方案详情
文
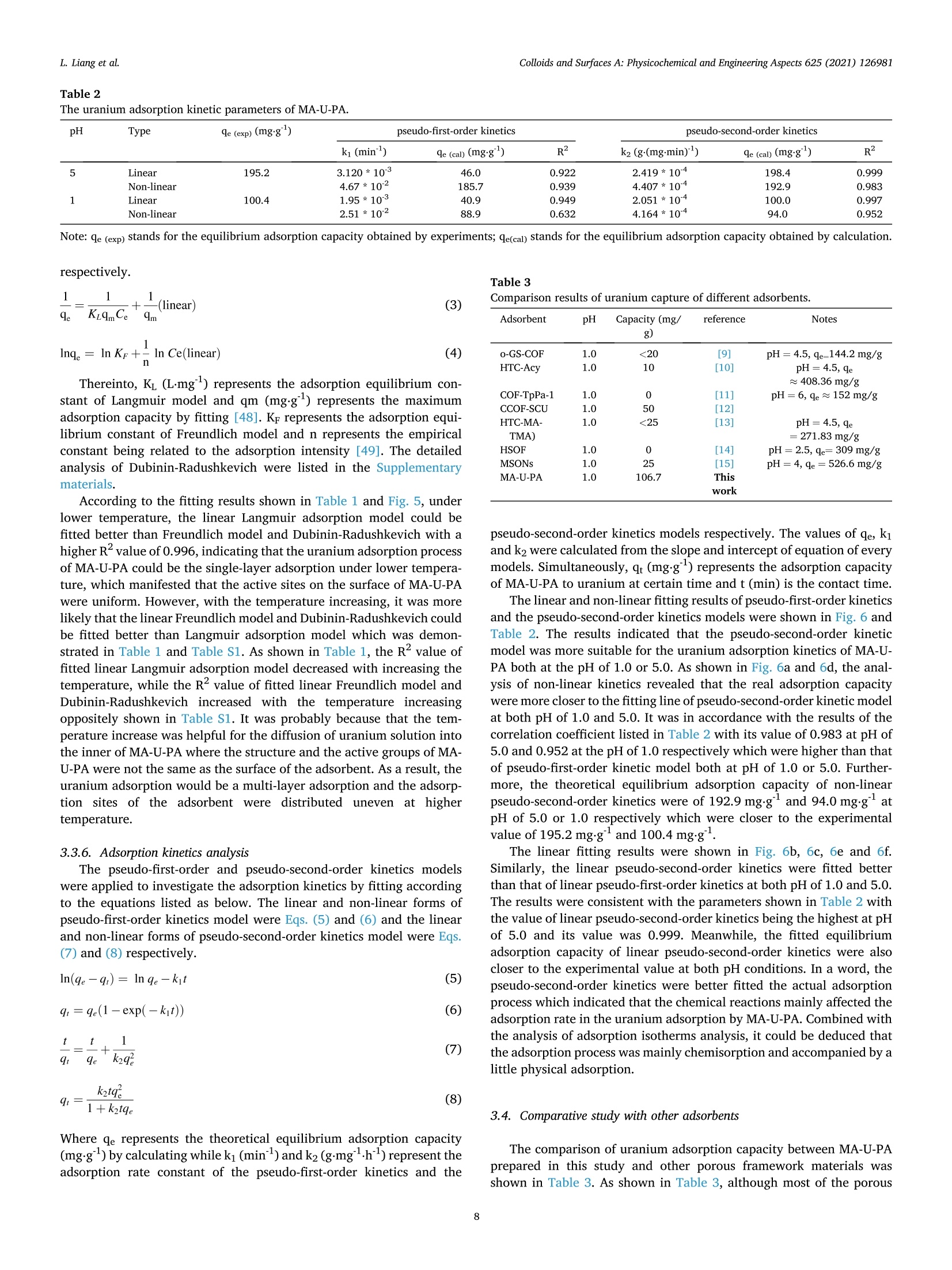
尽管各种吸附剂具有良好的铀吸附能力,但它们仍然面临着在高酸性条件下捕获铀的挑战。因此,在强酸性条件下有效地提取铀成为研究人员的追求。在这项研究中,一种新的植酸装饰的多孔有机聚合物(POPs)的制备方法是:用三聚氰胺和尿嘧啶合成一个有机框架(MA-U),然后用植酸进行改性。结果表明,所制备的有机框架具有良好的稳定性和较强的铀螯合作用。使所制备的材料在强酸条件下成为一种优良的吸附剂。在pH值为1的条件下,吸附能力达到106.7毫克⋅g-1,远远高于MA-U和其他大多数吸附剂。变化机制的研究证实植酸与MA-U的氢键作用导致了成功的改性。此外PA的膦酸基团主要是与铀螯合的活性基团。此外,PA对铀的MA-U-PA对铀的吸附过程符合伪二阶动力学和Langmuir等温模型。新制备的植酸装饰的多孔有机材料可以实现高效的从高酸性的核废水中捕获铀。
方案详情

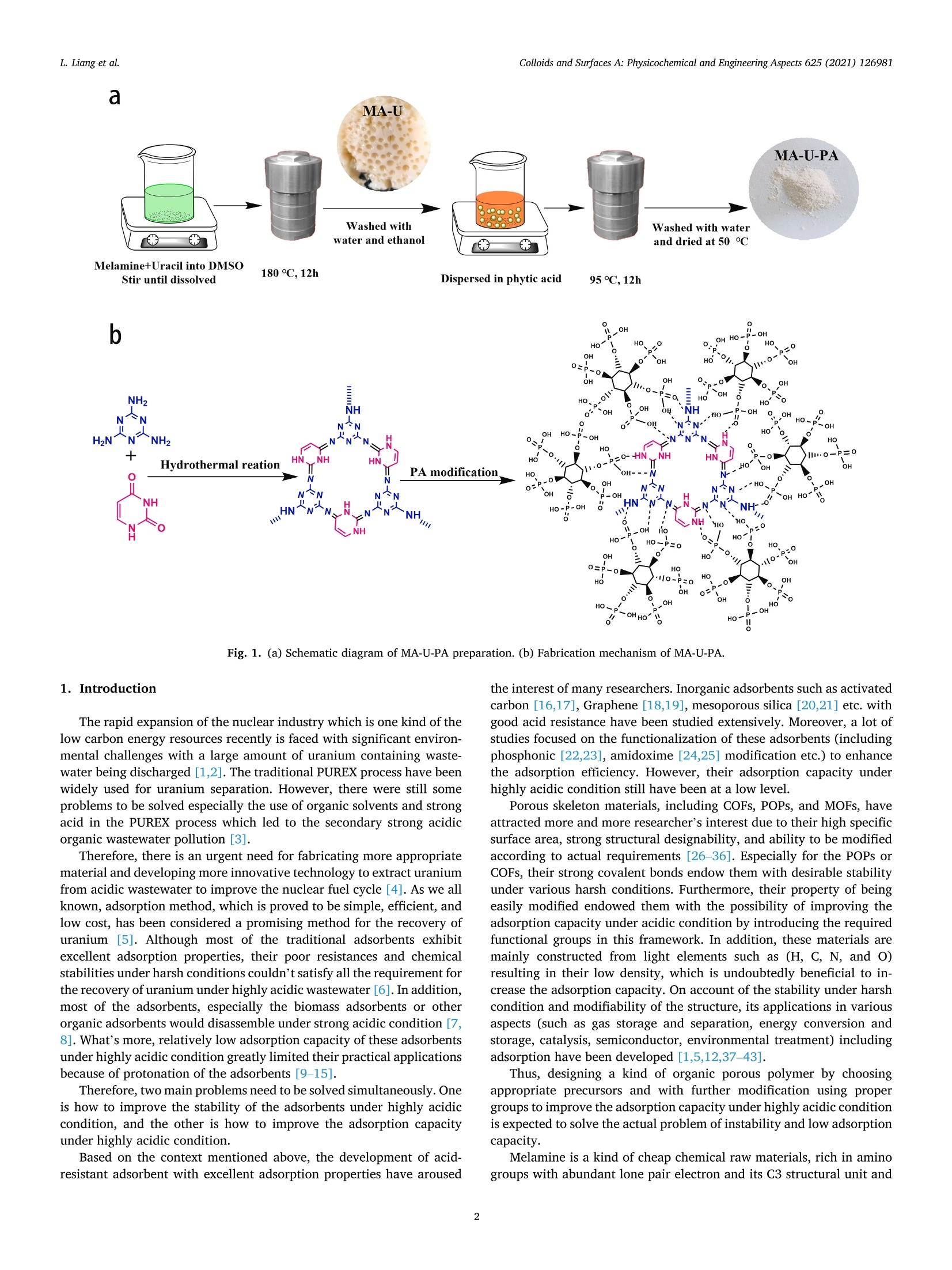
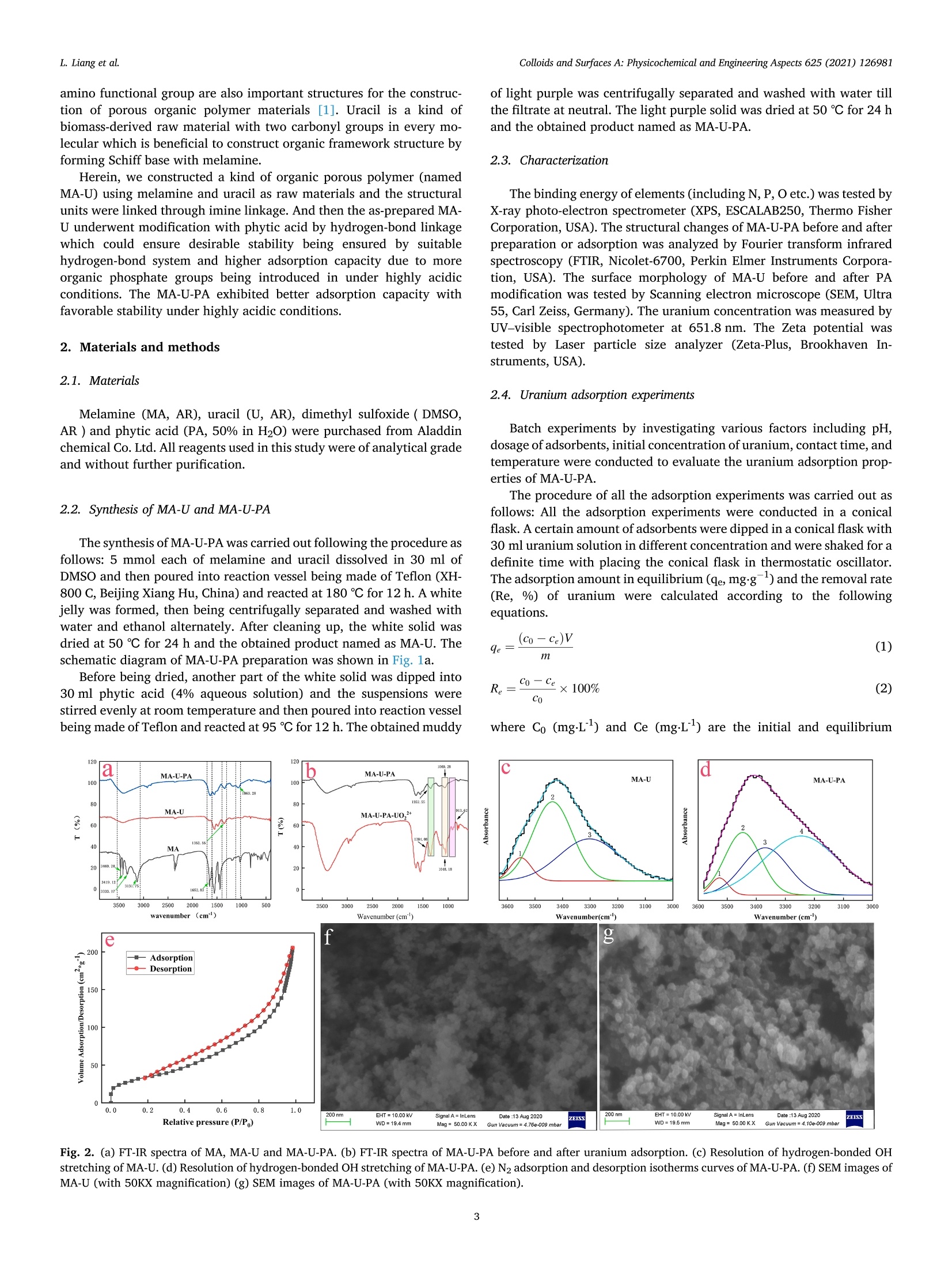
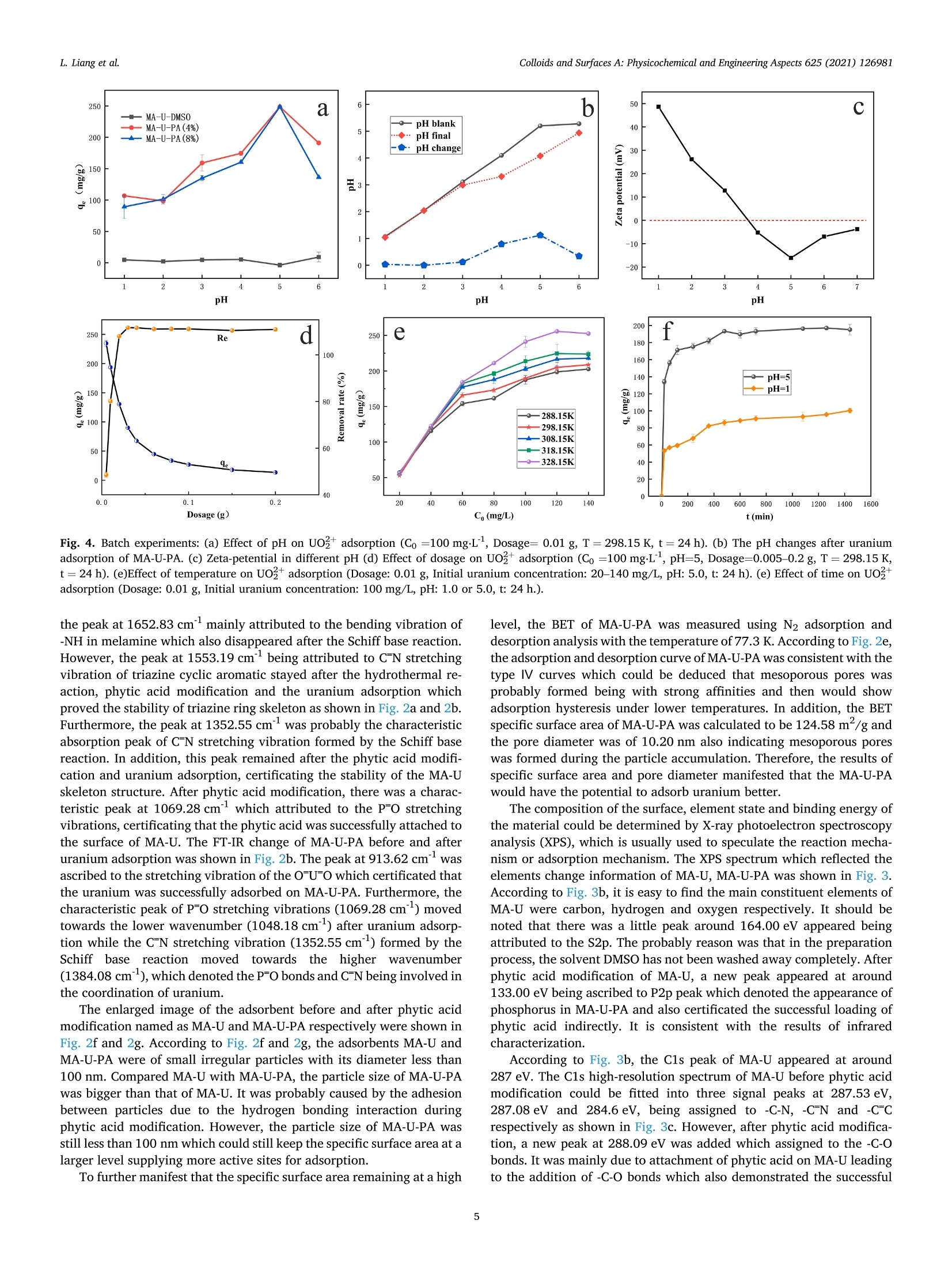
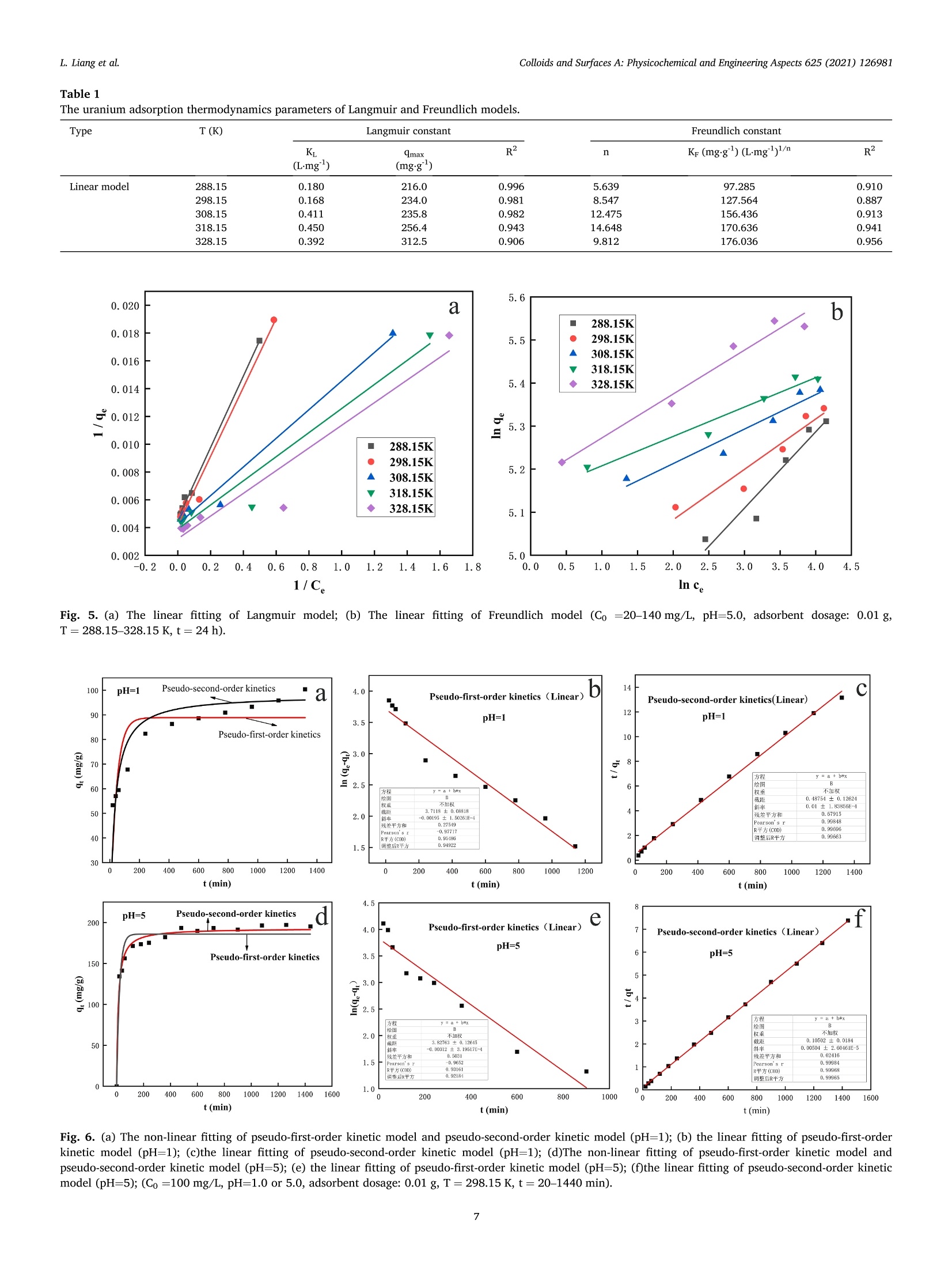
Colloids and Surfaces A: Physicochemical and Engineering Aspects 625 (2021) 126981Contents lists available at ScienceDirectColloids and Surfaces A: Physicochemical andEngineering Aspects Colloids and Surfaces A: Physicochemical and Engineering Aspects 625 (2021) 126981L. Liang et al. journal homepage: www.elsevier.com/locate/colsurfa Phytic acid-decorated porous organic polymer for uranium extractionunder highly acidic conditions Lili Liang a,b,c, Hao Zhang ac, Xiaoyan Lin a,c,", Kaiyin Yan, Mengsha Li , Xunhai Pan a,c,Yang Hua,c, Yan Chen ac, Xuegang Luoa, Ran Shang School of Materials Science and Engineering, Southwest University of Science and Technology, 621010 Mianyang, Sichuan, China Sichuan Preschool Educators College, No.16 of Education West Road, Fucheng District, 621010 Mianyang, Sichuan, China “Engineering Research Center of Biomass Materials, Ministry of Education, Southwest University of Science and Technology, 621010 Mianyang, Sichuan, ChinaState Key Laboratory of NBC Protection for Civilian, 102205 Beijing, China HIGHLIGHTS ·The raw materials were melamine, ura-cil and phytic acid which were relativelygreen and nontoxic. The organic framework made theadsorbent stable and phosphoric groupson the surface endowed the adsorbentcapturing uranium efficiently understrong acidic conditions. ·The uranium adsorption capacity of MA-U-PA attained to 106.7 mg.gunder pHof 1, higher than most of otheradsorbents. ARTICLEINFO High acidic wastewater A BSTRACT Keywords: Although various kinds of adsorbents have good uranium adsorption capacity, they still meet the challenge inPorous organic polymer capturing uranium under highly acidic condition. Therefore, efficiently extracting uranium under strong acidicPhytic acid condition become the pursuit of researchers. In this study, a new phytic acid decorated porous organic polymersUranium(POPs) named MA-U-PA was prepareCd by synthesizing a organic fraptureamework (MA-U) using melamine and uraciland with subsequent phytic acid modification. The results showed that the excellent stability of the preparedorganic framework and strong uranium chelating ability of phytic acid endowed the as-prepared material beingan excellent adsorbent under strong acidic condition. The adsorption capacity achieved 106.7 mg.gat the pH of1 which was much higher than that of MA-U and most of other adsorbents. The study of modification mechanismconfirmed that the hydrogen bonds interaction of phytic acid with MA-U lead to the successful modification. Inaddition, the phosphonate groups of PA was mainly the active groups chelated with uranium. Moreover, theuranium adsorption process of MA-U-PA was in accordance with the pseudo-second-order kinetic and Langmuirisothermal model. The newly prepared phytic acid decorated porous organic material could achieve efficienturanium capturing from highly acidic nuclear wastewater. a The rapid expansion of the nuclear industry which is one kind of thelow carbon energy resources recently is faced with significant environ-mental challenges with a large amount of uranium containing waste-water being discharged [1,2]. The traditional PUREX process have beenwidely used for uranium separation. However, there were still someproblems to be solved especially the use of organic solvents and strongacid in the PUREX process which led to the secondary strong acidicorganic wastewater pollution [3]. Therefore, there is an urgent need for fabricating more appropriatematerial and developing more innovative technology to extract uraniumfrom acidic wastewater to improve the nuclear fuel cycle [4]. As we allknown, adsorption method, which is proved to be simple, efficient, andlow cost, has been considered a promising method for the recovery ofuranium [5]. Although most of the traditional adsorbents exhibitexcellent adsorption properties, their poor resistances and chemicalstabilities under harsh conditions couldn’t satisfy all the requirement forthe recovery of uranium under highly acidic wastewater [6].In addition,most of the adsorbents, especially the biomass adsorbents or otherorganic adsorbents would disassemble under strong acidic condition [7,8]. What’s more, relatively low adsorption capacity of these adsorbentsunder highly acidic condition greatly limited their practical applicationsbecause of protonation of the adsorbents [9-15]. Therefore, two main problems need to be solved simultaneously. Oneis how to improve the stability of the adsorbents under highly acidiccondition, and the other is how to improve the adsorption capacityunder highly acidic condition. Based on the context mentioned above, the development of acid-resistant adsorbent with excellent adsorption properties have aroused the interest of many researchers. Inorganic adsorbents such as activatedcarbon [16,17], Graphene [18,19], mesoporous silica [20,21] etc. withgood acid resistance have been studied extensively. Moreover, a lot ofstudies focused on the functionalization of these adsorbents (includingphosphonic [22,23], amidoxime [24,25] modification etc.) to enhancethe adsorption efficiency. However, their adsorption capacity underhighly acidic condition still have been at a low level. Porous skeleton materials, including COFs, POPs, and MOFs, haveattracted more and more researcher’s interest due to their high specificsurface area, strong structural designability, and ability to be modifiedaccording to actual requirements [26-36]. Especially for the POPs orCOFs, their strong covalent bonds endow them with desirable stabilityunder various harsh conditions. Furthermore, their property of beingeasily modified endowed them with the possibility of improving theadsorption capacity under acidic condition by introducing the requiredfunctional groups in this framework. In addition, these materials aremainly constructed from light elements such as (H, C, N, and O)resulting in their low density, which is undoubtedly beneficial to in-crease the adsorption capacity. On account of the stability under harshcondition and modifiability of the structure, its applications in variousaspects (such as gas storage and separation, energy conversion andstorage, catalysis, semiconductor, environmental treatment) includingadsorption have been developed [1,5,12,37-43]. Thus, designing a kind of organic porous polymer by choosingappropriate precursors and with further modification using propergroups to improve the adsorption capacity under highly acidic conditionis expected to solve the actual problem of instability and low adsorptioncapacity. Melamine is a kind of cheap chemical raw materials, rich in aminogroups with abundant lone pair electron and its C3 structural unit and amino functional group are also important structures for the construc-tion of porous organic polymer materials [1]. Uracil is a kind ofbiomass-derived raw material with two carbonyl groups in every mo-lecular which is beneficial to construct organic framework structure byforming Schiff base with melamine. Herein, we constructed a kind of organic porous polymer (namedMA-U) using melamine and uracil as raw materials and the structuralunits were linked through imine linkage. And then the as-prepared MA-U underwent modification with phytic acid by hydrogen-bond linkagewhich could ensure desirable stability being ensured by suitablehydrogen-bond system and higher adsorption capacity due to moreorganic phosphate groups being introduced in under highly acidicconditions. The MA-U-PA exhibited better adsorption capacity withfavorable stability under highly acidic conditions. 