方案详情
文
鸡胴体酶解制备蛋白水解物生产Enzymatic hydrolysis of minced chicken carcasses for protein hydrolysate production
方案详情

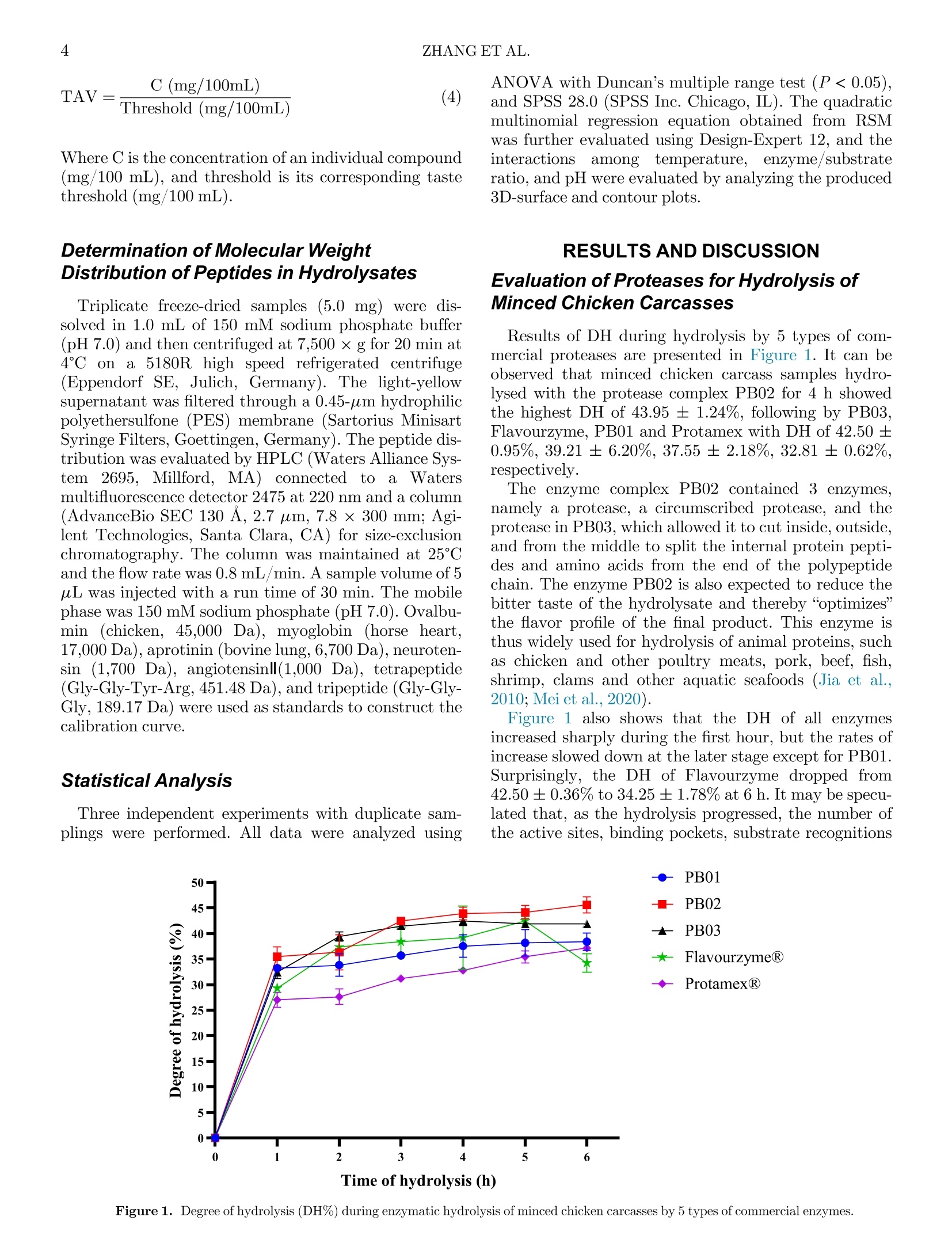
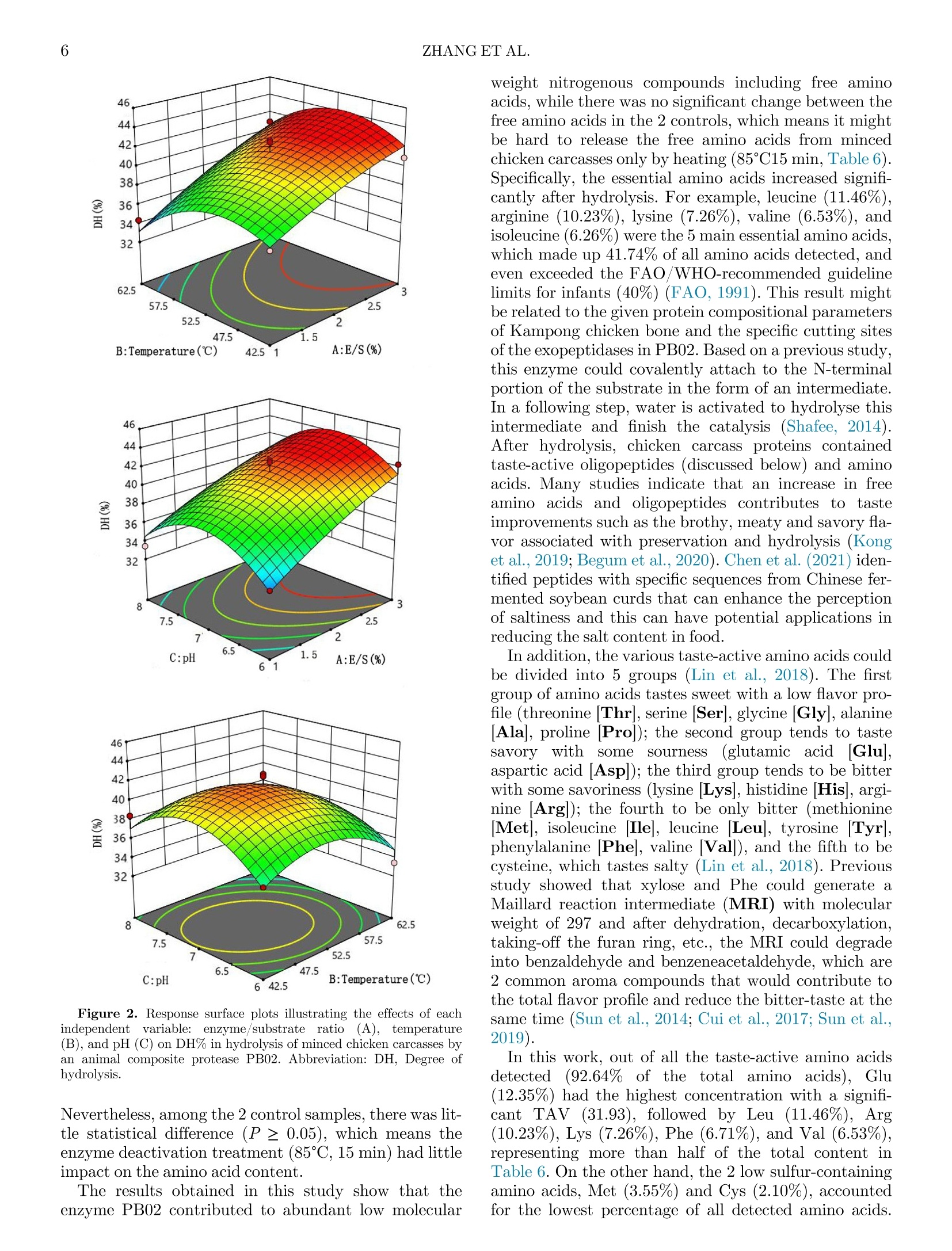
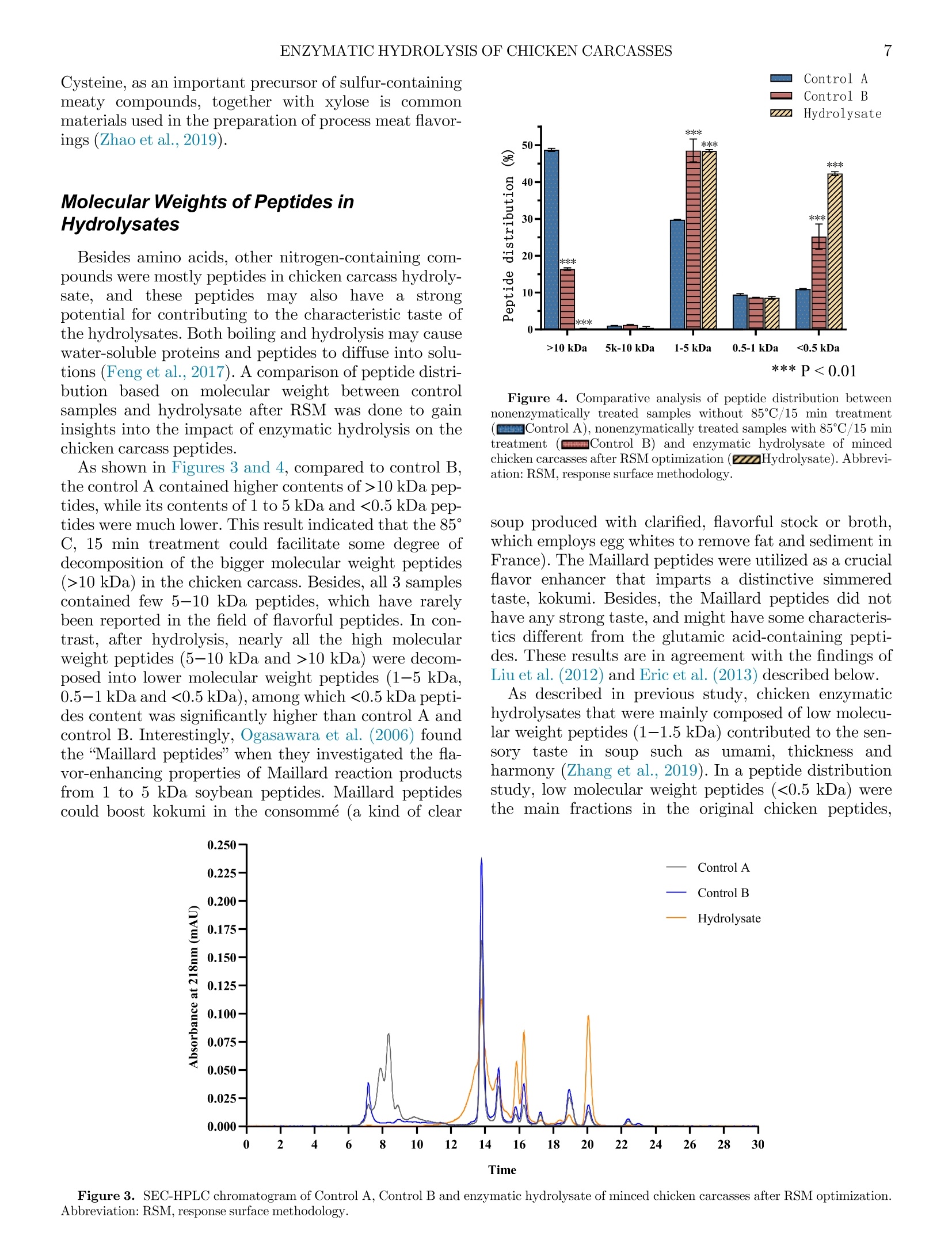
鸡胴体酶解制备蛋白水解物生产Enzymatic hydrolysis of minced chicken carcasses for protein hydrolysate production鸡胴体酶解制备蛋白水解物生产Poultry Science° 2ZHANG ET AL. Enzymatic hydrolysis ofminced chicken carcasses for protein hydrolysate production 新加坡国立大学食品科学与技术系新加坡国立大学(苏州)研究所 Xing Zhang,a Xinzhi Li,a ,c and Shao-Quan Liu a ,b ,1 广东海天创新科技有限公司 aDepartment of Food Science and Technology, National University of Singapore, Singapore 117543, Singapore; bNational University of Singapore (Suzhou) Research Institute, Jiangsu 215213, China; and cGuangdong Haitian Innovation Tech Co. Ltd. Foshan 528000, China ABSTRACT Animal and poultry processing generates signiicant volumes of by-products that can be further processedfor otheruses.In thisstudy,wetreated minced chicken carcasses with proteases to produce pro-tein hydrolysates that can be used as nutritional and/or lavor-enhancing ingredients. Five different microbial proteases were investigated for their abilities to hydro-lyse the minced chicken carcass: Flavourzyme, Prota-mex, PB01, PB02, and PB03, with PB02 demonstrating the highest degree of hydrolysis (DH ) of the minced chicken carcass (43.95%) after 4 h of hydrolysis. The essential hydrolytic parameters were optimized using response surfacemethodology in conjunction with Box-Behnken design. The optimal conditions were found to be: enzyme/substrate ratio of 3:100 (w/w), temperature of 51.20°C, pH of 6.62§ 0.05, and substrate/water ratio of 1:1 (w/v) for 4-h hydrolysis, which resulted in amaxi-mum DH of 45.44%. The protein recovery was 50.45 §2.05%, and the protein hydrolysate was high in free amino acids (7,757.31 mg/100 mL), of which essential and taste-active amino acids accounted for 41.74% and92.64%, respectively. The hydrolysate was comprised mainly of low molecular weight peptides (1-5 kDa, 0.5-1 kDa, and <0.5 kDa), which were potential taste sub-stances andlavor precursors. The resulting hydrolysate might be employed as a nutritive product, an ingredient forlavoring generation or a component of fermentation media. Keywords: chicken carcass, enzymatic hydrolysis, proteases, RSM, peptides 2023 Poultry Science 102:102791https://doi.org/10.1016/j.psj.2023.102791 INTRODUCTION Due to the feed price rises, international conlicts and COVID-related disruptions, global chicken meat pro-duction is expected to rise by only 0.43% in 2022 and in spite of this, still reaching an all-time high of 100.9mil-lion tons per year (USDA, 2022). It has also been found that chicken age can have a substantial inluence on dressing percentage and carcass quality, together with a clear trend toward a decrease in the proportion of bones and giblets in older chicken carcasses, resulting in signii-cant loss of sales (Poltowicz and Doktor, 2012). On the demand side, a sustained demand for low-cost animal protein continues to increase, which would result in a larger amount of waste such as skin, carcasses, vis-cera, and feathers being produced (Zhu et al., 2010; Lasekan et al., 2013; Alao et al., 2017). The eficient use C 2023 The Authors. Published by Elsevier Inc. on behalf of Poultry Science Association Inc. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/ 4.0/). ReceivedMarch 24, 2023. AcceptedMay 15, 2023. 