE power (W) 1.87 1.87 2.53 0.1310 Residual 11.81 0.74 Lack of Fit 11.78 1.07 247.97 <0.0001 Pure Error 0.022 4.320×10-3 Cor Total 54.98 The final extraction efficiency (Y) was given by Y=7.75+1.67A+ 0.49B +0.37C. The softwarepredicted that the yield of polysaccharides was 8.44 g/100g; however, in our realistic operation process,the parameters of ultrasoundpower, extraction time and solid-liquid ratio were selected as 550 W,40 min, and 1:30 respectively; the yield of polysaccharides was 8.56 g/100g,and error rate was 1.42%. Figure 4. The response surface for the effect of independent variables on ultrasound-assisted extractionefficiency of polysaccharides. (a) Solid-liquid ratio and extraction time, (b) solid-liquid ratio andultrasound power. 2.3.2. Optimization Parameters for Microwave-assisted Extraction (MAE) To further study the interactions between the factors, we optimized the solid-liquid ratio,extraction time, and microwave power. In Table 2, the maximum value of extraction efficiencywas defined as 100%, and compared with the other values. The model F-value of 17.30 indicated thatthe model was significant, but the probability for the occurring of such model F-value was only 0.01%,which can be treated as statistical noise. Values of"Probability> F"less than the 0.0500 indicated modelterms were significant. The "lack of fit F-value" of 47.86 implied that the lack of fit was significant.The probability of the occurrence of such a "lack of fit F-value" was only 0.03% and can be treated asstatistical noise. The response surfaces for the effect of independent variables on extraction efficiencyof lignans are shown in Figure 5. Besides, all results of the test of significance for regression coefficientfor microwave-assisted extraction can be checked in the Supplementary Information Table S2. Table 2. Test of significance for regression coefficient for microwave-assisted extraction. Source Sum of Squares df Mean Square F-Value p-Value Prob> F Model 34.91 9 3.88 17.30 <0.0001 A:liquid-solid(mL/g) 24.17 1 24.17 107.82 <0.0001 B:Extraction time (min) 3.45 3.45 15.39 0.0029 C:MAE power (W) 2.27 2.27 10.14 0.0098 AB 0.42 0.42 1.87 0.2017 AC 0.26 0.26 1.17 0.3043 0.023 0.023 0.10 0.7548 1.73 1.73 7.70 0.0196 2.90 2.90 12.93 0.0049 0.33 0.33 1.48 0.2523 Residual 2.24 0.22 Lack of Fit 2.20 0.44 47.86 0.0003 Pure Error 0.046 9.177×10-3 Cor Total 37.16 The final extraction efficiency (Y) was given by Y= 8.33+1.33A+0.50B +0.41C+0.23AB +0.18AC+0.054BC -0.35A2-0.45B2-0.15C. The software predicted the yield of polysaccharideswas 8.33 g/100g, however, in our realistic operation process, the parameters of microwave power,extraction time and solid-liquid ratio were selected 250 W, 20 min and 1:30 respectively, and the yieldof polysaccharides was 8.44 g/100 g, the error rate was 1.32%. B: Extraction time (min) (a) (b) (c) Figure 5. The response surface for the effect of independent variables on microwave-assisted extractionefficiency of polysaccharides. (a) Solid-liquid ratio and extraction time, (b) extraction time andmicrowave power, (c) solid-liquid ratio and microwave power. 2.4. Alternate Ultrasound/Microwave Extraction Design Based on the above optimum experiments, the experimental arrangement of alternatingultrasound and microwave-assisted extractions of polysaccharides was designed, and the essentialoil yield was also detected.The results showed that microwave-assisted extraction obtained a higherpolysaccharide yield (7.83±0.12 g/100g compared with 7.02±0.11 g/100g for ultrasound-assistedextraction).). In alternating experiments C-F, with extraction time under the same conditions,the polysaccharide yield obtained by microwave extraction was slightly higher than that by ultrasoundtreatment at first, with a higher number of alternations leading to a shortened extraction time andhigher polysaccharide yield (Figure 6). Figure 6. Alternate and synergistic ultrasound/microwave extraction of polysaccharides. Important factors for evaluating extraction methods are extraction rate, cost, ease of operation,time, and environmental pollution. Herein, the alternating ultrasound and microwave extractionmethods were evaluated with respect to the yield, extraction time, and environmental assessment ofthe crude polysaccharides. Refluxing extraction requires an extraction time of 4 h and uses a heatingsleeve that consumes 1 kW of power every hour, giving a total energy consumption of 4.0 kWh,and emits around 3200 g of carbon dioxide. However, on using microwave heating power of 0.25 kWand ultrasound auxiliary power of 0.55 kW, the alternating extraction method emitted only 360 gof carbon dioxide per experiment. Alternating experiment F achieved the highest polysaccharideextraction rate of 8.87 ±0.29 g/100g (Table3). Table 3. Experimental program of alternating ultrasound/microwave extractions of polysaccharides. Item Essential Oil Polysaccharide Environmental Impact Experimental Program Yield (mL/kg) Yield (g/100g) (g CO2 Rejected) A UAE 60 min 10.6 7.02±0.11 440 B MAE 60 min 10.9 7.83±0.12 200 C UAE 40 min + MAE 20 min 11.2 8.19±0.20 360 D MAE 20 min + UAE 40 min 11.5 8.48±0.33 360 E UAE 20 min +MAE10 min+ UAE 20 min +MAE 10 min 11.6 8.50±0.31 360 F MAE 10 min +UAE 20 min + MAE 10 min + UAE 20 min 12.2 8.87±0.29 360 G UAE & MAE 60 min 11.9 8.79±0.26 640 H Reflux extraction 4h 11.7 8.56±0.30 3200 In the electric field generated by microwaves, friction occurs between the moving ions and themedium, which may cause heating; besides, dipolar molecules attempt to align with alternating electricfields in the medium, and their oscillations cause collisions with surrounding molecules to generateheat. As the temperature inside the cell rises rapidly, the intracellular pressure increased beyond thecapacity tolerated by the expansion of the cell wall, resulting in cell rupture, which accelerated therate at which the polysaccharide molecules of S. chinensis diffused from the solid to the solid-liquidinterface. Then, at sufficiently high power, the sparse cycle produced by the ultrasound might exceedthe attractive