2. Materials and methods 2.1. Materials Melamine (MA, AR), uracil (U, AR), dimethyl sulfoxide (DMSO,AR ) and phytic acid (PA, 50% in H20) were purchased from Aladdinchemical Co. Ltd. All reagents used in this study were of analytical gradeand without further purification. 2.2. Synthesis of MA-U and MA-U-PA The synthesis of MA-U-PA was carried out following the procedure asfollows: 5 mmol each of melamine and uracil dissolved in 30 ml ofDMSO and then poured into reaction vessel being made of Teflon (XH-800 C, Beijing Xiang Hu, China) and reacted at 180 ℃ for 12 h. A whitejelly was formed, then being centrifugally separated and washed withwater and ethanol alternately. After cleaning up, the white solid wasdried at 50 ℃ for 24 h and the obtained product named as MA-U. Theschematic diagram of MA-U-PA preparation was shown in Fig. 1a. Before being dried, another part of the white solid was dipped into30 ml phytic acid (4% aqueous solution) and the suspensions werestirred evenly at room temperature and then poured into reaction vesselbeing made of Teflon and reacted at 95℃ for 12 h. The obtained muddy of light purple was centrifugally separated and washed with water tillthe filtrate at neutral. The light purple solid was dried at 50 ℃ for 24 hand the obtained product named as MA-U-PA. 2.3. Characterization The binding energy of elements (including N, P, O etc.) was tested byX-ray photo-electron spectrometer (XPS, ESCALAB250, Thermo FisherCorporation,USA). The structural changes of MA-U-PA before and afterpreparation or adsorption was analyzed by Fourier transform infraredspectroscopy (FTIR, Nicolet-6700, Perkin Elmer Instruments Corpora-tion, USA). The surface morphology of MA-U before and after PAmodification was tested by Scanning electron microscope (SEM, Ultra55, Carl Zeiss, Germany). The uranium concentration was measured byUV-visible spectrophotometer at 651.8 nm. The Zeta potential wastested by Laser particle size analyzer (Zeta-Plus, Brookhaven In-struments, USA). 2.4. Uranium adsorption experiments Batch experiments by investigating various factors including pH,dosage of adsorbents, initial concentration of uranium, contact time,andtemperature were conducted to evaluate the uranium adsorption prop-erties of MA-U-PA. The procedure of all the adsorption experiments was carried out asfollows: All the adsorption experiments were conducted in a conicalflask. A certain amount of adsorbents were dipped in a conical flask with30 ml uranium solution in different concentration and were shaked for adefinite time with placing the conical flask in thermostatic oscillator.The adsorption amount in equilibrium (qe, mg.g)and the removal rate(Re, %) of uranium were calculated according to the followingequations. where Co (mg.L) and Ce (mg.L) are the initial and equilibrium Fig. 2. (a) FT-IR spectra of MA, MA-U and MA-U-PA. (b) FT-IR spectra of MA-U-PA before and after uranium adsorption. (c) Resolution of hydrogen-bonded OHstretching of MA-U. (d) Resolution of hydrogen-bonded OH stretching of MA-U-PA. (e) N2 adsorption and desorption isotherms curves of MA-U-PA. (f) SEM images ofMA-U (with 50KX magnification) (g) SEM images of MA-U-PA (with 50KX magnification). Fig. 3. (a) U4f of MA-U-PA-U. (b) XPS of MA-U, MA-U-PA and MA-U-PA-U. (c) C1s of MA-U, MA-U-PA and MA-U-PA-U. (d) N1s of MA-U,MA-U-PA and MA-U-PA-U. concentration of uranium respectively, V (ml) is the volume of uraniumsolution used for adsorption and m (g) is the weight of the adsorbent.Three parallel tests were required for all adsorption tests and adoptedthe average of three data as the final results. In order to explore the uranium adsorption properties of MA-U-PAunder highly acidic conditions, the effect of pH on adsorption capacitywas performed with the pH range of 1.0-6.0. Moreover, the effect ofdosage on adsorption was studied with varying the dosage of adsorbentfrom 0.0050 g to 0.2000 g. The effects of the initial uranium concentration were studied withthe initial uranium concentration varying from 20 to 140 mg.L bydipping 0.0100 g MA-U-PA in 30 ml uranium solution at pH5.0 for 24 hand the effects of temperature on uranium adsorption was studied withtemperatures ranging from 288.15 K to 328.15 K. The adsorptionisotherm was investigated by fitting Langmuir and Freundlich models.The effect of adsorption time on uranium adsorption and the adsorptionkinetics were investigated with the contact time at 20-1440 min whilethe initial concentration of uranium at 100 mg.L and the adsorbentdosage being of 0.0100 g at pH 5.0 or pH 1.0. 3. Results and discussion 3.1. Preparation of MA-U and MA-U-PA The preparation process and mechanism of MA-U-PA was shown inFig.1a and 1b respectively. Generally, melamine includes threecentrosymmetric amino groups while two carbonyl groups exist in uracilmolecular. Under the condition of hydrothermal reaction, the Schiff base was formed between the amino group and the carbonyl groupwhich construct a new porous skeleton structure named MA-U. Never-theless, due to the two carbonyl groups of uracil not in the para position,the porous skeleton structure was not of ordered structure. Meanwhile,in the formation process of skeleton structure, almost all the aminogroup of melamine was involved in the reaction, so there was no excessamino group to react with uranyl ions. As a result, the uraniumadsorption of MA-U was not well. This was definitely consistent with theresults in Fig. 4a. However, the porous skeleton of MA-U was a structurewith a lot of nitrogen which could donate the lone pair to form ahydrogen bond with the hydroxy hydrogen in phytic acid. Therefore, theMA-U-PA was obtained by hydrogen bonds formed by hydroxyl of phyticacid with adjacent nitrogen-contained groups or oxygen of P-O withadjacent hydrogen in imino groups after hydrothermal reaction. Afterthat, the phytic acid on the surface of the MA-U porous skeletonendowed it with better uranium adsorption capacity of capturinguranium. 