1Corresponding author: fstlsq@nus.edu.sg of by-products is one of comprehensive approaches to reducing these losses and to assisting the chicken indus-try in valorization of food waste. In industrial poultry processing,most parts of a chicken are carefully cut out, but the carcasses remain as a by-product that have a low value. Many attempts have been made to evaluate the feasibility of various chicken by-products valoriza-tionmethods in order to produce high-value goods such as protein hydrolysates. For instance, chicken bones weretransformed intohydrolysateswhichcouldbe nutritional and lavoring food substances (Dong et al.,2014). products produced from chicken bone extract, wheat protein, and rice protein hydrolysatesmight be exploited as possible natural lavorings in food applications(Chiang et al., 2022). Li et al. (2021) also found that the pork trimmings hydrolysate contained a high amount of taste-active amino acids thatmight serve as nutritional and/orlavoring ingredients. The goal of this research was to evaluate and optimize hydrolysis of minced chicken carcasses using several commercial proteolytic enzymes and response surface methodology (RSM ) with a view to producing a hydro-lysate that can be used as alavoring per se or as a sub-strate for subsequent biotransformation into novel lavoring substances. MATERIALS ANDMETHODS Materials and Enzymes Fresh chicken carcasses were purchased from a local supermarket (Aw’s Market Fresh Anxin Kampong Chicken Bones, NTUC Fairprice Co Ltd, Singapore). The chicken carcasses were washed by the running tap water to remove the residual blood, and then were cut into blocks at a size of approximately 1£ 3£ 5 cm2 and minced using a handheld blender (HR1613/00, Philips, Koninklijke, Netherland). All minced chicken carcasses were pooled, packed in food grade air-tight bags and kept at -20°C before use. Table 1 shows the 5 different microbial proteases that were procured from various suppliers. Flavour-zyme_500MG is a fungal protease/peptidase complex made by Aspergillus oryzae with a stated enzymatic activity of 500 LAPU per g (Novozymes, Bagsvard, Denmark). Protamex, with a declared enzymatic activ-ity of 1.5 AU/g (Anson unit per gram, Novozymes, Bagsvard, Denmark), is a complex of protease pro-duced by Bacillus licheniformis . Papain (800 000 units1, Nanning Pangbo Biological Engineering Co., Ltd.[Nanning, China], PB01) is aproteasepreparation extracted from raw papaya fruits. MEAP (mixed enzymes for animal proteolysis, Nanning Pangbo Bio-logical Engineering Co., Ltd. [Nanning, China], PB02), is a protease/peptidase that includes endoprotease, mixed exopeptidases and fungal protease and peptidase made from A. oryzae. Flavourzyme_Pangbo (20 000units g 010, Nanning Pangbo Biological Engineering Co., Ltd. [Nanning, China], PB03) is amixture of protease and peptidase from B. licheniformis . Proximate Composition Before compositional measurements, raw and hydro-lysed samples were freeze-dried in a freeze dryer (VirTis-Benchtop Freeze Dryers, SP Industries TM,Warminster, PA). The moisture content of the minced chicken car-casses was determined using an electronic moisture bal-ance (MOC-120H, Shimadzu Co Ltd., Kyoto, Japan). The ash content was determined based on the standard Association of Oficial Analytical Chemists (AOAC ,1995)method with slightmodiications. Total crude pro-tein content was determined using the standard AOAC (1995) procedure with the Kjeldahl system(VAPODEST20, Gerhardt GmbH & Co. KG, Germany). Total fat con-tent was also determined according to the AOAC (1995) procedure using a SoxthermRapid Soxhlet System(SOX-THERMTM, C. Gerhardt GmbH & Co. KG, Germany). The defatted sample was collected and kept in a sealed bag at - 20°C for