force of the molecules of the fluid and form cavitation bubbles. The suspended solidpromotes the collapse of the asymmetric bubble, which produces extremely high-speed jets that aredirected at S. chinensis, thereby promoting its extraction. Using SEM to observe the fruit before and after different extraction methods, it was concludedthat ultrasound expanded and ruptured the cells through shaking and cavitation, while microwavedestroyed most of the cell walls in the alternating treatment, which greatly improved the rate ofheterogeneous reaction and achieving uniform mixing between the heterogeneous reactants (Figure 7).As such rupture has rarely been observed using ultrasound, it is conceivable that essential oilspermeated the cuticle to be extracted during ultrasound treatment. The schematic representation of the analysis mechanism of optimal alternating ultrasound andmicrowave-assisted extraction of S. chinensis polysaccharides is shown in Figure 8. The dried fruit ofS. chinensis has a high degree of lignification and the surface of the raw material is dense. The first stepis microwave-assisted extraction-the microwave selectively heats the intracellular polar molecules,such that the intracellular pressure of the cells is larger than the extracellular pressure; and ruptures andsome effective substances are dissolved from the cells. In the ultrasound method, due to its cavitationeffect, the bubble mix in the extracellular solvent collapses, and the jet is formed outside the cell toincrease the flow rate of the solvent on the cell surface and enter the cell interior. In the microwavetreatment (third step), the thermal effect not only cause the cells to swell again, but also rapidlytransfers the solvent of the dissolved polysaccharide to the extracellular, which greatly increasesextraction efficiency and allows more effective substances to be dispersed outside the cells. Therefore,when the fourth step uses more ultrasound, the permeability of the cell membrane is further increaseddue to the oscillating effect, and the instantaneous large pulling force generated by the gas blastingcauses more solvent to enter the cell; the solvent and gas dissolved in the solvent also enter the cell,and bubble collapse occurs, such that the effective substance is better dissolved in the solvent. Figure 7. SEM images of S. chinensis fruits. (a) S. chinensis fruit raw materials, (b) S. chinensis fruitstreated by ultrasound digestion, (c) S. chinensis fruits treated by microwave digestion, and (d) S. chinensisfruits treated by alternate ultrasound/microwave digestion. Figure 8. The mechanism of alternating ultrasound and microwave-assisted extraction. In summary, alternate ultrasound/microwave extraction treatment destroys the samplemicrostructure without a high-temperature heat source, which could eliminate the temperaturegradient and heating speed, while avoiding decomposition and isomerization of natural products dueto long extraction times. Therefore, compared with reflux extraction, this method is more conducive forthe extraction of heat-sensitive substances. It is a more efficient extraction method in the pretreatmentof plant material in the food chemistry field. 2.5. Polysaccharide Deproteinization An increasing amount of deproteinized proteins causes the protein content in the crudepolysaccharide solution to gradually decrease, and the polysaccharide content to diminish (Figure 9).In the first four deproteinizations, the protein content reduction ratio and polysaccharide loss werelarger. After four rounds, the protein and polysaccharide content slowly decreased. In order to ensurepolysaccharide content, deproteinization was performed no more than four times. The deproteinizedpolysaccharide solution was concentrated and lyophilized to obtain refined S. chinensis polysaccharidesin a yield of 5.11 g/100 g S. chinensis. This process eliminates DES residues and completely separatesDES from the polysaccharides. Figure 9. Polysaccharide content after removing protein 12 times with the Sevag method. 2.6. Free Radical Scavenging Activity of Polysaccharides Excellent antioxidant activity of S. chinensis polysaccharide was demonstrated in hydroxylradical scavenging tests (Figure 10). The hydroxyl radicals were produced by Fenton reaction.Hydrogen peroxide/Fe+ system can produce free radicals as follows: H2O2+Fe2+→·OH+OH+ Fe+. It can be seen that the generation of a hydroxyl radical depends on the content of Fe2+ andH202. The polysaccharide scavenges the excessive oxygen free radicals produced in vivo and blocksthe free radical reaction chain in vivo. The polysaccharide was formulated to a concentration of0.5 mg/mL, and serially diluted to 0.4, 0.3, 0.2, 0.1 mg/mL. Polysaccharide sample solutions andvitamin C (VC) solutions with the same concentrations were prepared. At low concentrations (lessthan 0.20 mg/mL), the polysaccharide free radical scavenging activity was similar to that ofVC.At a concentration of 0.20 mg/mL, the free radical scavenging activities were 28.02%(polysaccharide)and 23.68% (VC). However, at sample concentrations of more than 0.20 mg/mL, the scavengingactivity of the polysaccharide increased with increasing concentration, while the hydroxyl radicalscavenging activity of VC gradually plateaued. At a concentration of 0.50 mg/mL, the clearancerates using the polysaccharides and VC were 66.3% and 36.49%, respectively. Therefore, the hydroxylradical scavenging activity of S. chinensis polysaccharides was better than that of VC at sampleconcentrations of more than 0.20 mg/mL. This was because according to the Fenton reaction, there arethree reagents-oxidant, reducer and metal ion-and the generation of a hydroxyl radical depends onthe content of Fe+and H2O2. However, there are several nature metal ions (such as Fe, Zn, Cu) inpolysaccharide. Moreover, if the absolute concentrations of Fe+ are higher than H2O2 and VC, a partof VC was used to reduce Fe+, which results in inhibition of H2O2. Finally, the Fenton reaction was inhibited and OH was not generated [22,23]. Therefore, the hydroxyl radical scavenging activity wasbetter at higher concentrations of polysaccharides compared to VC. Concentration of polysaccharides (mg/mL) Figure 10. Hydroxyl radical scavenging activity of polysaccharide. 