3.2. Characterization of MA-U and MA-U-PA The infrared spectrum of MA-U adsorbent before and after prepara-tion shown in Fig. 2a was investigated using FT-IR spectrometer in therange of 400-4000 cm. The results showed that the antisymmetricstretching vibrations of -NH2 in melamine at 3469.28 cm and3419.12 cm°disappeared obviously after the Schiff base reaction be-tween the amino group of melamine and the carbonyl group of uracilwhich indicated that almost all the -NH2 of melamine reacted withcarbonyl groups to form the MA-U skeleton structure. Simultaneously, pH 250 Re d 250e 1100 200 200 15( 80 —0-288.15K 1(100 298.15K 100 308.15K 60 5( -318.15K 9e -328.15K 0 50 40 0.0 0.1 0.2 20 40 60 80 100 120 140 Dosage (g) C(mg/L) Fig. 4. Batch experiments: (a) Effect of pH on UO?adsorption (Co =100 mg.L, Dosage=0.01 g, T=298.15 K, t=24 h). (b) The pH changes after uraniumadsorption of MA-U-PA. (c) Zeta-petential in different pH (d) Effect of dosage on UOadsorption (Co =100 mg.L, pH=5, Dosage=0.005-0.2 g,T=298.15 K,t=24 h). (e)Effect of temperature on UO+adsorption (Dosage: 0.01 g, Initial uranium concentration: 20-140 mg/L, pH: 5.0, t:24 h). (e) Effect of time on UO+adsorption (Dosage: 0.01 g, Initial uranium concentration: 100 mg/L, pH: 1.0 or 5.0, t:24 h.). the peak at 1652.83 cmmainly attributed to the bending vibration of-NH in melamine which also disappeared after the Schiff base reaction.However, the peak at 1553.19 cmbeing attributed to CN stretchingvibration of triazine cyclic aromatic stayed after the hydrothermal re-action, phytic acid modification and the uranium adsorption whichproved the stability of triazine ring skeleton as shown in Fig. 2a and 2b.Furthermore, the peak at 1352.55 cmwas probably the characteristicabsorption peak of CN stretching vibration formed by the Schiff basereaction. In addition, this peak remained after the phytic acid modifi-cation and uranium adsorption, certificating the stability of the MA-Uskeleton structure. After phytic acid modification, there was a charac-teristic peak at 1069.28 cmwhich attributed to the P-O stretchingvibrations, certificating that the phytic acid was successfully attached tothe surface of MA-U. The FT-IR change of MA-U-PA before and afteruranium adsorption was shown in Fig. 2b. The peak at 913.62 cmwasascribed to the stretching vibration of the OU-O which certificated thatthe uranium was successfully adsorbed on MA-U-PA. Furthermore, thecharacteristic peak of P-O stretching vibrations (1069.28 cm) movedtowards the lower wavenumber (1048.18 cm) after uranium adsorp-tion while the CN stretching vibration (1352.55 cm’) formed by theSchiff base reaction movedd towards the higher wavenumber(1384.08 cm), which denoted the P-O bonds and C-N being involved inthe coordination of uranium. The enlarged image of the adsorbent before and after phytic acidmodification named as MA-U and MA-U-PA respectively were shown inFig. 2f and 2g. According to Fig. 2f and 2g, the adsorbents MA-U andMA-U-PA were of small irregular particles with its diameter less than100 nm. Compared MA-U with MA-U-PA, the particle size of MA-U-PAwas bigger than that of MA-U. It was probably caused by the adhesionbetween particles due to the hydrogen bonding interaction duringphytic acid modification. However, the particle size of MA-U-PA wasstill less than 100 nm which could still keep the specific surface area at alarger level supplying more active sites for adsorption. To further manifest that the specific surface area remaining at a high level, the BET of MA-U-PA was measured using N2 adsorption anddesorption analysis with the temperature of 77.3 K. According to Fig. 2e,the adsorption and desorption curve of MA-U-PA was consistent with thetype I curves which could be deduced that mesoporous pores wasprobably formed being with strong affinities and then would showadsorption hysteresis under lower temperatures. In addition, the BETspecific surface area of MA-U-PA was calculated to be 124.58 m/g andthe pore diameter was of 10.20 nm also indicating mesoporous poreswas formed during the particle accumulation. Therefore, the results ofspecific surface area and pore diameter manifested that the MA-U-PAwould have the potential to adsorb uranium better. The composition of the surface, element state and binding energy ofthe material could be determined by X-ray photoelectron spectroscopyanalysis (XPS), which is usually used to speculate the reaction mecha-nism or adsorption mechanism. The XPS spectrum which reflected theelements change information of MA-U, MA-U-PA was shown in Fig. 3.According to Fig. 3b, it is easy to find the main constituent elements ofMA-U were carbon, hydrogen and oxygen respectively. It should benoted that there was a little peak around 164.00 eV appeared beingattributed to the S2p. The probably reason was that in the preparationprocess, the solvent DMSO has not been washed away completely. Afterphytic acid modification of MA-U, a new peak appeared at around133.00 eV being ascribed to P2p peak which denoted the appearance ofphosphorus in MA-U-PA and also certificated the successful loading ofphytic acid indirectly. It is consistent with the results of infraredcharacterization. According to Fig. 3b, the C1s peak of MA-U appeared at around287 eV. The C1s high-resolution spectrum of MA-U before phytic acidmodification could be fitted into three signal peaks at 287.53 eV,287.08 eV and 284.6 eV, being assigned to -C-N, -C N and -C Crespectively as shown in Fig. 3c. However, after phytic acid modifica-tion, a new peak at 288.09 eV was added which assigned to the -C-0bonds. It was mainly due to attachment of phytic acid on MA-U leadingto the addition of -C-O bonds which also demonstrated the successful modification of phytic acid. Simultaneously,the electron binding energyof-C-N had shifted to a lower degree. The reason might be -C-N beinginvolved in the hydrogen bond interaction during modification. Never-theless, there was little shift in the four signal peaks of C1s in MA-U-PAafter uranium adsorption. It is indirectly proved that the uraniumadsorption has little effect on the structural stability of the MA-U-PA. As for N1s, there were three peaks located at 400.95 eV, 399.65 eVand 398.29 eV, attributing to -N-H,-C-N and-CN respectively of MA-Ushown in Fig. 3d. After modification, the -N-H, -C-N and -CN peakshifted to 400.34 eV, 399.34 eV and 398.25 eV respectively. Thesechanges suggested that hydrogen bond interaction occured duringphytic acid modification. Furthermore, the XPS spectra of MA-U-PA after uranium adsorption(MA-U-PA-U)and the high resolution spectrum of U4f, shown in Fig. 3band Fig. 3a respectively, confirmed the successful uranium adsorptionon MA-U-PA. The high resolution XPS spectra of U4f appeared at393.10 eV and 382.30 eV being separately ascribed to U 4 fs/2 and U4 f7/2 which could be assigned to the multiple splitting and spins ofunpaired electrons in the atomic shells [44]. Furthermore, the spin-orbitseparation energy between U4f 7/2 and U4f 5/2 was 10.8 eV, whichsuggested that there were no redox reaction occured during uraniumadsorption on MA-U-PA and the uranium species could be a single statebeing of uranyl ions [45]. 