further analysis. Enzymatic Hydrolysis Before the experiment, 40 g of thawed minced chicken carcasses was weighed and suspended in distilled water at a ratio of 1:1 (w/v) in a Duran laboratory bottle (Merck, Darmstadt, Germany). Aftermixingmanually, themixture wasrrst placed in a water bath (SW22, Julabo, Seelbach, Germany) for 10min to reach 50°C. One type of enzyme was added to the mixture according to the conditions in Table 1, based on the results frompreliminary experiments. Speci cally, the pH values of the samples were kept natural(6.62§0.05) initially. In the RSM stage, the pH values of the samples were adjusted accordingly using 1 M lactic acid (Merck) or 1MNaOH (Merck) solutions. Subsequently, 1 type of protease was added into each bottle at an enzyme/substrate ratio of 2:100 (w/w) for alltve proteases andmixedmanually. All samples were then placed back in the same water bath at 50°C and incubated at a shaking rate of 160 rpmfor 6 h. After the hydrolysis, all the samples were heated at 85°C for 15 min to terminate the reaction. Samplings were con-ducted at time intervals of 0, 1, 2, 3, 4, 5, and 6 h. The slurry obtained was ltered through a coconut clothTl-ter to remove the insolublematerials. Determination of Degree of Hydrolysis The degree of Hydrolysis (DH ) of the samples was calculated using the relationship between a -amino Table 1. Optimal parameters of 5 proteases used inminced chicken carcass hydrolysis. Code of enzyme PB01 PB02 PB03 Flavor protease and peptidasemixture from B. Flavourzyme_500MG A fungal protease/ peptidase complexmade by Protamex A complex of protease produced by B. MEAP (mixed enzymes for Basic property Papain animal proteolysis) licheniformis A. oryzae licheniformis E/S1, (%, w/w) 2:100 2:100 2:100 2:100 2:100 Temperature (°C) 50°C−55°C 50°C−52.5°C 50°C 50°C 50°C pH 6−7 6.5−7 6.5−7 6.0−6.5 6.5 1E/S: Enzyme/substrate ratio (%, w/w). nitrogen (AN) and total nitrogen according to the equa-tion: The amount of a -amino nitrogen present in the hydro-lysate was determined using themodiied formaldehyde titrationmethod described by Li et al. (2021). Controls were also prepared by incubating minced chicken car-casses in the same water bath without adding enzymes. Experimental Design for Hydrolysis Optimization and Determination of Protein Recovery Preliminary single-factor experiments revealed that theimportantfactorsthataffectedtheamino acid release from chicken carcass protein were A, enzyme/substrate ratio (E/S, %), B, temperature (°C) and C, pH. And the initial screening of proteases showed that PB02 was the best protease that gave a relative high DH with a lower price among the 5 commercial proteases. The RSM-based on Box-Behnken design was applied to optimize the effects of the above-mentioned factors on the amino acid releases after chicken carcass hydroly-sis and to build an empirical model. Additionally, the independent variables and their associated values were also chosen based on theindings of preliminary screen-ing. Tables 1 and 2 summarize the experimental range and levels for the 3 independent variables in terms of their actual and coded values, respectively. Using this approach, an experimental design of 1-response (DH) and 3-factors with 3 levels containing 5replicates at the center point was generated in Design-Expert 12 and 17 experimental runs in random order were required. In actual experiments, all the 17 experi-mentalruns were conductedin this random order accordingly to reduce potential biases. For each run, one40-galiquotof mincedchickencarcasssample was weighed accurately in a Duran