2.7. In vitro MTT Activity Test of Polysaccharides S. chinensis polysaccharides have strong immune activities that can significantly promote theproliferation of mouse splenic lymphocytes, with increasing polysaccharide concentration alsoincreasing the cell proliferation rate (Table 4). At a polysaccharide concentration of 0.20 mg/mL,the cell proliferation rate was the largest, at about 30%. When the polysaccharide concentrationreached 0.80 mg/mL, the cells showed almost no proliferation, with a proliferation rate of less than5.00%. Therefore, 0.20 mg/mL was selected as the optimal S. chinensis polysaccharide concentration topromote cell growth. Table 4. Effect of polysaccharide concentration on mouse spleen cell proliferation. Concentration Parallel Sample 1 Parallel Sample 2 Parallel Sample 3 (mg/mL) PR/% A PR/% A PR/% A 0.80 1.54 0.7268 2.53 0.7339 4.62 0.7489 0.40 24.35 0.8914 24.36 0.8902 25.44 0.8979 0.20 29.48 0.9268 30.58 0.9347 29.30 0.9255 0.10 29.66 0.9281 23.54 0.8843 26.15 0.9030 0.05 20.86 0.8651 22.63 0.8778 23.40 0.8833 Control group 0.7194 0.7118 0.7162 ConA and Lps are effective mitogens that promote lymphocyte transformation. This experimentconfirmed the synergistic effect of S. chinensis polysaccharides and ConA or Lps cells. The role of ConAand Lps was to proliferate more than twice as much as the polysaccharides (Table 5). Meanwhile,adding polysaccharides and ConA or Lps cells significantly increased lymphocyte proliferation capacity.The synergistic effect of S. chinensis polysaccharides with the Lps cells was about 1.5 times better thanthat with the ConA cells. Table 5. Effect of adding ConA and Lps on mouse spleen cell proliferation. Concentration Parallel Sample1 Parallel Sample 2 Parallel Sample 3 (mg/mL) PR/% A PR/% A PR/% A 0.20 29.48 0.9268 30.58 0.9347 29.30 0.9255 0.20 +ConA 108.76 1.4943 107.11 1.4825 108.97 1.4958 0.20+Lps 146.76 1.7663 143.15 1.7405 150.42 1.7925 ConA 79.04 1.2816 80.33 1.2908 80.53 1.2922 Lps 79.06 1.2817 79.23 1.2829 78.85 1.2802 Controlgroup 0.7194 0.7118 0.7162 2.8. Kinetic Study ofEssential Oils The traditional hydro-distillation method is used to extract essential oil; essential oils precipitateswith boiling water at 100°C, and the polysaccharide is extracted by aqueous extraction-alcoholprecipitation method. Thus, the DES hydro-distillation method was suitable for simultaneouslyextracting crude polysaccharides and essential oils, and the crude polysaccharide was precipitated bysubsequently increasing the alcohol content of the DES solution, and then filtered, which completelyseparated polysaccharide from DES solution. When the extraction time of MHD and DES UMHDtreatment was increased from 0 min to 20 min, the average essential oil extraction efficiencyincreased dramatically. Therefore, 20 min was selected as the extraction time for further experiments.The essential oil yield showed little change after 25 min of MHD and DES UMHD treatment. However,in the HD reaction, essential oil precipitation slowly began to appear after 30 min, with 120 minrequired to reach the essential oil yield obtained with the other methods, and further treatmentresulted in a small steady increase (Figure 11). This phenomenon, which is attributed to heat transfer,is mainly achieved through conduction and convection only in the HD process. In contrast, in the MHDand UMHD processes, heat transfer was achieved through radiation, conduction, and convection.The ionization of DES in water produced salts that make the essential oils more likely to precipitate.Among them, DES UMHD produced the highest essential oil yield. As for ChCl-based acidic DESs,studies have inferred that CF of ChCl (HBA) with strong H bond acceptability could help to disruptthe intermolecular hydrogen bonding network of biomass and facilitate its dissolution, which is benefitfor the subsequent HBD access and attack on the acid catalytic sites of biomass. Thus, better biomassdeconstruction and hydro-distillation performance of DESs solution was achieved as compared to thewater, thereby increasing the yield of the essential oil [24]. Figure 11. Kinetic study of essential oil extraction. 2.9. Antioxidant Analysis of Essential Oils In our study, the antioxidant capacity of S. chinensis essential oils obtained using differentextraction methods was studied in vitro using three complementary test systems, namely DPPH,FRAP, and B-carotene scavenging activity assays. Freshly prepared DPPH solution has a deep purple color with maximum absorption (1.24) at517 nm that generally fades when an antioxidant is present in the medium. Therefore, antioxidantmolecules can annihilate hydrogen atoms or electrons provided by DPPH and convert them intocolorless products, resulting in a decrease in absorbance at 517 nm. With increasing essential oilconcentration, a significant increase in inhibition rate was observed, with the low IC50 value indicatingthat the sample had strong antioxidant activity.The IC50 values of DES UMHD and HD samples were29.82 ug/mL and 52.34 ug/mL, respectively (Figure 12a). In summary, the DPPH scavenging effect wasreduced in the order VC>BHA> DES UMHD>HD. In this study, the antioxidant activity of essentialoils has shown that monoterpenes have a higher antioxidant effect, while the major component ofS. chinensis essential oil were sesquiterpenes. Therefore, in my opinion, the lack of monoterpenes inessential oil was one of the possible reasons for its weak antioxidant activity [25]. This result indicatedthat DES UMHD extracts more essential oils, which had a more pronounced effect on DPPH than HD. Concentration of essential oil (ug/mL) (a) (b) Figure 12. Cont. (c) Figure 12. Antioxidant abilities of essential oils: (a) DPPH free radical scavenging activity of essentialoils, (b) FRAP ferric-reducing antioxidant power of essential oils, and (c) B-carotene scavengingcapacity