3.3. Uranium adsorption of MA-U-PA 3.3.1. Effect of pH on uranium adsorption The influence of pH on the uranium adsorption capacity of MA-U andMA-U-PA were investigated with the pH ranging from 1.0-6.0 and theresults were shown in Fig. 4a. According to Fig. 4a, the uraniumadsorption of MA-U at all pH conditions were almost zero. That wasprobably due to the -NH2 disappearance after the MA-U synthesis andthere was no active group existing. However, the uranium adsorptioncapacity of MA-U-PA after the phytic acid modification increased a lotand the concentration of phytic acid during the modification processnearly had no effect on the adsorption.As a result, we chose the con-centration of phytic acid at 4% during the modification process.Furthermore, the uranium adsorption capacity of MA-U-PA increasedwith increasing the pH value and while the pH value was at 5.0, theuraniumcadsorption capacity reachedthe maximumlvalue of248.7 mg.g.Combined with Fig. 4c, the zeta-potential was at the mostnegative level at pH of 5.0 which was more attractive to the positivelycharged uranium. That was the probable reason why the maximaladsorption capacity was got at pH of 5.0. Nevertheless, the uraniumadsorption of MA-U-PA at pH of 1.0 was still at a higher level of106.7 mg.g. The probable reason could be that the weaker alkalinity ofamide hindered the proton combination of the framework of MA-U-PAand kept the surface negatively or neutral at a low pH which would bebeneficial to capture the metal cation from an acidic solution [4].Simultaneously, the pH changes of the uranium solution before and afteradsorption were monitored at all pH conditions shown in Fig. 4b. It wasfound that the pH values of uranium solution after adsorption nearlyunchanged while the pH was less than 3.0. However, the pH values ofuranium solution after adsorption had a minor decrease while the pHwas at 4.0-6.0. It may be due to the dissociation of small amounts ofphytic acid at the pH of 4.0-6.0 which could confirm in a certain extentthat the loading of phytic acid is more favorable under acidic conditions. 3.3.2. Effect of adsorbent dosage on uranium adsorption The adsorbent dosage is an important parameter which would affectits actual usage. When the dosage of adsorbent is too small, it couldn’tremove all the uranium. However, if the dosage of adsorbent is exces-sive, it potential of capturing uranium couldn’t be fully explored. Todetermine the optimal adsorbent dosage, the effects of dosage onremoval rate and adsorption capacity of MA-U-PA were investigated. Asshown in Fig. 4d, with the adsorbent dose increasing from 0.0050 g to 0.2000 g, the adsorption capacity continued to decrease which denotedthat the utilization efficiency of adsorbents would decrease whileincreasing the dosage. Furthermore, the effective active site of the MA-U-PA had not reached adsorption saturation when its adsorption equi-librium reached while increasing the adsorbent dosage. Simultaneously,the removal efficiency continued to increase until it reached 100% as theamount of adsorbent increasing. Both the removal rate which reflect thefinal removal effects and adsorption capacity reflecting the utilizationefficiency and usage cost of MA-U-PA should be considered in actualapplication. According to Fig. 4d, although the removal rate was notbalanced after 0.0100 g attaining to around 80%, the adsorption ca-pacity still kept at a higher level. As a result, we selected 0.0100 g as theadsorbent dosage for the following adsorption experiment in order toensure both adsorption capacity and utilization efficiency being of thebetter values. 3.3.3. Effect of initial uranium concentration and temperature The effect of temperature on the uranium adsorption capacity of theMA-U-PA with the temperature ranging from 288.15 K to 328.15 K wasinvestigated and the results were shown in Fig. 4e. The temperature hadlittle effect on the adsorption capacity while the initial uranium con-centration was under 40 mg.L. However, with increasing the initialuranium concentration, the temperature increase would promote theuranium adsorption slightly by MA-U-PA. The probable truth may bethat, when the concentration of MA-U-PA attained to a higher level, thetemperature rise could increase the collision efficiency of uranium andMA-U-PA obviously with the temperature increasing. To the contrary,the collision efficiency of uranium and MA-U-PA was almost constantwith increasing the temperature while the concentration of uranium wasat a lower level. In a word, it was beneficial for the uranium adsorptionof MA-U-PA by increasing the temperature to some extent. Simultaneously, the influence of the initial uranium concentrationranging from 20 mg.Lto 140 mg.L’ on the adsorption capacity of theMA-U-PA was also investigated with the temperature ranging from288.15 K to 328.15 K. As can be seen from Fig. 4e,with increasing theinitial concentration of uranium, the uranium adsorption capacity ofMA-U-PA gradually increased and when the initial concentration ofuranium was 120 mg.L, the adsorption capacity of MA-U-PA reachedequilibrium. 3.3.4. Effect of time on the uranium capacity of MA-U-PA As shown in Fig. 4f, with the contact time increasing, the uraniumadsorption capacity of the MA-U-PA increased rapidly within the first20 min both under the pH condition of 5.0 or 1.0, and then slowlyincreased until it reached equilibrium in less than 480 min. The rapidincrease of uranium adsorption capacity at the beginning stage of thewhole adsorption might be caused by the surface adsorption of uraniumby MA-U-PA where a large number of active sites mainly being phyticacid on the surface played a major role. The adsorption rate becameslower with the contact time continuing to increase, which resulted fromthe longer diffusion time of adsorption solution into the inner of MA-U-PA because of the microcellular structure of MA-U-PA which was inaccordance with the results of BET analysis. 3.3.5. Adsorption isotherms analysis The Langmuir, Freundlich models and Dubinin-Radushkevichmodels were applied to investigate the adsorption isotherm processwhich could further reflect the adsorption mechanism and performance.According to reference [46,47], when the adsorption sites on theadsorbent were basically the same and the adsorption process was asingle-layer adsorption with the adsorption energy being generallyequal, the Langmuir isotherm was predicted to be fitted well. While theFreundlich isotherm assumed that the adsorption process was amulti-layer adsorption with the adsorption sites of the adsorbent beingdistributed uneven, and the adsorption energy were not equal. The Eqs.(3) and (4) were the linear of Langmuir model and Freundlich model Table 1The uranium adsorption thermodynamics parameters of Langmuir and Freundlich models. Type T (K) KL Langmuir constant 9max R2 n Freundlich constant Ke(mgg) (Lmg)1/n R2 (Lmg}) (mgg) Linear model 288.15 0.180 216.0 0.996 5.639 97.285 0.910 298.15 0.168 234.0 0.981 8.547 127.564 0.887 308.15 0.411 235.8 0.982 12.475 156.436 0.913 318.15 0.450 256.4 0.943 14.648 170.636 0.941 328.15 0.392 312.5 0.906 9.812 176.036 0.956 1.8 In c。 Fig. 5. (a) The linear fitting of Langmuir model; (b) The linear fitting of Freundlich model (Co =20-140 mg/L,pH=5.0, adsorbent dosage: 0.01g,T=288.15-328.15 K, t=24h). 