laboratory bottle with triplicate samplings. Independent triplicate experiments followingthesame random run ordersatdifferent dates/times to reduce potential systematic errors. The estimated response surface DH could be described by the following quadratic equation (Shankar et al., 2010): Table 2. Values of coded levels used in optimization of hydrolysis of minced chicken carcasses using an animal composite protease PB02. Process variables Coded levels -1 0 1 E/S,%(A) 1:100 2:100 3:100 Temperature, °C(B) 42.5 52.5 62.5 pH (C) 6 7 8 where Y is a dependent variable (DH), b 0 is a constant; b i, b ii, and b ji are coeficients that would be estimated using the equation; and Xi and Xj are values of the inde-pendent variables that represent the linear or quadratic effects of the A, B, and C values on the response, respec-tively. Evaluation of designs and calculation of predicted data were conducted automatically by using Design-Expert 12.0.1.0 32-bit version (Stat-Ease, Inc.,Minneap-olis,MN), and statistical analysis of the regressionmodel was conducted using ANOVA. The key values such as the F -statistic, R 2, P -values, and lack of vt were calcu-lated by the software. From this, a proposed equation was presented based on the results obtained. Determination of Free Amino Acids in Protein Hydrolysates The amino acid proile of the hydrolysate was deter-mined using the ARACUS Amino Acid Analyzer’s pre-set physiological separation algorithm (MembraPure, Berlin, Germany). A lithium-cation exchange column was used along with ninhydrin (a reagent for postcol-umn derivatization) and eluents (Eluent A to Eluent F) provided by the manufacturer. Except for proline and hydroxyproline, which were identiied at 440 nm, all other amino acids were detected at 570 nm. The amino acids were quantiied using a calibration factor gener-ated by MembraPure. The data evaluation was con-ducted with a Chromatography Software. Firstly, the chromatogram of an standard amino acid mixture(“physiological standard” from the supplier) was inte-grated as a template. Secondly, the chromatogram(s) of unknown samples was loaded to calibrate the values of each peak (concentration / nmol, area, height) at a proper dilution factor by the sample dilution buffer, fol-lowed by transferring the results to an Excel table and calculating the concentration by molar mass of each amino acid. Where C is the concentration of an individual compound(mg/100 mL), and threshold is its corresponding taste threshold (mg/100mL). Determination ofMolecularWeight Distribution of Peptides in Hydrolysates Triplicate freeze-dried samples (5.0 mg) were dis-solved in 1.0 mL of 150 mM sodium phosphate buffer (pH 7.0) and then centrifuged at 7,500£ g for 20min at 4°C ona 5180R highspeedrefrigeratedcentrifuge(EppendorfSE,Julich,Germany). Thelight-yellow supernatant wasiltered through a 0.45-m mhydrophilic polyethersulfone (PES) membrane (Sartorius Minisart Syringe Filters, Goettingen, Germany). The peptide dis-tribution was evaluated by HPLC (Waters Alliance Sys-tem 2695, Millford, MA) connected to a Waters multiluorescence detector 2475 at 220 nmand a column (AdvanceBio SEC 130 A nc , 2.7m m, 7.8 £ 300 mm; Agi-lent Technologies, Santa Clara, CA) for size-exclusion chromatography. The column was maintained at 25°C and thelow rate was 0.8mL/min. A sample volume of 5m L was injected with a run time of 30min. Themobile phase was 150mMsodiumphosphate (pH 7.0). Ovalbu-min (chicken,45,000 Da), myoglobin (horse heart,17,000 Da), aprotinin (bovine lung, 6,700 Da), neuroten-sin (1,700 Da), angiotensin l (1,000 