of essential oils. The reduction capacity of a compound can indicate its potential antioxidant activity. A higherabsorbance indicates a higher ferric-reducing power, with the essential oils from different extractionmethods and standards showing increasing ferric-reducing power as the concentration increased(Figure 12b). At 0.15 mg/mL, the highest reducing power of DES UMHD (A593=1.817) was 856.05 (TE)/g,while the reducing power of HD (A593=0.365) was 130.05 (TE)/g. Therefore, the reducing power ofessential oils and antioxidants was in the order of DES UMHD>VC> BHA>HD. Among the essential oils prepared by different methods, the essential oil resulting from UMHDhad the highest inhibition capacity (SC%=18.12%) in Figure 12c. The inhibition capacity of linoleic acidoxidation by HD was 14.25%. Therefore, for the B-carotene-linoleic acid bleaching assay, a descendingorder of inhibition capacity of VC > DES UMHD>HD> BHA is shown. 2.10. Chemical Composition of S. chinensis Essential Oil by Different Extraction Methods The compositions of S. chinensis essential oil were obtained through HD,MHD and DES UMHD.Thirty-six components of essential oil obtained by DES UMHD and 18 components obtained by HD andMHD were investigated. The relative amount percent of individual volatile compounds was expressedas percent peak areas relative to total peak areas by GC-MS analysis (RA %) (Table 6). The maincomponents were the same-Ylangene, (E)-c-bergamotene and β-himachalene-above 50% of thetotal peak area (Figure 13). Olefin had high unsaturation and strong reducing ability. As can be seenfrom Figure 13, the olefin content was relatively high—Ylangene (RA=28.63%), (E)-c-bergamotene(RA=10.62%), and β-himachalene (RA=10.60%)-thus, showing that the essential oil had a certainantioxidant capacity. Figure 13. Volatile compounds in S. chinensis obtained by DES UMHD. 3. Materials and Methods 3.1. Raw Materials and Chemical Reagents S. chinensis fruit was purchased in October 2017 from Three Trees Market in Harbin City,Heilongjiang Province, China. Mature fruit was picked in September 2017 in the Greater-LesserKhingan Mountains region of Northeast China and identified by Professor Yongzhi Cui of NortheastForestry University (Harbin, China) as S. chinensis fruit originating from Heilongjiang Province.The S. chinensis fruits were air-dried and sieved. The resultant 20-40 mesh powder was cryopreservedat -20C, and uniformly mixed before sampling. Choline chloride (ChCl) was purchased from MacklinBiochemical Co., Ltd. (Shanghai, China). The chemical reagents used in this experiment, Coomassiebrilliant blue G250, bovine serum albumin (BSA), fetal calf serum, dimethyl sulfoxide (DMSO),2,2-Diphenyl-1-picrylhydrazyl (DPPH) and vitamin C (VC), were purchased from Sigma-Aldrich(Louis, Missouri, USA), and the other reagents were purchased from Tianjin Kemiou Chemical ReagentCo., Ltd. (Tianjin, China). All solvents and chemicals were analytical grade. 3.2. Preparation of DES Because ChCl has strong water absorption, it was placed in a vacuum oven at 80 C for 24 hbefore the experiment. A certain amount of ChCl and EG was weighed and placed into a conical flask;the composition of DES was uniformly mixed according to the molar ratio, and the specific molarratios (1:1, 1:2, 1:3,1:4,1:5) were operated [4], heated, melted in an 80 °C oil bath, and magneticallystirred until it became a colorless clear liquid-ChCl-EG mixed with a given volume of water beforeuse. Similarly, ChCl and GI, ChCl and BDO were weighed to obtain ChCl-GI and ChCl-BDO. 3.3. Methods 3.3.1. Polysaccharide Extraction Methods Determination Method of Polysaccharide The phenol-sulfuric acid method uses sulfuric acid to hydrolyze polysaccharides intomonosaccharides, followed by rapid dehydration to result in aldehyde derivatives that formorange-yellow compounds with phenol [26,27]. The absorbance of these compounds was thenmeasured at 490 nm at room temperature using a WFJ2100 UV-visible spectrophotometer, which wasobtained from UNICO (Shanghai, China). Accurately weighed standard glucose (10.0 mg) was placed in a 100-mL volumetric flask andwater was added to the scale of 1.0, 2.0, 4.0, 8.0 and 10.0 mL, respectively, to produce a 10.0 mLsolution. Glucose solution concentration gradient of 2.0 mL was taken and mixed with distilledwater to 10.0 mL. Six percent phenol solution 1.0 mL was then added to it with concentrated sulfuricacid 5.0 mL, followed by shaking and then cooling. The glucose standard curve used the formulaY=13.302X- 0.0469 (R2=0.9994). Determination Method of Protein This method combines Coomassie Brilliant Blue G-250 dye with a basic amino acid and proteinaromatic amino acid residues in an acidic solution, which changes the position of the maximumabsorption peak of the dye from 465 nm to 595 nm at room temperature, with a corresponding colorchange from brown-black to blue. Accurately weighed Coomassie brilliant blue G-250 (100.0 mg) was dissolved in 95% ethanol(50.0mL);85% phosphoric acid (100 mL) was added, and the mixture was diluted to 1,000 mL withdistilled water. Accurately weighed crystal bovine serum protein standard (10.0 mg) was dilutedto 100 mL in a volumetric flask with NaCl solution (0.15 M), giving a 0.1 mg/mL albumin standardsolution. 2.0, 4.0, 6.0,8.0 and 10.0 mL of NaCl solution (0.15 M) was added to produce 10.0 mL of thesolution. To 0.1 mL of the above albumin concentration gradient solution was added 5.0 mL Coomassiebrilliant blue G-250 solution, shaking well. Using BSA as the standard, a protein standard curve wasprepared using this Coomassie brilliant blue G-250 staining method [28]. The standard curve wasdrawn and used the formula Y= 5.865X+0.0009 (R2=0.9994). Compared Extraction Method of Polysaccharides Dried fruit of S. chinensis (10.0 g) was placed in a round bottom flask and hydrothermallydistilled with DES (300 mL). The mixture was refluxed for 4h. After the extraction was complete,the supernatant was filtered, cooled, and anhydrous ethanol was slowly added to give an ethanolvolume fraction of 80%. After centrifugation at 6000 rpm for 10 min with a 3K-30 ultracentrifuge(Sigma-Aldrich), the crude polysaccharide yield was calculated, and the sample was stored at -20 °Cin an LGJ-10 freeze dryer, which was purchased from Brothers Equipment Co., Ltd. (Henan,China).The polysaccharide was extracted using the aqueous extraction-alcohol precipitation method, and DESwas dissolved in alcohol solution and removed with liquid phase after centrifugation. Recovery ofDES was done through decompression concentration. Ultrasound-assisted Extraction (UE) of Polysaccharides Dried fruit of S. chinensis (10.0 g) was placed in a round bottom flask and DES (300 mL) was added.Ultrasound-assisted extraction was conducted in an ultrasound/microwave extraction apparatus(XH-300A, Xianghu Sci.