100-pH=1 Pseudo-second-order kinetics _a ■ 90 ■ 80 Pseudo-first-order kinetics 70 60 50 40 30 0 200 400 600 800 1000 1200 1400 t (min) pH=5 Pseudo-second-order kinetics 200 T Pseudo-first-order kinetics 150 三100 50 0 200 400 600 800 1000 1200 1400 t (min) t (min) Fig. 6. (a) The non-linear fitting of pseudo-first-order kinetic model and pseudo-second-order kinetic model (pH=1); (b) the linear fitting of pseudo-first-orderkinetic model (pH=1); (c)the linear fitting of pseudo-second-order kinetic model (pH=1);(d)The non-linear fitting of pseudo-first-order kinetic model andpseudo-second-order kinetic model (pH=5); (e) the linear fitting of pseudo-first-order kinetic model (pH=5); (f)the linear fitting of pseudo-second-order kineticmodel (pH=5); (Co=100 mg/L, pH=1.0 or 5.0, adsorbent dosage: 0.01 g,T=298.15 K, t= 20-1440 min). Table 2The uranium adsorption kinetic parameters of MA-U-PA. pH Type ge(exp) (mg.g) pseudo-first-order kinetics pseudo-second-order kinetics k1 (minl) qe (cal) (mgg) R2 k2 (g(mgmin)) qe (cal) (mg.g) R² 5 Linear 195.2 3.120*10 46.0 0.922 2.419*10 198.4 0.999 Non-linear 4.67*10° 185.7 0.939 4.407*10 192.9 0.983 1 Linear 100.4 1.95*10 40.9 0.949 2.051*10 100.0 0.997 Non-linear 2.51*10~ 88.9 0.632 4.164*10 94.0 0.952 Note: ge (exp) stands for the equilibrium adsorption capacity obtained by experiments; ge(cal) stands for the equilibrium adsorption capacity obtained by calculation. Thereinto, KL (Lmg) represents the adsorption equilibrium con-stant of Langmuir model and qm (mg.g) represents the maximumadsorption capacity by fitting [48]. Kp represents the adsorption equi-librium constant of Freundlich model and n represents the empiricalconstant being related to the adsorption intensity [49]. The detailedanalysis of Dubinin-Radushkevich were listed in the Supplementarymaterials. According to the fitting results shown in Table 1 and Fig. 5, underlower temperature, the linear Langmuir adsorption model could befitted better than Freundlich model and Dubinin-Radushkevich with ahigher Rvalue of 0.996, indicating that the uranium adsorption processof MA-U-PA could be the single-layer adsorption under lower tempera-ture, which manifested that the active sites on the surface of MA-U-PAwere uniform. However, with the temperature increasing, it was morelikely that the linear Freundlich model and Dubinin-Radushkevich couldbe fitted better than Langmuir adsorption model which was demon-strated in Table 1 and Table S1. As shown in Table 1, the R value offitted linear Langmuir adsorption model decreased with increasing thetemperature, while the R value of fitted linear Freundlich model andDubinin-Radushkevich increased with the temperature increasingoppositely shown in Table S1. It was probably because that the tem-perature increase was helpful for the diffusion of uranium solution intothe inner of MA-U-PA where the structure and the active groups of MA-U-PA were not the same as the surface of theadsorbent. As a result, theuranium adsorption would be a multi-layer adsorption and the adsorp-tion sites of the adsorbent were distributed uneven at highertemperature. 3.3.6. Adsorption kinetics analysis The pseudo-first-order and pseudo-second-order kinetics modelswere applied to investigate the adsorption kinetics by fitting accordingto the equations listed as below. The linear and non-linear forms ofpseudo-first-order kinetics model were Eqs. (5) and (6) and the linearand non-linear forms of pseudo-second-order kinetics model were Eqs.(7) and (8) respectively. Where ge represents the theoretical equilibrium adsorption capacity(mg.g) by calculating while ki(min)and k2 (g.mgh) represent theadsorption rate constant of the pseudo-first-order kinetics and the Table 3Comparison results of uranium capture of different adsorbents. Adsorbent pH Capacity (mg/ reference Notes g O-GS-COF 1.0 <20 [9] pH=4.5, qe144.2 mg/g HTC-Acy 1.0 10 [10] pH=4.5, qe ≈408.36 mg/g COF-TpPa-1 [11] pH=6,9e≈152 mg/g CCOF-SCU [12] HTC-MA- <25 [13] pH=4.5, ge TMA) =271.83 mg/g HSOF [14] pH=2.5, ge=309 mg/g MSONs [15] pH=4, ge=526.6 mg/g MA-U-PA 1.0 106.7 This work pseudo-second-order kinetics models respectively. The values of qe, kiand k2 were calculated from the slope and intercept of equation of everymodels. Simultaneously, qt (mg.g) represents the adsorption capacityof MA-U-PA to uranium at certain time and t (min) is the contact time. The linear and non-linear fitting results of pseudo-first-order kineticsand the pseudo-second-order kinetics models were shown in Fig. 6 andTable 2. The results indicated that the pseudo-second-order kineticmodel was more suitable for the uranium adsorption kinetics of MA-U-PA both at the pH of 1.0 or 5.0. As shown in Fig. 6a and 6d, the anal-ysis of non-linear kinetics revealed that the real adsorption capacitywere more closer to the fitting line of pseudo-second-order kinetic modelat both pH of 1.0 and 5.0. It was in accordance with the results of thecorrelation coefficient listed in Table 2 with its value of 0.983 at pH of5.0 and 0.952 at the pH of 1.0 respectively which were higher than thatof pseudo-first-order kinetic model both at pH of 1.0 or 5.0. Further-more, the theoretical equilibrium adsorption capacity of non-linearpseudo-second-order kinetics were of 192.9 mg.gand 94.0 mg.gatpH of 5.0 or 1.0 respectively which were closer to the experimentalvalue of 195.2 mg.gand 100.4 mg.g. The linear fitting results were shown in Fig. 6b, 6c, 6e and 6f.Similarly, the linear pseudo-second-order kinetics were fitted betterthan that of linear pseudo-first-order kinetics at both pH of 1.0 and 5.0.The results were consistent with the parameters shown in Table 2 withthe value of linear pseudo-second-order kinetics being the highest at pHof 5.0 and its value was 0.999. Meanwhile, the fitted equilibriumadsorption capacity of linear pseudo-second-order kinetics were alsocloser to the experimental value at both pH conditions. In a word, thepseudo-second-order kinetics were better fitted the actual adsorptionprocess which indicated that the chemical reactions mainly affected theadsorption rate in the uranium adsorption by MA-U-PA. Combined withthe analysis of adsorption isotherms analysis, it could be deduced thatthe adsorption process was mainly chemisorption and accompanied by alittle physical adsorption. 3.4. Comparative study with other adsorbents The comparison of uranium adsorption capacity between MA-U-PAprepared in this study and other porous framework materials wasshown in Table 3. As shown in Table 3, although most of the porous Fig.7. Schematic diagram of mechanism of MA-U-PA capturing uranium. framework materials including COFs and porous organic polymers(POPs) exhibited a good uranium adsorption capacity under their bestpH condition and good stability in higher acidic condition, the uraniumadsorption capacity in higher acidic condition were still not high withmost of their uranium adsorption being less than 50 mg.gat pH of 1.0.However, the uranium adsorption capacity of MA-U-PA at pH of 1 was106.7 mg.g, exceeding most of the porous framework materials. 