Da), tetrapeptide(Gly-Gly-Tyr-Arg, 451.48 Da), and tripeptide (Gly-Gly-Gly, 189.17 Da) were used as standards to construct the calibration curve. Statistical Analysis Three independent experiments with duplicate sam-plings were performed. All data were analyzed using ANOVA with Duncan’smultiple range test (P < 0.05), and SPSS 28.0 (SPSS Inc. Chicago, IL). The quadratic multinomial regression equation obtained from RSM was further evaluated using Design-Expert 12, and the interactions among temperature, enzyme/substrate ratio, and pH were evaluated by analyzing the produced 3D-surface and contour plots. RESULTS AND DISCUSSION Evaluation of Proteases for Hydrolysis of Minced Chicken Carcasses Results of DH during hydrolysis by 5 types of com-mercial proteases are presented in Figure 1. It can be observed that minced chicken carcass samples hydro-lysed with the protease complex PB02 for 4 h showed the highest DH of 43.95 § 1.24%, following by PB03, Flavourzyme, PB01 and Protamex with DH of 42.50 §0.95%, 39.21 § 6.20%, 37.55 § 2.18%, 32.81 § 0.62%, respectively. The enzyme complex PB02 contained 3 enzymes, namely a protease, a circumscribed protease, and the protease in PB03, which allowed it to cut inside, outside, and from themiddle to split the internal protein pepti-des and amino acids from the end of the polypeptide chain. The enzyme PB02 is also expected to reduce the bitter taste of the hydrolysate and thereby “optimizes”the lavor proile of the inal product. This enzyme is thus widely used for hydrolysis of animal proteins, such as chicken and other poultry meats, pork, beef, ish, shrimp, clams and other aquatic seafoods (Jia et al.,2010;Mei et al., 2020). Figure 1 also shows that the DH of all enzymes increased sharply during theirst hour, but the rates of increase slowed down at the later stage except for PB01. Surprisingly, the DH of Flavourzyme dropped from 42.50§ 0.36%to 34.25§ 1.78%at 6 h. Itmay be specu-lated that, as the hydrolysis progressed, the number of the active sites, binding pockets, substrate recognitions Figure 1. Degree of hydrolysis (DH%) during enzymatic hydrolysis ofminced chicken carcasses by 5 types of commercial enzymes. as well as transition-state binding in Flavourzyme decreased, leading to the increase of the activation energy of the reaction, and allowing kinetically unfavor-able reactions to proceed through to product formation (Nothling et al., 2018). Optimization of Chicken Carcass Hydrolysis by RSM For the response factor DH, RSM was applied to the chosen PB02 composite proteases as it showed the high-est DHmentioned above. The independent variables are shown in Table 2. The quadraticmodel below was gener-ated by analyzing the obtained experimental data: The equation shows a goodit with the data obtained experimentally as the adjusted coeficient of determina-tion (R 2adj) was 0.8422, which suggests that this equation canexplain84.22% of thevariabilityinthe model (Table 3). The P -values were generally low (P = 0.0027), with an insigniecant (P = 0.1942) residual lack of=t. The coefacient of determination (R 2 = 0.9309) was considered acceptable, relecting low experimental errors based on the ANOVA results (Table 4). An examination of the effects of different variables on themaximum DH revealed that the ratio of enzyme to substrate (A) had themost signiicant effect on themax-imum DH (0.0004), while temperature (B) also signii-cantly affected themaximum DH (0.0322), but pH (C) Table 3. Coded level combinations fromthe Box-Behnken design matrix and the response of dependent variable DH (%) forminced chicken carcasses