& Tech. Co. Ltd., Beijing, China) for 60 min at 550 W [29]. A sample was takenevery 10 min to obtain the extraction kinetic curve. The same operation methods used in the section“Compared Extraction Method of Polysaccharides" were employed after the extraction was complete. Microwave-assisted Extraction (ME) of Polysaccharides Dried fruit of S. chinensis (10.0 g) was placed in a round bottom flask and DES (300 mL) wasadded. The mixture was placed in the ultrasound/microwave extraction apparatus, and microwaveirradiation was conducted for 30 min at 250 W. A sample was taken every 5.0 min to obtain theextraction kinetic curve. The same operation methods used in the section "Compared ExtractionMethod of Polysaccharides" were employed after the extraction was complete. The extracted essentialoil was dried with anhydrous sodium sulfate and stored in a freezer. Alternate Ultrasound/Microwave Extraction of Polysaccharides Dried fruit of S. chinensis (10.0 g) was placed in a round bottom flask and DES (300 mL) wasadded. The reaction mixture was extracted using alternating microwave and ultrasound treatmentsin the ultrasound/microwave extraction apparatus, and the extraction procedure was optimized.According to Table 3, the experiments A-G used total polysaccharide extraction time of 60 min.The extraction process was optimized by changing the extraction time and extraction sequence of theultrasound/microwave under the same conditions, and using a reflux extraction method (experimentH) for comparison. The same operation methods used in the section "Compared Extraction Method ofPolysaccharides" were employed after the extraction was complete. RSM Optimization Parameters of Polysaccharides To further study the interaction between the factors, we optimized the operating conditions byRSM and used the Box-Behnken software for data processing [29]. The Box-Benhnken design withthree factors was applied using Design-Expert 8.0 without any blocking. The bounds of the ultrasoundfactors were 1:20-1:40 solid-liquid ratio, 400-700 W ultrasound power, 30-50 min extraction time.Specific protocols for experimental conditions are shown in Table 7. Table 7. Experimental design matrix to screen important variables for ultrasound-assisted extraction. Run 3 Factor A Factor B Factor C Response L-S(mL/g) Extraction Time (min) UAE (W) Yield of Polysaccharides (g/100g) 40.00 50.00 400.00 9.39 40.00 50.00 700.00 9.45 40.00 30.00 400.00 9.11 46.82 40.00 550.00 9.77 30.00 40.00 297.73 5.59 20.00 30.00 700.00 6.09 40.00 30.00 700.00 9.26 30.00 40.00 550.00 8.47 30.00 56.82 550.00 8.74 20.00 50.00 400.00 6.82 30.00 40.00 550.00 8.52 30.00 40.00 550.00 8.56 20.00 50.00 700.00 6.22 13.18 40.00 550.00 3.55 30.00 40.00 550.00 8.44 20.00 30.00 400.00 5.76 30.00 23.18 550.00 5.73 30.00 40.00 550.00 8.37 30.00 40.00 550.00 8.46 30.00 40.00 802.27 8.63 Similarly, the bounds of the microwave factors were 1:20-1:40 solid-liquid ratio, 200-250 Wmicrowave power, 15-25 min extraction time. Specific protocols for experimental conditions are shownin Table 8. Table 8. Experimental design matrix to screen important variables for microwave-assisted extraction. Run Factor A Factor B Factor C Response l-s (mL/g) Extraction Time (min) MAE (W) Yield of Polysaccharides (g/100g) 2 40.00 15.00 200.00 7.33 40.00 25.00 250.00 9.56 46.82 20.00 225.00 10.11 30.00 20.00 225.00 8.34 7 30.00 20.00 225.00 8.43 20.00 25.00 250.00 6.84 20.00 15.00 250.00 6.33 30.00 20.00 225.00 8.21 30.00 20.00 225.00 8.44 30.00 20.00 225.00 8.24 20.00 25.00 200.00 6.02 13.18 20.00 225.00 4.45 40.00 15.00 250.00 8.66 30.00 28.41 225.00 8.32 30.00 20.00 267.04 8.52 30.00 20.00 225.00 8.37 20.00 15.00 200.00 6.25 30.00 11.59 225.00 5.66 40.00 25.00 200.00 8.54 30.00 20.00 182.96 7.14 3.3.2. Sevag Deproteinization Method for Polysaccharides For preparation of 2.0% (mass fraction) crude polysaccharide solution, a volume of 1/3chloroform-n-butanol (4:1 v/v) solution was added, full oscillated for 15 min, oscillated twice,centrifuged to remove the precipitate. A small amount of water was taken to measure protein contentand polysaccharide content, then the aqueous phase was added to the 1/3 chloroform-n-butanolsolution and repeated twelve times. The deproteinized polysaccharide solution was concentrated andlyophilized at -20 ℃ [30], and the protein removal efficiency and reserved amount of polysaccharidewere calculated. 3.3.3.Hydroxyl Free Radical Scavenging Method for Polysaccharides S. chinensis polysaccharide (0.05-0.50 mg/mL) was added to a mixed solution (3 mL) containing2-deoxyribose (3 mM), ferric chloride (0.1 mM), hydrogen peroxide (1 mM), ethylenediaminetetraaceticacid (0.1 mM), VC (0.1 mM), and phosphate buffer solution (0.02M, pH 7.4), and heated at 37 °Cfor 1 h. Next, tert-Butanol (1 mL, 1%) and trichloroacetic acid (1 mL, 2.8%) solutions were added,followed by heating at 100 °C for 20 min. The reaction was then cooled to room temperature andanalyzed at 536 nm. The ability of S. chinensis polysaccharide to scavenge hydroxyl free radical withpolysaccharide concentration was analyzed. The unit of polysaccharide concentration was mg/mL,and the unit of cell proliferation rate was %. 