3.5. Mechanism analysis ofMA-U-PA preparation and uraniumadsorption In preparation of MA-U-PA, the hydrogen bond played an importantrole in the step of phytic acid modification of MA-U. According toreference [50-52], the hydrogen bond could be analyzed by resolvingthe peak of FTIR at 3000 cm~3700 cm°by which every fitted peakrepresented one kind of hydrogen bond. In order to testify the change ofhydrogen bond before and after phytic acid modification, the IR peak at3000 cm²~3700 cmhad been resolved into three or four bands whilewe assumed that all the hydrogen bonds followed a Gaussian distribu-tion. As shown in Fig. 2c, there were three intermolecular hydrogenbonds being appeared at 3551.81 cm°, 3436.87 cm°, 3302.82 cmrespectively. They were formed by the H of NH in uracil unit with twokinds of N in melamine unit in the MA-U framework or with the N of NHin uracil unit of another framework. Nevertheless, there was one morepeak appearing at 3246.25 cmshown in Fig. 2d which represented thehydrogen bond formed by the O of P=O in PA with H of NH in uracil unitand the relative strength of this peak was strong denoting that this kindof hydrogen bond played an more important role in forming MA-U-PA. Itwas in accordance with the speculation mechanism in phytic acidmodification. The uranium adsorption mechanism of MA-U-PA was deduced by thecombined analysis of FT-IR, XPS, adsorption isothermal analysis andadsorption kinetics analysis. The probable uranium adsorption mecha-nism was shown in Fig. 7. During the uranium adsorption, the uraniumchelated with the active groups in the state of uranylion according to theXPS analysis. The active groups involved in the coordination mainly wasthe PO of PA and the nitrogen-containing group in the MA-U-PA skel-eton played a little part role in uranium capturing according to the XPSanalysis. It was worthy noting that the framework of MA-U-PA providingthe stable structure and the weaker alkalinity of amide in MA-U-PAbeing beneficial to the deprotonation of MA-U-PA facilitated the ura-nium adsorption under highly acidic condition [4]. 4. Conclusions A kind of newly porous organic framework decorated by phytic acidnamed MA-U-PA was successfully prepared by hydrothermal synthesisusing melamine and uracil as building unit. The morphological andphysicochemical properties of MA-U-PA were characterized by FT-IR,SEM, BET, XPS and Z-potential. Furthermore, due to its stable frame-work and phosphoric groups on the surface, the MA-U-PA being of alight purple powder, could achieved efficient removal of uranium underhighly acidic conditions with the maximum uranium capacity being of106.7 mg.gat the pH of 1.0 which was much higher than most porousorganic adsorbents. Moreover, the results showed that the adsorptionprocess was mainly chemisorption and accompanied by a little physicaladsorption.Due to the framework structure and rich phosphate groupsbeing favorable for extracting uranium under strong acidic condition,MA-U-PA could be an ideal candidate adsorption material for uraniumcapture in acidic nuclear fuel effluents. CRediT authorship contribution statement Lili Liang: Conceptualization, Methodology, Software, Formalanalysis, Investigation, Data curation, Writing-original draft. HaoZhang:Writing-review & editing. Xiaoyan Lin: Methodology, Super-vision, Funding acquisition, Project administration. Kaiyin Yan:Investigation, Validation. Mengsha Li: Formal analysis, Investigation,Validation. Xunhai Pan: Writing-review & editing. Yang Hu: Char-acterization. Yan Chen: Conceptualization, Characterization. XuegangLuo: Methodology. Shang Ran: Formal analysis, Revision. Declaration of Competing Interest The authors declare that they have no known competing financialinterests or personal relationships that could have appeared to influencethe work reported in this paper. Acknowledgements This work was financially supported by State Key Laboratory of NBCProtection for Civilian (SKLNBC2020-21), Longshan academic talentresearch support plan of Southwest University of Science and Technol-ogy (18lzx315), Nuclear energy development project of EngineeringResearch Center for Biomass Materials of Ministry of Education, China(12zg610202). Appendix A. Supporting information ( Supplementary data associated with this article ca n be found in t he online version at do i :10.1016/ j .co l surfa.2021. 12 6981 . ) ( References ) ( [1] M . Chaudhary, L. Singh, P . Rekha,V.C. Srivastava, P. Mohanty, Adsorption ofuranium from aq u eous sol u tion as w ell as s eawater conditions by n i trogen-enriched n anoporous polytriazine, C hem. E n g.J. 3 7 8 (2019), 122236, ht t ps:// do i. or g/ 1 0. 10 16/ j. c ej. 2019 . 1 22236 . ) ( [2] H . Z hu, B . W ang, W . K. Z h u, T. D u an, X.G. Luo, D.Q. Sun, J. Zhou, S p ace and s tructure activation of collagen fiber for h i gh efficient capture io d ine in off-gas, C olloids Surf. A Physicochem. E n g. Asp. 6 1 7 (2021), 126389, htt ps :// d oi .or g / 1 0 . 10 1 6 /j . c o l s u rf a.2021 . 126389 . ) ( [3] Y . Xie, C.L. Chen, X.M. Ren, X.X . Wang, H.Y. Wang, X.K. Wang, Emerging natural and t ailored materials for uranium-contaminated water treatment a nd environmental r e mediation, P r og. M a ter. Sci. 1 03 (2019) 1 8 0-234, ht t p s :// do i. o rg / 1 0. 1 0 16 / j. pmats c i . 20 1 9 .0 1 . 005 . ) ( [4 ] J .P. Yu, L.Y. Yuan, S. Wang, J. H . L an, L .R. Zheng, C. X u, J. Chen, L . Wang, Z. W. Huang, W.Q. Ta o , Z. R . Li u , Z.F. Chai, J.K. G ibson, W.Q. S h i, P h osphonate- decorated covalent o r ganic frameworks for actinide extraction: a breakthrough u nder highly acidic c onditions, CCS Chem. 1 (2019) 28 6 -295, http s ://d o i . o r g / 1 0. 3 1 635 / cc sc h e m . 01 9 . 2 0190005. ) ( [5] Y . Yuan, Y.J. Yang, X.J. M a, Q.H . Meng, L. L . Wang, S. Zhao, G.S. Zhu, Molecularly i mprinted p orous a romatic frameworks and their composite components forselective extraction of uranium i o ns, Adv. Mater. 30 (2018),1706507, https :/ /doi . o rg / 10 . 1 002 /a dma.201706507. ) ( [ 6] H . Liao, J . Yu, W.K. Z hu, M . Kuang, T. D u an, Y.D. Zhang, X.Y. Li n , X.G. Luo,J. Zhou, Nano-zero-valent Fe/Ni particles loaded on collagen fibers immobilized by b ayberry t a nnin as a n effective reductant for uranyl in a q ueous solutions, Appl. Surf. Sci. 507 (2020), 145075, https: // doi. o r g / 10.1016/ j .apsu s c.2019.145075. ) ( [7] L .L.L i ang, X . Y. L i n, Y.F. Liu, S.Y. S un, H.H. C h u, Y. Chen, D. Liu, X. G . Luo,J. Zhang, R. Shang, Carboxymethyl k onjac glucomannan mechanically reinforcing g ellan gum microspheres for u ranium removal, Int J. Biol. Macromol. 145 (2020) 5 35-546, h t tps: //d oi.org/ 1 0 . 1 0 16 / j . i jb i o m ac.2 0 19.12. 1 8 8 . ) ( [8] 1 L .L. L iang, X.Y. Lin, S.Y. Sun, Y. Chen, R. Shang, X.G. Luo, Stereoscopic porous g ellan gum-based microspheres as high performance adsorbents for U(VI) removal,J.Radioanal. Nucl. C hem. 3 1 9 (2019) 213-225, htt p s:/ /d oi . o r g /10 . 100 7 /s10 9 67- 018-6323- 1 . ) ( [ 9] R . Wen, Y. Li,M.C. Zhang, X.H. Guo,X. Li , X . F. Li, J . H an, S. Hu, W. T an, L.J. Ma, S.J. Li, Graphene-synergized 2D covalent organic framework for adsorption: a m utual p r omotion strategy to achieve stabilization an d functionalizationsimultaneously, J.H a zard. Mater. 358 (2018) 273-285, http s : //d o i .org /1 0 .1 0 1 6/j. ihazm a t.2018.06.059 . ) ( [10 ] Q . Song, L.J. M a , J. Liu, C.Y. Bai, J.X. G e ng, H. Wang, B. L i , L .Y. Wang, S.J. L i, P reparation and a d sorption p e rformance of 5-azacytosine-functionalizedhydrothermal ca r bon for selective solid-phase extraction of uranium, J. Colloid I nter. Sci. 386 (2012) 291 - 299, ht t ps : / / do i . o rg / 1 0 . 1 0 1 6/ j . j cis .201 2. 0 7 . 0 7 0. ) ( [ 11 ] Z .D. L i,H.Q. Z h ang, X.H. Xiong, F. L u o, U ( VI) adsorption onto covalent or g anic f rameworks-TpPa-1, J. S o lid State Chem. 2 7 7 (2019) 48 4 -492, htt p s :// d o i .org/ 1 0 . 1 016/ j.j s sc. 2019.06 . 044. ) ( [12 ] C .Y.Bai, J. Li, S.B. Liu, X.Y . Yang, X.D. Yang, Y . Tian, K .C. Cao, Y . Huang, L . J. Ma, S .J. L i, In situ preparation o f n itrogen-rich a n d functional ul t ra-microporous c arbonaceous COFs by “segregated”microwave irradiation, M icroporous M esoporous Mat. 1 9 7 (2014) 14 8 -155, htt ps : / /doi . o rg/1 0 . 