hydrolysed by an animal composite protease PB02. Run order1 Coded levels A B C DH (%)a 1 0 0 0 42.76§ 0.39 2 0 0 0 40.26§ 1.72 3 1 0 -1 42.30§ 5.12 4 -1 -1 0 36.62§ 1.72 5 -1 1 0 34.35§ 2.46 6 1 -1 0 40.94§ 6.18 7 0 -1 -1 36.39§ 1.80 8 0 0 0 40.71§ 3.93 9 0 0 0 41.39§ 5.29 10 0 1 1 32.99§ 2.97 11 0 0 0 42.53§ 4.47 12 -1 0 -1 34.35§ 3.12 13 0 -1 1 38.44§ 4.47 14 1 1 0 40.94§ 2.75 15 1 0 1 40.03§ 1.04 16 0 1 -1 33.44§ 4.54 17 -1 0 1 33.44§ 3.43 Abbreviation: DH, Degree of hydrolysis. aAll the values stated asmean § SD (standard deviation) of 3 values%variability explained R 2 = 0.9309, R2adj = 0.8422. 1Randomized. showedthe minimal impacton the maximum DH(0.7048). In addition, the quadratic terms (B2, C2) had signiicant effects on the DH with their respective P val-ues of less than 0.01. The 3D illustrations of the DH responses using Eq. (4) are presentedin Figure2,andthe maximum DH obtained is indicated in the contour plot by the surface conined in the smallest ellipse. The optimal conditions adopted by Design-Expert methods were: enzyme/sub-strate ratio (A) of 3.00: 100 (w/w), temperature of 51.20°C (B), and pH of 6.935. Here, the desirability is0.410, and themaximumDH predicted under these con-ditions was 44.228%. Applying these optimum hydroly-sis conditions, we conducted tests to check the validation of DH and found that it had amean value of44.198%with a standard error of 1.07%(n = 3). In order to keep the cost of production down, we tested the optimal conditions with unadjusted pH (6.62), and the result showed an even higher DH value of 45.436% than that (44.198%) obtained under the optimal conditions adopted by Design-Expertmethods. The inal optimal conditions using RSM for the PB02animal composite protease were thus determined as:enzyme/substrate ratio of 3.00:100 (w/w), temperature at 51.20°C, unadjusted pH (6.62), substrate/water ratio of 1:1 (w/v), and 4 h. The PR was 50.45%, which indi-cates that our methodmay help with better utilization of chicken carcasses. Proximate Composition of Chicken Carcass and Hydrolysate The proximate composition of minced raw chicken carcasses and hydrolyzed samples obtained under opti-mal hydrolysis conditions are presented in Table 5. Compared with the freeze-driedminced raw chicken car-casses, there was little compositional change before and afteriltration. Afteriltration, the total ash and fat con-tents of the freeze-dried hydrolysate decreased by over50% and 90%, respectively, because iltration with a coconut cloth ilter had removed the insoluble substan-ces. Simultaneously,most of the fat was removed by the naturally separation from the aqueous solution and by coconut clothilter; only 1.25%fat remained. Due to the removal of fat and insoluble matters that would have trapped ashes, the crude protein content increased by 21.38%. Amino Acid Proiles ofMinced Chicken Carcasses and Hydrolysates The amino acid compositions of nonenzymically treated control A (4 h in the water bath without 85°C treatment), nonenzymically treated control B (4 h), and enzymatic hydrolysate (4 h) are presented in Table 6. Thirty-nine amino acids with ammonium chloride were detected and all detected amino acids increased signii-cantly (P < 0.05) except Ans after 4 h of enzymatic hydrolysis under RSM-optimized conditions. Figure 2. Response surface plots illustrating the effects of each independent variable: enzyme/substrate ratio (A), temperature (B), and pH (C) on DH% in hydrolysis ofminced chicken carcasses by an animal composite protease PB02. Abbreviation: DH, Degree of hydrolysis. Nevertheless, among the 2 control