3.3.4. In vitro MTT Activity Test Method for Polysaccharides Polysaccharides are a class of active ingredients that can regulate immune activity and delayaging [31]. The amount of crystals produced was proportional to the number of living cells(succinate dehydrogenase in dead cells disappeared and MTT cannot be reduced). The mouse spleniclymphocytes were cultured in DMEM with 10% FBS. First, cell suspensions were seeded in 96-wellplates (1×105/well),and incubated at 37 °C for 12 h; then the polysaccharides were added. After 6 h,a certain amount of MTT were added into each well and the plate was further incubated for 4 h. Finally,the medium was removed and 200 uL DMSO was added to dissolve the formazan. After 10 min,absorbance at 490 nm was measured [321. 3.3.5. Essential Oil Extraction Methods Hydro-distillation (HD) Extraction of Essential Oils Dried fruit (10.0 g) was hydrodistilled using a Clevenger-type instrument and extracted with DES(300 mL) for 4h until no further essential oil was obtained. The essential oil was collected, dried overanhydrous sodium sulfate, and stored at 0 °℃ [33]. Microwave-assisted Hydro-distillation Extraction (MHD) of Essential Oils The MHD process is based on a conventional hydro-distillation system, but with microwaveirradiation used during the heating process. An ultrasound/microwave extraction apparatus wasused to heat the sample, a mixture of S. chinensis (10.0g) and DES (300 mL), in which the microwavepower was 250 W. Ultrasound/Microwave-assisted Hydro-distillation (UMHD) Extraction of Essential Oils Dried fruit (10.0 g) were mixed with DES (300 mL, 0.75M) in a round bottom flask. The ultrasoundpower was 550 W, and the microwave power was 250 W. After UMHD extraction, the essential oilswere collected. 3.3.6. Chemical Composition Analysis of Essential Oil GC-MS analysis of the essential oil was carried out on an Agilent 6890N-5973 insert gaschromatograph (Agilent Technologies, Palo Alto, CA, USA) with a capillary column (30mm x0.25 mm,film thickness 0.25 um) equipped with an Agilent 6890N-5973 mass selective detector in the electronimpact mode. The GC was operated under the following conditions: manual injection 1 pL; injectortemperature, 270°C; carrier gas (He) flow 1 mL/min; oven temperature programmed 40 °C to 165℃at a rate of 10 °C/min, from 165 °C to 200 °C at a rate of5°C/min and 200 °C to 250°C at a rateof 10°C/min. The detector temperature was 280 °C. The MS was operated under 70 eV, scan range15-500 amu, scan-TIC. The chemical compositions of essential oils were identified by direct comparisonof their mass spectral pattern in NIST02 Mass Spectral Library. 3.3.7. Scanning Electron Microscopy (SEM) Microstructure analysis was performed using a scanning electron microscope (FEI QUANTA200,Eindhoven, Netherlands). To obtain conductivity, a thin layer of gold was sputtered onto the samplesurface using an SCD 005 Sputter Coater (BAL-TEC, Aavorstadt, Switzerland). 3.3.8. Antioxidant Analysis Methods for Essential Oils Free Radical Scavenging Activity A 1.0 mL methanolic solution of essential oil was mixed with 2.0 mL methanolic solution of DPPH(35 mg/L). The absorbance of mixture was measured at 517 nm after 30 min in darkness. VC was usedas the positive control [34,35]. Ferric Reducing/Antioxidant Power (FRAP) Assay The capacity for reducing antioxidants was determined using the FRAP assay [36]. Tests wereperformed in triplicate and repeated with VC as the control. The reducing antioxidant power of thesample was expressed in Trolox equivalents, which is the ratio of the slope of the sample regressionline to the Trolox slope. Scavenging efficiency of β-carotene A stock solution of -carotene-linoleic acid was prepared as follows [37,38]. The procedurewas repeated with the same concentration of VC and a blank containing only ethanol (500 uL).After incubation, the absorbance of the mixture was measured at 490 nm. 4. Conclusions DES was found as a green medium to successfully extract high purity S. chinensis polysaccharidesand essential oils. The optimal reagent was prepared from choline chloride and ethylene glycol withmolar ratio 1:3 and solvent ratio 7:3 v/v. By optimizing the ultrasonic/microwave, an extractioncondition of 550W (UE), 250W (ME), and solid-liquid ratio of 1:30 w/v was obtained. Besides,in the experiment of S. chinensis polysaccharides, it was found that the crude polysaccharide wasdeproteinized four times by Sevag method to obtain maximum fine polysaccharide (5.11 g/100g);in the scavenging activity of S. chinensis polysaccharide toward hydroxyl radical, we found whenthe sampling concentration was more than 0.20 mg/mL, and the scavenging activity had almostdoubled. The in vitro activity test of polysaccharides showed that 0.20 mg/mL was the optimalconcentration of S. chinensis polysaccharides with Lps cells-it was about 1.5-fold better than thatwith ConA cells. Similarly, the essential oil extraction method was compared and 400 W (UE) and20 min were selected. In the antioxidant experiments of essential oils, the free radical scavengingactivity of DES UMHD extracts determined by B-carotene is first-rate. Furthermore, the FRAP assay ofessential oils showed that the DES UMHD sample had optimal antioxidant activity. This indicates thatDES presents an efficient separation of essential oils and polysaccharides from natural plants underultrasound/microwave digestion. Supplementary Materials: The following are available online, Table S1: The credibility analysis of the regressionequations for ultrasound-assisted extraction, Table S2: The credibility analysis of the regression equations formicrowave-assisted extraction. Author Contributions: J.-H.L. and S.L. conducted experiment; W.L. and C.-H.M. analyzed data and preparedthe tables and figures; J.-H.L., W.L. and C.-H.M. designed this research work and wrote the manuscript;S.