10 1 6 /j. m icrom e so.2014.06.004 . ) ( [13] H.L. Li, Y . Li, Y.Z. Zhou, B.L. Li, D.B. Liu , H.Y. Liao, Efficient removal of uranium using a melamine/trimesic acid-modified hydrothermal carbon-basedsupramolecular organic framework,J. S o lid State Chem. 544 ( 2 019) 1 4 -24, ht t ps :// do i. o r g / 1 0 . 1 016/j .j ci s . 2 01 9 .02. 0 79. ) ( [14] B . L i , L . Wang, Y . L i , D.Q. W a ng, R. We n , X.H. Guo, S.J. Li, L .J. Ma, Yin Tian , Conversion of supramolecular organic framework to ur a nyl-organic coordination c omplex: a new“matrix-free”strategy fo Hri r highly efficient capture of uranium, RSC Adv. 7 (2017) 8985- 8 993, htt ps : / /doi.or g / 10 . 1039/C6R A 28356J. ix ) ( [15] H .L. L i, Y. Li , B.L. L i , Y. Dai, X. Chen, Melamine-induced novel MSONs h eterostructured f ramework: c o ntrolled-switching between MOF and S OF via a self- a ssembling approach for rapid uranium sequestration, Ch e m. Eng . J. 3 7 9 (202 0 ), 122279, h ttps ://do i . o r g/ 1 0. 1 016/j . c e j . 2 019 . 122279 . ) ( [16] L. Liu, X.Y. Lin, M.S. Li, H .H. Chu, H.Y. Wang, Y. X ie , Z.C. Du, M.J. Liu, L.L. Liang, H .Y. G ong, J. Zhou, Z.G. Li, X.G. Luo, Microwave-assisted hydrothermal sy n thesisof carbon doped with phosphorus for u r anium (VI) a d sorption, J. R adioanal. N ucl. Chem. 327 (2021)73-89, ht tp s : / / do i . o r g/1 0 . 1 007 / s1 0967-020-0 7 453-6. ) ( [17] Y .B. S un, S.B. Yang, Y . C hen, C.C. D ing, W .C. Cheng, X.K. W a ng, Adsorption anddesorption of U(VI) on functionalized graphene oxides: a combined experimental and theoretical study, Environ. Sci. Technol. 49 (2015) 4255-4262, h t tps: / / d o i . o rg /1 0.1021/ es 505590j . ) ( [1 8 ] S .J. Yu,X . X. W a ng, X.L. Tan, X.K. Wang, Sorption of ra d ionuclides from aqu e ous s ystems o nto graphene oxide-based materials: a review, Inorg. Chem. 2 (2015) 5 93-612, h ttp s: / / doi.org / 10 . 1039 / C4QI00221K. ) ( [19] X . Wang, R.M. Li , J.Y. Liu, R .R. Chen, H .S. Z hang, Q. Li u , Z .S. Li, J. Wang, M elamine modified graphene hydrogels for the removal of uranium (VI) from a queous s o lution, N ew J. C h em. 19 (2017) 1-10, htt p s://d oi .org/10. 1 03 9 / C7N J 01 9 27K . ) ( [ 20] O . A D u darko,C. G unathilake, N.P . Wic k ramaratne, V.V. Sliesarenko, Y.L. Zub, J . Gorka, S. Dai, M. Jaroniec, Synthesis of mesoporous silica-tethered phosphonic a cid sorbents for uranium species from aq u eous solutions, Col l oid Surf. A 482 ( 2015) 1- 8 , h t tps: // doi.or g/ 1 0. 10 1 6 /j .co ls u r f a.2015. 0 4 . 0 1 6 . ) ( [21]LI.Y. Yuan, L . Zhu, C.L. Xiao, Q.Y. Wu, N. Zhang, J.P. Yu, Z.F. Ch a i, W.Q. Shi , Lar g e- pore 3 D cubic m esoporous ( K IT-6) hybrid bearing a hard-soft donor combined l igand for enhancing U(VI) capture: a n experi m ental a n d theoretical investigation, A CS Appl. Mater. Inter 9 (2017) 3774-3784, h tt ps : / /d o i .or g /10 . 1 02 1 / acsam i. 6b 1 5642 . ) ( [22] E 1 .A. Imam, I.E. El-Sayed,M.G. Mahfouz, A.A. T o lba, T. Akashi, A.A. G alhoum, E . G uibal, S ynthesis of o-amino phosphonate fun c tionalized chi t osan sorbents: effect of methyl vs phenyl group on uranium sorption, Chem. Eng. J. 352 (2018) 1 022-1 0 34, h t tps: / /d oi . org /1 0. 1 01 6 / j . c e j.2 0 1 8.06 . 00 3 . ) ( [ 23] S . A nnam, G. G o pakumar, C.V.S.B. Ra o , N. Sivaraman, A. S iv a ramakrishna, K. V ijayakrishna, E xtraction o f actinides by T r i-n-butyl phosphate d erivatives: e ffect of substituents, Inorg. Chim. Acta 469 (2018) 123-132, http s ://do i. o r g / 1 0. 1 016/ j . i c a . 201 7 . 07.048 . ) ( [24] J . Xiong, S. Hu, Y. Liu, J . Yu, H.Z. Y u, L . Xie, J. Wen, X.L. Wang, P o lypropylene m odified with a midoxime/carboxyl groups in s e parating uranium(VI) from t horium(IV) i n aqueous solutions , ACS Sustain. Chem . Eng. 5 (2017)1924- 1 930, h t tps :// doi.or g / 1 0 . 1021 / acss u schemeng. 6 b0 2 663 . ) ( [25] S .D. Alexandratos, X.P. Zhu, M. Florent, R. Sellin, Polymer-supported bifunctional a midoximes fo r the s o rption o f uranium fr o m seawater, Ind. Eng. Chem. Res. 55 ( 2016) 4208-4216, ht tps:// d o i. org /1 0 .1021/ac s .i e cr. 5 b 037 4 2. ) ( [ 26] A .P. Cote, A.I. Benin, N.W. Ockwig, M. O'Keeffe, A.J. Matzger,O.M. Y a ghi, Porous,crystalline, covalent organic frameworks, S c ience 310 (2005)1166-1170. https : / /science.sciencemag . org / cont e nt / 310/ 5 751 / 1 16 6 . ) ( [27] M.A. Trevor, J .R. Li, W.G. Lu, H.C. Zhou, Methane storage in advanced porous m aterials, Chem. Soc. Rev. 41 ( 2 012) 7761-77 7 9, htt p s:// d oi .or g /1 0 .1 03 9 /C 2 C S 35251F . ) ( [ 28] L. Tiziana, C. A l essia, Z . Antonio, C. Al e ssandra, Use of natural zeol i tes charged w ith a mmonium or carbon dioxide in ph y toremediation of lead and zinc c ontaminated s o ils, J. Chem. Technol. Biot. 87 (2012) 1 342- 1 348, h ttps ://d o i . o r g / 1 0. 10 02/ jc tb.3788. ) ( [ 29] W . J. Dominic, G. Ju l ien, T.P. Valeska, B. D . Ri c hard, E.J. Ka r en, B. R . Chris, M . Svetlana, B .D. A ndres, Gas sensing using porous m a terials f o r automotiveapplications, Chem. Soc. Rev. 44 (2015) 4290-4321, htt p s://d oi .org/10. 1 03 9 / C5C S 00040H . ) ( [ 30] Y .F.Z e ng, R . Q. Z o u, Y . L. Zhao, Covalent o r ganic f r ameworks for CO2 capture, Ad v . M ater. 2 8 ( 2016) 2855-2873, ht tp s: / /d oi. o rg /1 0 . 1 00 2 /a d ma.201505 0 04. ) ( [31] L .Y. Wang, B. Dong, R.L. Ge, F. X . Jiang, J.K. X u , Fluorene-based two-dimensionalcovalent organic framework with thermoelectric properties through doping, ACS Appl. Mater. Inter9 (2017)7108-7114, h t tps ://d o i. or g /1 0. 1 021/a csa mi.6 b 14 9 1 6. ) ( [ 32] Z .J. Yin, S.Q. Xu, T.G. Zhan, Q.Y. Qi , z.Q. Wu , X. Zha o , Ultrahigh volatile iodineuptake by h ollow microspheres formed f r om a h e tero pore covalent organic f ramework, Chem. Commun.53 (2017) 726 6 -7269, http s : // d o i . or g /10 . 1 039/ C7CC01045A . ) ( [ 33] D . Urbano,C. Avelino, Ordered co v alent organic frameworks, COFs and PAFs, frompreparation to application, Coord. Chem. Rev. 3 11 (2016) 85-124, h t tps: // d o i. o rg / 1 0. 10 16/ j . cc r . 20 1 5.12 . 010 . ) ( [34] W \ .J. Zhang, P . P. Jiang, Y . Wang, J. Z hang, Y.X. Gao, P.B. Z hang, Bottom-up a pproach to engineer a molybdenum-doped covalent-organic framework catalyst f or selective oxidation reaction, RSC Adv. 4(2014) 51544-51547,ht tp s :/ /do i.org/ 1 0.1039 / C 4 RA09304F . ) ( [ 35] H. Yang, S.L. Zhang, L.H. Han, Z. Z hang, Z. Xue , J. Gao, Y.J. Li, C.S. Huang, Y.P. Yi,H.B. Liu, High conductive two-dimensional covalent organic framework for lithiumstorage w ith large capacity, ACS Appl. Mater. Interfaces 8 (2016) 5366-5375 , h tt ps :// d oi. or g /1 0 . 1021 / ac s a mi.5b12 3 70 . ) ( [36] S . Wang, Q.Y. Wang, P . P. Shao, Y .Z. H an, X. Gao,L. M a, S. Y uan, X .J. Ma, J. W . Zhou, X. Feng, Exfoliation of covalent organic frameworks into few-layer redox- a ctive n anosheets as cathode materials fo r lithium-ion ba t teries, J. Am. Chem. Soc. 1 39 ( 2017) 4 258-4261,ht t p s : / / d o i.org/ 1 0 .1 0 2 1 / ja cs . 