samples, there was lit-tle statistical difference (P ≥ 0.05), which means the enzyme deactivation treatment (85°C, 15min) had little impact on the amino acid content. The results obtained in this study show that the enzyme PB02 contributed to abundant low molecular weight nitrogenous compounds including free amino acids, while there was no signiicant change between the free amino acids in the 2 controls, whichmeans itmight be hard to release the free amino acids from minced chicken carcasses only by heating (85°C15min, Table 6). Speciically, the essential amino acids increased signii-cantly after hydrolysis. For example, leucine (11.46%), arginine (10.23%), lysine (7.26%), valine (6.53%), and isoleucine (6.26%) were the 5main essential amino acids, whichmade up 41.74% of all amino acids detected, and even exceeded the FAO/WHO-recommended guideline limits for infants (40%) (FAO, 1991). This resultmight be related to the given protein compositional parameters of Kampong chicken bone and the speciic cutting sites of the exopeptidases in PB02. Based on a previous study, this enzyme could covalently attach to the N-terminal portion of the substrate in the form of an intermediate. In a following step, water is activated to hydrolyse this intermediate and inish the catalysis (Shafee, 2014). Afterhydrolysis,chickencarcassproteinscontained taste-active oligopeptides (discussed below) and amino acids. Many studies indicate that an increase in free amino acids andoligopeptides contributes to taste improvements such as the brothy,meaty and savoryla-vor associated with preservation and hydrolysis (Kong et al., 2019; Begumet al., 2020). Chen et al. (2021) iden-tiled peptides with speci2c sequences from Chinese fer-mented soybean curds that can enhance the perception of saltiness and this can have potential applications in reducing the salt content in food. In addition, the various taste-active amino acids could be divided into 5 groups (Lin et al., 2018). The orst group of amino acids tastes sweet with a low.avor pro-gle (threonine [Thr ], serine [Ser ], glycine [Gly ], alanine [Ala ], proline [Pro ]); the second group tends to taste savory with some sourness (glutamic acid [Glu ], aspartic acid [Asp ]); the third group tends to be bitter with some savoriness (lysine [Lys ], histidine [His ], argi-nine [Arg ]); the fourth to be only bitter (methionine[Met ], isoleucine [Ile ], leucine [Leu ], tyrosine [Tyr ], phenylalanine [Phe ], valine [Val ]), and the ifth to be cysteine, which tastes salty (Lin et al., 2018). Previous study showed that xylose and Phe could generate a Maillard reaction intermediate (MRI) with molecular weight of 297 and after dehydration, decarboxylation, taking-off the furan ring, etc., the MRI could degrade into benzaldehyde and benzeneacetaldehyde, which are2 common aroma compounds that would contribute to the totallavor proile and reduce the bitter-taste at the same time (Sun et al., 2014; Cui et al., 2017; Sun et al.,2019). In this work, out of all the taste-active amino acids detected (92.64% of the total amino acids), Glu (12.35%) had the highest concentration with a signii-cantTAV(31.93),followedbyLeu(11.46%), Arg (10.23%), Lys (7.26%), Phe (6.71%), and Val (6.53%), representing more than half of the total content in Table 6. On the other hand, the 2 low sulfur-containing amino acids, Met (3.55%) and Cys (2.10%), accounted for the lowest percentage of all detected amino acids. Control A Control B ZZZZ Hydrolysate ***P <0.01 Figure 4. Comparative analysis of peptide distribution between nonenzymatically treated samples without 