-X.L. contributed chemical reagents and analytical tools. Funding: This research was funded by the National Natural Science Foundation of China (31890773),the Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province in 2016 (LBH-Q16001),the Natural Science Foundation of Heilongjiang Province for Young Scholar (QC2015034), the Research Start-upFunding of Introduce Talents in Northeast Forestry University (YQ2015-02), and the Fundamental Research Fundsfor the Central Universities (2572017ET02). Acknowledgments: We thank Simon Partridge from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac),for editing the English text of a draft of this manuscript. Conflicts of Interest: The authors declare no conflict of interest. References ( 1. Jeong, K.M.; Yang, M . ; Jin, Y.; K im, E.M.; Ko, J.; L ee, J. Identification of major flavone C-glycosides andtheir optimized extraction from C y mbidium kanran using deep eutectic solvents. Molecules 2017, 22, 2006. [ CrossRe f ) ( 4. Saravana, P . S.; H o, T.C.; Chae, S.J.; Cho, Y.J.; Park, J.S.; Lee, H.J . ; Chun , B.S . Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohyd. Polym. 2018, 195, 622-630. CrossRe f l ) ( 5. Sun, J.X.; Mei, Z.X.; Tang, Y.J; Ding, L.J;Jiang, G.C.; Zhang, C.; Sun, A.D. ; Bai, W.B. Stability, Antioxidant capacity and d egradation kinetics of pelargonidin-3-glucoside exposed to ultrasound power a t low temperature.Molecules 2016,21,1109. [C rossRef ] [ Pu b M e d] ) ( 6. Vauchel, P.; Colli, C.;Pradal, D.; Philippot, M.; Decossin, S.; Dhulster, P.;Dimitrov,K. Comparative LCA ofultrasound-assisted extraction of polyphenols from chicory grounds und e r different operational conditions. J.Clean. Prod. 2018 , 196,1116-1 1 23. [ CrossRef ] ) ( 7. Otu, P.N.Y.; Jiang, H.N.;Zhou,C.S.; Yang, H.P. Sorghum Bicolor L. leaf sheath polysaccharides: Dual frequencyultrasound-assisted extraction and desalination. Ind. Crop. Prod.2018,126,368-379. [ C rossR e f] ) ( 8. Ma, C.H.; Yang, L.; Wang, W.J.; Yang, F.J.; Zhao, C.J.; Zu, Y.G. Extraction of dihydroquercetin from Larixgmelinii with ultrasound-assisted and microwave-assisted alternant digestion. Int. J. M o l . Sci. 20 1 2, 13 , 8789-8804. [ C rossRef] [ PubMed] ) ( 9. Yuan,Y.; Xu, X.; Jing,C.L.; Zou, P.; Zhang, C.S.; Li, Y.Q. Microwave assisted hydrothermal extraction ofpolysaccharides f r om Ul v a prolifera: Functional properties and bi o activities. Carbohyd. Po l ym. 201 8 , 181, 902-910. [ CrossRef] ) ( 10. Albalawi, A.H.; E l-Sayed, W .S.; A ljuhani, A .; A lmutairi, S .M.; Rezki, N .; A ouad, M . R.; Messali, M.Microwave-assisted s ynthesis of some p otential bioactive imidazolium-based room-temperature i onicliquids. Molecules 2018,23,1727. [ C rossRef] ) ( 11. L . Liu, Z.Z.;Deng, B.Q.; L i , S.L.; Zou, Z.R. Optimization of solvent-free microwave assi s ted extraction ofessential oi l from Cinnamomum camphora le a ves. Ind. C r op.Prod. 2018,124,353-362. [Cr o s sRef] ) ( 12. Liew, S.Q.;Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Sequential ultrasound-microwave assisted acid extraction(UMAE) of pectin from pomelo peels. Int. J. Biol. Macromol. 2016, 93,426-435.[ C r ossRef][P ub Med] ) ( 13. Raone,., AA.; Cassanelli, 1, A.; Scheggi, S.; Rauggi, R.; Danielli. B.; Montis, M.G.D. Hypothalamus-pituitary-adrenal modifications consequent to chronic stress exposure in an experimentalmodel o f depression in rats. Neuroscience 2 0 07, 146, 1734-1742.[Cro ssRef] ) ( 14. Ma, C.H.; Yang, L.; Zu, Y.G.; Liu, T.T. Optimization of conditions of solvent-free microwave extraction andstudy on antioxidant capacity of essential oil fr o m Sch i sandra chinensis (Turcz.) Baill. Food. Che m . 2012, 134, 2532-2539. [ CrossRe f ) ( 15. Zeng, H.L.; Zhang, Y.; Lin, S.; Jian, Y.Y.; Miao, S.;Zheng,B.D. Ultrasonic-microwave synergistic extraction(UMSE) and molecular weight distribution of polysaccharides from Fortunella margarita (Lour.) Swingle. Sep.Purif. Technol. 2015, 144,97-106. [ C rossRef] ) ( 16. Yue, C.J.; Chen, J; Hou, R.R.; T ian, W .J.; L iu, K.H.; Wang, D . Y.; L u , Y.; L iu, J.G.; Wu, Y.; H u, Y . L. The antioxidant action and mechanism of selenizing Schisandra chinensis polysaccharide in chicken embryo hepatocyte. I nt. J . Bio l . Macromol. 2017, 98,506-514 . [ CrossRef] ) ( 17. . Cheng, Z.Y.; Y ang, Y.J; L iu, Y.; Liu, Z.G.; Zhou, H.L.; H u, H . B. T w o-steps extraction of es s ential o i l,polysaccharides a n d biphenyl cyclooctene lignans from Schis a ndra chine n sis Baill frui t s. J. Ph armaceut.Biomed.2014,96,162-169.[C rossRef] [ P ubMed] ) ( 18 Lokesh, K.N.; C hannarayappa; Venkatarangana, M. Exe m plified screening standardization of potentantioxidant nutraceuticals by principles of design of experiments. J. Funct. Foods.2015,17,260-270. ) ( 19. Gao, X.X.;Meng, X.J.; Li, J.H. Hypoglycemic effects of a water-soluble polysaccharide isolated from Schisandrachinensis (Turcz.) Bail. i n alloxan-induced diabetic mice. J. B i o t echnol. 2008, 136,725. ) ( 20. Baj, T.; Baryluk, A.; Sieniawska, E . Application of mixture design fo r optimum anti o xidant activity ofmixtures of essential o ils from Ocimum basilicum L., Origanum majorana L. a n d Rosmarinus officinalis L. Ind . Crop. Prod. 2018,115,52-61.[ CrossRef ] ) ( 22. Zhang, Z.S.; Wang, X.M.; Zhao, M.X.; Qi, H.M. Free-radical degradation by Fe2+/Vc/H2O2 and antioxidant activity of polysaccharide from Tremella fuciformi s . Carbohyd. Polym. 2014,112,578-582. [CrossRe f ] [P u b M ed] ) ( 23. Xiong, X .; Huang, G.L.; H uang, H.L. T h e antioxidant activities of phosphorylated polysaccharide fromnative ginseng. Int. J. Biol. Macromol. 2019,126, 842-845. [C rossR e f] [ P ub M ed ] ) ( 24. Li, A.L.; Hou, X.D.; L in, K.P.; Zhang, X.; Fu, M.H. Rice straw pretreatment using deep eutectic solvents withdifferent constituents molar ratios : Biomass fractionation, polysaccharides enzymatic digestion and solventreuse. J. Biosci. Bioeng. 2018, 126,346-35 4.[CrossR ef ][Pu b Me d ] ) ( 25. Hasheminya, S . M.; M o karram, R. R .; Ghanbarzadeh, B.; Ha m ishekar, H.; Kafil, H. S .; Dehghannya, J.Development a n d characterization o f biocomposite films made from kefiran, carboxymethyl cellulose and S atureja Khuzestanica essential oil. Food. Chem. 2019,18. [ C r oss R ef ) ( 26. Cao, D.Q.; S o ng, X. ; Hao, X.D.; Yang, W.Y.; Iritanib, E.; Katagiri, N. Ca²+-aided separation of polysac c harides and proteins by microfiltration: I m plications for sludge processing. Sep. Purif. Technol. 