7b02 6 48. ) ( [37] J. Li, X.D. Yang, C.Y. Ba i , Y. Ti a n, B. Li, S. Zhang, X.Y. Yan g , S.D. Ding, C.Q. Xia, X. Y . Tan, L.J. Ma, S.J. Li, A novel benzimidazole-functionalized 2-D COF material:synthesis and a p plication as a selective solid-p h ase ex t ractant for separation ofuranium, J. C olloid I nter. Sci. 437 (2015) 211-218, h ttps: / /d o i . o rg/ 10 .1 0 1 6/j. j c i s. 201 4 .09.046. ) ( [38] 4 C .Y. Bai, M.C. Zhang, B. Li, Y. Tian, S. Zhang, X.S. Zhao, Y. Li, L . Wan g , L.J. Ma, S. J.Li, Three novel triazine-based materials with different O/S/N s et of donor atoms:one-step p reparation and comparison of their c a pability in selective separation of u ranium, J. Hazard. Mater.300 (2015) 368-377, h t t ps : // d o i. o rg /1 0 . 1 0 16/ j . ihazmat.201 5 .07.020. ) ( [ 39] S . Zhang, X.S. Z h ao, B.Li , C.Y. B a i, Y . Li, L . Wang, R. Wen, M.C. Zh a ng, L.J . Ma , S.J. Li,“Stereoscopic”2D super-microporous phosphazene-based covalent organic framework: design, synthesis a n d selective sorption towards uranium at high acidic condition, J. Hazard. Mater. 3 1 4 (2016) 95-104 , http s://d o i . o r g / 10 . 1 0 1 6 / j. i h a z ma t .201 6 .04. 0 31. ) ( [ 40] Y . Yuan, Q.H. Meng, M. Faheem, Y.J . Yang, Z.N. Li, Z.Y. W a ng, D. De n g, F.X. Su n , H .M. He, Y.H. H u ang, H.Y. Sha, G. S . Zhu, A mo l ecular coordination tem p latestrategy f or designing selective porous aromatic f r amework mat e rials for uranylcapture, ACS C ent. Sci. 5 (2019) 1432-1439, https :// d o i . org / 1 0 . 1 021 / ac s cen t sc i .9b00494 . ) ( [ 41] B .Y. Li, Qi Sun, Y.M. Z hang, C.W. A b ney, B. Aguila, W.B. Lin , S . Q. M a , F unctionalized p orous aromatic framework fo r efficient u ranium a d sorption f r om a queous s o lutions, A CS Appl. M a ter. Interfaces 9 (2017) 12511-12517, htt p s :// doi. org / 1 0 . 1 021 / a c s am i .7 b 0 1 7 1 1. ) ( [42] Q . Sun, B. Aguila, L .D. E arl, C.W. Abney, L. W ojtas, P .K. T hallapally, S.Q. Ma,Covalent o rganic frameworks as a decorating platform for utilization and a f finityenhancement of chelating sites for radionuclide sequestration, Adv. M ater. 30 (2018), 1705479, http s:// d o i . o r g /10.1002 / adma.201 7 05479. ) ( [43 ] C .C. D ing, W.C. Cheng, X.Q. Nie, Z . W. Niu, T. D u an, Y. Y . Zhang, A. M . Asiri, H. M . Marwani, Y. L i , Y .B. Sun, S pectroscopic and theoretical investigation on efficient removal of U(VI) by amine-containing polymers, Chem. Eng. J. 367 ( 2019) 94-101, ht tp s: / / do i .o rg / 1 0. 10 1 6/j. c e j . 20 1 9.02.142. ) ( [44] X . Z hong, W. Liang, Z.P. L u, B.W. Hu, Highly efficient enrichment mechanism of U(VI) and Eu(III) by covalent or-ganic frameworks with intramolecular hydrogen- b onding f r om s o lutions, Appl. Surf. S ci. 504 ( 2020), 1 44403, h t t ps: //d oi . or g / 1 0 .101 6 /j .aps u sc.20 1 9 . 14 4 4 03 . ) ( [45] X . Zhong, Z.P. Lu, W. Liang, X .J. Guo, B.W. H u, Fabrication o f 3D hierarchical f lower-like 8-MnO2@COF nanocomposites for t h e efficient and ultra-fast removalof UOion from aqueous solution, Environ. Sci. Nano 7 ( 2 0 20) 3303-3317, h tt ps :// d o i. or g /10.1039 / D0EN00793E. ) ( [46] z .X. Y ou,N. Z h ang, Q.L. G u an, Y.H. Xing, F . Y. Bai, L.X. Sun, High sorption capacity o f U (VI) b y C OF-based material doping hy d roxyapatite microspheres: kinetic,equilibrium and m echanism investigation, J. Inorg. Organomet. Polym. Mater. 30 (2020)1 9 66-1 9 79,htt ps: //doi . o r g /1 0 .1 0 07/s 1 0 9 04-019- 0 1420- 9 . ) [47] H.Y. Gong, X.Y. Lin, Y. Xie, L. Liu, J. Zhou, H. Liao, R. Shang, X.G. Luo, A novelself-crosslinked gel microspheres of premna microphylla turcz leaves for theabsorption of uranium, J. Hazard. Mater. 404 (2021),124151,https://doi.org/10.1016/j.jhazmat.2020.124151. [48] I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum,J. Am. Chem. Soc. 40 (1918) 361-1368, https://doi.org/10.1021/ja02242a004. ( [ 49] B . N agy, C. Manzatu, A . M a icaneanu, C. Indolean,B.T. Lucian, C. Majdik, Linear a nd nonlinear regression analysis for heavymetals removal using Agaricus bisporusmacrofungus, Arab J. Chem. 1 0 (2017) S3569-S3579, h t tps:/ /d oi. o rg/ 1 0.1016/j . ar a bj c . 201 4 . 0 3 . 004 . ) [50]1D.Y. Huang, M. Wu, C. Wang, S. Kuga, Y. Huang, Effect of partial dehydration onfreeze-drying of aqueous nanocellulose suspension, ACS Sustain Chem. Eng. 8(2020) 11389-11395, https://doi.org/10.1021/acssuschemeng.0c03688. [51] S.Y. Oh, D.I. Yoo, Y. Shin H.C. Kim, H.Y. Kim, Y.S. Chung, W.H. Park, J.H. Youk,Crystalline structure analysis of cellulose treated with sodium hydroxide andcarbon dioxide by means of X-ray diffraction and FTIR spectroscopy, Carbohydr.Res. 340 (2005) 2376-2391, https://doi.org/10.1016/j.carres.2005.08.007. [52] J.G. Zhang, X.M. Chen, J. Zhou, X.G. Luo, Uranium biosorption mechanism modelof protonated Saccharomyces cerevisiae, J. Hazard. Mater. 385 (2020), 121588,https://doi.org/10.1016/j.jhazmat.2019.121588. 植酸装饰的多孔有机聚合物用于高酸性条件下的铀提取前言尽管各种吸附剂具有良好的铀吸附能力,但它们仍然面临着在高酸性条件下捕获铀的挑战。因此,在强酸性条件下有效地提取铀成为研究人员的追求。在这项研究中,一种新的植酸装饰的多孔有机聚合物(POPs)的制备方法是:用三聚氰胺和尿嘧啶合成一个有机框架(MA-U),然后用植酸进行改性。结果表明,所制备的有机框架具有良好的稳定性和较强的铀螯合作用。使所制备的材料在强酸条件下成为一种优良的吸附剂。在pH值为1的条件下,吸附能力达到106.7毫克⋅g-1,远远高于MA-U和其他大多数吸附剂。变化机制的研究证实植酸与MA-U的氢键作用导致了成功的改性。此外PA的膦酸基团主要是与铀螯合的活性基团。此外,PA对铀的MA-U-PA对铀的吸附过程符合伪二阶动力学和Langmuir等温模型。新制备的植酸装饰的多孔有机材料可以实现高效的从高酸性的核废水中捕获铀。Insects Awaken /简介 最近,作为低碳能源之一的核工业的快速发展面临着重大的环境挑战,大量的含铀废水被排放出来[1,2]。传统的普雷克斯工艺已被广泛用于铀分离。然而,仍有一些问题需要解决,特别是在普雷克斯工艺中使用有机溶剂和强酸导导致了二次强酸有机废水的污染。因此,迫切需要制造更合适的材料和开发更多的创新技术来从酸性废水中提取铀,以改善核燃料循环[4]。正如我们所知道的,吸附法被证明是简单、有效和低成本的方法。尽管大多数传统的吸附剂表现出优异的吸附性能,但它们在恶劣条件下的抵抗力和化学稳定性较差。在高酸性废水中对铀的回收[6]。此外。大部分的吸附剂,特别是生物质吸附剂或其他有机吸附剂在强酸性条件下会被分解[7。8]. 更重要的是,在强酸条件下,这些吸附剂的吸附能力相对较低。大大限制了其实际应用。因为吸附剂的质子化[9-15]。因此,有两个主要问题需要同时解决。一个是如何提高吸附剂在高酸性条件下的稳定性。另一个是如何提高高酸条件下的吸附能力在高酸性条件下的吸附能力。基于上述背景,开发具有优良吸附性能的耐酸吸附剂已经引起了许多研究人员的兴趣。无机吸附剂,如活性炭炭[16,17]、石墨烯[18,19]、介孔二氧化硅[20,21]等具有良好耐酸性的无机吸附剂已被广泛研究。此外,大量的研究集中在这些吸附剂的功能化上(包括膦酸[22,23],酰胺肟[24,25]修饰等)以提高吸收效率。然而,它们在高酸性条件下的吸附能力但它们在高酸性条件下的吸附能力仍处于较低水平。多孔骨架材料,包括COF、POPs和MOFs,由于其高的比表面积、强的结构可设计性,吸引了越来越多的研究人员的兴趣。特别是对于POPs或COFs,它们的强共价键赋予了它们在各种苛刻条件下理想的稳定性此外,它们容易被修改的特性使它们有可能在酸性条件下提高在酸性条件下通过引入所需的在这个框架中引入所需的官能团。此外,这些材料是主要由轻型元素构成,如(H、C、N和O)。导致其密度低,这无疑对提高吸附能力是有益的。考虑到在恶劣条件下的稳定性,其在各方面的应用(如气体储存和分离、能源转换和储存、催化、半导体、环境等),包括吸附已被开发[1,5,12,37-43]。因此,通过选择适当的前驱体并进一步修改,并使用适当的基团进一步改性来设计一种有机多孔聚合物,以适当提高的基团来提高高酸性条件下的吸附能力。三聚氰胺是一种廉价的化工原料,含有丰富的氨基,具有丰富的孤对电子,其C3结构单元和氨基官能团也是构建多孔有机高分子材料的重要结构[1]。脲嘧啶是一种从生物质中提取的原料,每个分子中都有两个羰基,有利于通过构建有机框架结构。与三聚氰胺形成席夫碱。在此,我们用三聚氰胺和尿素构建了一种有机多孔聚合物(名为MA-U)。结构单元通过亚胺连接连接起来。然后将制备好的MA-U与植酸通过氢键连接进行改性。这可以确保理想的稳定性,因为合适的氢键系统和更高的吸附力确保了理想的稳定性,并且由于在下层引入了更多的有机磷酸盐基团而具有更高的吸附能力,可以确保理想的稳定性条件下引入更多的有机磷酸盐基团。MA-U-PA表现出更好的吸附能力和在高酸性条件下具有良好的稳定性。Insects Awaken /祥鹄仪器在文献中的使用过程2.2. MA-U和MA-U-PA的合成 MA-U-PA的合成是按照以下程序进行的流程如下。三聚氰胺和尿嘧啶各5毫摩尔溶解在30毫升的DMSO中,然后倒入由Teflon(XH-800C,北京祥鹄,中国)制成的反应容器中,在180℃下反应12小时。形成白色果冻,然后被离心分离,用水和乙醇交替清洗。清理后,白色固体在50℃下干燥24小时,得到的产品命名为MA-U。图1a所示为MA-U-PA的制备示意图。在干燥前,将另一部分白色固体浸入30毫升植酸(4%的水溶液),并在室温下将悬浮液均匀搅拌,然后倒入用Teflon制成的反应容器,在95℃下反应12小时。泥状物被离心分离并用水洗涤,直到滤液呈中性。浅紫色固体在50℃下干燥24小时。得到的产品被命名为MA-U-PA。Insects Awaken /结论一种植酸修饰的新型多孔有机骨架,通过水热合成法成功制备MA-U-PA使用三聚氰胺和尿嘧啶作为构建单元。 形态学和MA-U-PA的理化性质通过FT-IR表征SEM、BET、XPS和Z电位。此外,由于其稳定的骨架和表面的磷酸基团,MA-U-PA 是一种淡紫色粉末,可实现对铀的高效脱除高酸性条件,最大铀容量为在pH值为1.0时为106.7 mg⋅g-1,远高于大多数多孔材料有机吸附剂。此外,结果表明吸附过程以化学吸附为主,同时伴有少量物理吸附。由于框架结构和丰富的磷酸基团有利于在强酸性条件下提取铀,MA-U-PA可能是铀的理想候选吸附材料酸性核燃料流出物中的捕获。
确定





还剩9页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《核废水中铀的提取(多孔有机聚合物)检测方案(微波消解仪)》,该方案主要用于废水中(类)金属及其化合物检测,参考标准--,《核废水中铀的提取(多孔有机聚合物)检测方案(微波消解仪)》用到的仪器有微波消解工作站 Honeycomb、微波消解工作站 Acidcube XH-800DE型
相关方案
更多