2018,202,318-325. C rossRef l ) ( 27. Yang, B.;Jiang, Y.M.; Zhao, M.M. Effects of ultrasonic extraction on the physical and chemical properties ofpolysaccharides from longan fruit pericarp. Polym. Degrad. Stabil. 2008,93,268-272.[C r o ssRef] ) ( 28. Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Zhao, Q.; Li, W.J.; Xie, M.Y. Sulfated modification,characterization and antioxidant activities o f polysaccharide from Cyclocarya paliurus. Foo d Hydrocolloid. 2016,53,7-15.[C rossRef] ) ( 29. Ma, C.H.; Liu, T.T.; Yang, L.;Zu, Y.G.;Wang, S.Y.; Zhang, R.R. Study on i onic liquid-based ultrasonic-assistedextraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill. A n al. Ch i m. Acta. 2011, 689,110-116.[C rossRef] ) ( 30. Xiong, Q.P.; H uang, S.;Chen, J.H.; Wang, B.L.; He, L.;Zhang, L.; Li, S.J.; Wang,J.Z.; Wu, J.G.; Lai, X.P.; et al.A novel green method for deproteinization of polysaccharide f rom Cipangopaludina chinensis by freeze-thawtreatment. J. Clean. Prod. 2017,142,3409-3418. [Cr ossRef] ) ( 31. Vian,M.A.; Fernandez, X.; Visinoni, F.; Chemat, F . Microwave hydrodiffusion an d gravity, a new techniquefor extraction of essential oils. J. Chromatoor.A. 2008,1190,14-17. [Cross R e f ] [Pu b Me d ] ) ( 32. Zhang, W.X.; Chen, L.; Li, P.;Zhao,J.Z.; Duan, J.Y. Antidepressant and i mmunosuppressive activities of two polysaccharides from Por i a coc o s (Schw.) Wolf. Int . J. B i ol. Macromol. 2018, 12 0 ,1696-1704. [Cro s s Ref] PubMed l ) ( 33. Filly, A . ;Fernandez,X.; Minuti, M.; Visinoni, F . ; Cravotto, G.; Chemat, F. Solvent-free microwave extractionof essential oil from aromatic herbs: F r om laboratory to pilot and indu s trial scale. Food. Chem . 2014, 150,193-198. [ CrossRef ] [ Pu b Med] ) ( 34. Rostaei, M.; F allah, S.; L origooini, Z.; Surki, A .A. The effect of organic manure and chemical fertilizer on essential oil, chemical compositions and antioxidant activity of dill (Anethum graveolens) in sole and intercropped with soybean (Glycine max). J. C lean. P rod. 2018,199,18-26.[C r o s s Ref] ) ( 35. Wang, L.B.; Liu, F.C.; Wang, A.X.; Yu, Z.Y.; Xu, Y.Q.; Yang, Y. Purification, characterization and bioactivitydetermination of a n ovel polysaccharide from pumpkin (Cucurbita moschata) see d s. Food Hydrocolloid. 2017,66,357-364. [ CrossRef] ) ( 36. Muinoa, I.; Diaz,M.T.; Apeleo, E.; P erez-Santaescolastica, C.; Rivas-Canedo, A.; Perez, C.; Caneque, V . ;Lauzurica,S.; Fuente, J. Valorisation of an extract from olive oil waste as a natural antioxidant for reducingmeat waste resulting f r om oxidative processes. J. Clean. P r od. 2017, 1 4 0,924-932. [C r o ssRef] ) 37. Liang, R.; Han, R.M.; Fu, L.M.; Ai,X.C.; Zhang, J.P.; Skibsted, L.H. Baicalin in radical scavenging and itssynergistic effect with B-Carotene in antilipoxidation. J. Agric. Food Chem. 2009, 57,7118-7124. [CrossRef] ( 38. Kivrak,S. Essential oil composition and antioxidant activities of eight cultivars of Lavender and Lavandin from western Anatolia. Ind. Crop. Prod. 2018,117,88-96. [Cro ssRef] ) Sample Availability: Samples of the compounds are not available from the authors. @ 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open accessCC article distributed under the terms and conditions of the Creative Commons Attribution(CC BY) license (http://creativecommons.org/licenses/by/4.0/). 超声波/微波消解法深层共晶水蒸气萃取五味子精油和多糖的替代方法 (Turcz.) Baill01摘要采用超声/微波交替协同的方法从五味子中同时提取精油和多糖。用深层共晶溶剂(DES)从五味子中提取精油和多糖。在最佳条件下,在选定的氯化胆碱-乙二醇1:3溶剂中提取,分别得到12.2毫升/千克和8.56克/100克的精油和多糖。还研究了多糖的自由基清除和免疫活性以及精油的抗氧化活性。淋巴细胞的增殖能力通过在多糖中加入协和素A或脂多糖而得到大幅提高。多糖(0.20毫克/毫升)精油清除DPPH的IC50值为水力蒸馏法和超声/微波辅助水力蒸馏法(DES UMHD)获得的精油清除DPPH的IC50值为52.34微克/毫升和29.82微克/毫升。通过DES UMHD获得的精油具有在150克/毫升时具有最高的还原力(856.05(TE/克),具有最强的抑制能力(SC%= 18.12%). S. chinensis具有作为天然抗氧化剂开发的潜力。02 简介深层共晶溶剂(DES)是一种可持续的替代离子液体的方法,它来自于绿色的天然和可再生成分。与传统的提取方法相比。DES预处理可以提高消化率,减少能源消耗,并简化溶剂回收的程序。DES可以通过将简单的季铵盐,如氯化胆碱(ChCl)与适当的氢键供体(HBD)(如金属卤化物、酸和醇)在加热的情况下形成[1]。这些制备的DES是无毒的,容易合成,而且制备成本低。有关于DES在提取酚类化合物方面的应用报告[2]。多糖[3]和蛋白质[4],而且研究还在继续探索更多的应用,如超声辅助,它利用超声波的机械、空化和热效应,通过提高其运动速度来增加对介质的穿透力。最近几年已经进行了研究。超声波产生的空化作用大大提高了异质反应的速率,实现了异质反应物之间的均匀混合反应物,加速反应物和产物的扩散,促进新固相的形成。并控制颗粒的大小和分布[6,7]。相比之下,微波辐照是作为一种能量源,诱导分子运动和具有永久偶极子的液体旋转[8]。因此,生物质材料吸收了微波能量,细胞温度迅速上升,从而使细胞的内部压力超过了细胞壁的膨胀能力,导致细胞分解。这使得细胞内的活性成分可以自由流动,并在较低的温度下被捕获和溶解。因此,它具有广泛的应用范围[9,10]。替代的超声/微波萃取技术,直接结合了这两种萃取模式,充分利用超声波的振动能量和微波的热效应。微波辐照[ 11 ]。这种方法有很多优点,如加热速度快,能耗低、溶剂要求低、回收率高。同时进行的超声/微波萃取也有利于提取极性和热不稳定的成分,避免了萃取或合成过程中处理时间过长而导致的分解现象。高温和高压的提取或合成过程中产生的分解[12],这种技术已经被应用于提取生物碱[13]、黄酮[14]、多糖[15]和其他植物的活性物质。五味子多糖以其护肝、补肾、抗疲劳、抗氧化、增强免疫力、抗衰老、抗肿瘤等功效吸引了研究人员的注意。增强、抗衰老、抑制肿瘤和抗糖尿病的作用[16,17]。S. chinensis 精油是提取自植物特有的芳香物质,可以防止咳嗽,促进DNA合成,并成为血浆蛋白原激活的抑制剂[17,18]。天然成分的生产需要初步研究化合物的分离,并建立最佳的提取方法和条件确立[19]。虽然各种固液萃取方法可以用来获得天然成分,但这些方法都有缺点,包括萃取时间长、溶剂需求量大、以及部分天然分子(如酚类化合物)的损失。在食品化学领域,经常使用超声/微波同步提取法来提取天然产品。与此相反,超声/微波交替提取技术很少被报道[ 15 ]。然而,协同超声/微波萃取的效率不一定比交替超声/微波消解的效率高,而且天然产品的提取效率在很大程度上受到交替组合提取模式的影响[ 14]。因此,本工作旨在开发一种超声/微波交替提取方法与DES,同时提取更高的多糖和精油产量,以研究超声/微波交替提取方法的最佳组合,并分析了其机制。此外,还详细说明去蛋白后,自由基清除活性和细胞增殖的多糖,对精油的抗氧化活性进行了研究[ 20, 21]。这也证明了S. chinensis多糖和精油可以作为潜在的天然抗氧化剂和免疫刺激剂,符合可持续性和绿色化学的原则。03祥鹄仪器在此文献中的使用多糖的超声辅助提取(UE) 将S. chinensis的干果(10.0克)置于圆底烧瓶中,加入DES(300毫升)。在超声/微波提取仪中进行超声辅助提取。(XH-300A, Xianghu Sci. & Tech. Co. Ltd., Beijing, China),在550 W下进行60分钟[29]。每隔10分钟取一个样品,每隔10分钟取一个样品以获得萃取动力学曲线。在 "聚苯乙烯的提取方法比较 "一节中使用了相同的操作方法。萃取完成后,采用与 "多糖的比较萃取法 "相同的操作方法。04结论发现DES是一种绿色介质,可以成功地提取高纯度的S.chinensis多糖和精油。最佳试剂是由氯化胆碱和乙二醇制备的,摩尔比为1:3,溶剂比为7:3 v/v。通过优化超声波/微波,550W(UE),250W(ME),固液比为1:30 w/v的萃取条件。此外。在S. chinensis多糖的实验中,发现粗制多糖被Sevicolina的四次脱蛋白处理后,就可以得到多糖。用Sevag法进行四次脱蛋白,得到最大的细多糖(5.11 g/100g)。在S. chinensis多糖对羟基自由基的清除活性中,我们发现当取样浓度超过0.20mg/mL时,其清除活性几乎增长了一倍。多糖的体外活性试验表明,0.20 mg/mL是秦艽多糖的最佳浓度。多糖在Lps细胞中的最佳浓度,比在ConA细胞中的最佳浓度高1.5倍左右。同样,对精油的提取方法进行了比较,选择了400W(UE)和20分钟被选中。在精油的抗氧化实验中,DES UMHD提取液的自由基清除活性β-胡萝卜素测定的DES UMHD提取物的自由基清除活性是一流的。此外,精油的FRAP检测显示DES UMHD样品具有最佳的抗氧化活性。这表明这表明DES可以有效地分离天然植物的精油和多糖。THIS IS TITLE
确定







还剩21页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《五味子精油和多糖中超声波/微波消解法萃取检测方案(微波合成仪)》,该方案主要用于中药材和饮片中前处理检测,参考标准--,《五味子精油和多糖中超声波/微波消解法萃取检测方案(微波合成仪)》用到的仪器有电脑微波超声波组合合成萃取仪 Soniwave XH-300A+型 、Magicube 高压超声波微波协同组合工作站
相关方案
更多