85°C/15 min treatment ( Control A), nonenzymatically treated samples with 85°C/15min treatment ( Control B) and enzymatic hydrolysate of minced chicken carcasses after RSMoptimization ( Hydrolysate). Abbrevi-ation: RSM, response surfacemethodology. soup produced with clariied, lavorful stock or broth, which employs egg whites to remove fat and sediment in France). TheMaillard peptides were utilized as a crucial lavor enhancer that imparts a distinctive simmered taste, kokumi. Besides, the Maillard peptides did not have any strong taste, andmight have some characteris-tics different from the glutamic acid-containing pepti-des. These results are in agreement with theindings of Liu et al. (2012) and Eric et al. (2013) described below. As described in previous study, chicken enzymatic hydrolysates that weremainly composed of lowmolecu-lar weight peptides (1−1.5 kDa) contributed to the sen-sory taste in soup such as umami, thickness and harmony (Zhang et al., 2019). In a peptide distribution study, low molecular weight peptides (<0.5 kDa) were the main fractions in the original chicken peptides, Time Figure 3. SEC-HPLC chromatogram of Control A, Control B and enzymatic hydrolysate ofminced chicken carcasses after RSM optimization. Abbreviation: RSM, response surfacemethodology. Table 4. ANOVA for responses of DH. Source Sumof squares Degree of freedom Mean square F-value P-value Model 188.99 9 21.00 10.49 0.0027 A-E/S 80.96 1 80.96 40.43 0.0004 B-Temperature 14.23 1 14.23 7.11 0.0322 C-pH 0.3120 1 0.3120 0.1558 0.7048 AB 1.29 1 1.29 0.6433 0.4489 AC 0.4624 1 0.4624 0.2309 0.6455 BC 1.56 1 1.56 0.7802 0.4064 A2 1.28 1 1.28 0.6389 0.4504 B2 32.22 1 32.22 16.09 0.0051 C2 50.08 1 50.08 25.01 0.0016 Residual 14.02 7 2.00 Lack ofat 9.20 3 3.07 2.55 0.1942 Pure error 4.82 4 1.20 Cor total 203.01 16 Abbreviation: DH, Degree of hydrolysis. Table 5. Proximate composition ofminced chicken carcasses and optimized hydrolysates. Component Raw chicken carcass (%) Hydrolysate after RSM(%, beforeiltration) Hydrolysate after RSM(%, afteriltration) Moisture Ash1 68.27§ 0.4115.35§ 1.76 ND14.57§ 0.36 ND 7.29§ 0.01 Crude protein1 Fat1 55.75§ 2.1123.03§ 0.28 51.86§ 0.42 25.88§ 0.31 67.67§ 1.55 1.25§ 0.04 Abbreviations: ND, not determined; RSM, response surfacemethodology. 1Expressed on dry weight basis. Table 6. Amino acid composition and taste activity values (TAV) of nonenzymically treated controls A, B and enzymatic hydrolysate ofminced chicken carcass (4 h). *Control A (4h) **Control B (4h) Hydrolysate (4h) Content TAV Content TAV Content TAV Asp Thr Ser 18.29§ 2.88c 37.02§ 3.87c42.04§ 5.87c 0.18 0.14 0.28 17.44§ 5.11c 30.24§ 7.99c 35.14§ 10.08c 0.17 0.12 0.23 262.89§ 35.36b 344.88§ 60.65b198.94§ 45.55b 2.63 1.33 1.33 Glu 119.39§ 3.83c 3.98 99.02§ 18.43c 3.30 957.89§ 123.13b 31.93 Gly 47.79§ 2.90 c 0.37 38.47§ 7.94c 0.30 98.55§ 12.36b 0.76 Ala 86.46§ 5.51c 1.44 66.99§ 13.08cc 1.12 409.81§ 54.09b 6.83 Val 39.50§ 1.17c 0.99 31.50§ 5.81c 0.79 506.59§ 70.14b 12.66 (Cys)2 4.11§ 1.61c ND 2.09§ 1.15c ND 163.13§ 15.07b ND Met 17.72§ 0.20c 0.59 13.80§ 2.26c 0.46 275.29§ 29.53b 9.18 Ile 25.65§ 0.75c 0.28 20.38§ 3.47c 0.23 485.76§ 63.14b 5.40 Leu 45.26§ 0.95c 0.24 35.67§ 4.99c 0.19 888.97§ 74.26b 4.68 Tyr 25.48§ 1.85c ND 19.64§ 4.34c ND 349.00§ 126.53b ND Phe 23.45§ 1.00c 0.26 17.99§ 2.82c 0.20 520.14§ 71.69b 5.78 Cys 0.04§ 0.08c ND 0.00§ 0.00c ND 2.59§ 2.51b ND His 20.70§ 5.93c 1.04 18.81§ 8.67c 0.94 185.91§ 73.75b 9.30 Trp 6.88§ 0.73c ND 4.95§ 1.66c ND 59.34§ 6.38b ND Ans 165.89§ 12.12c ND 132.38§ 26.41c ND 150.03§ 25.84c ND Hylys 8.00§ 0.95c ND 6.19§ 2.46c ND 57.80§ 23.80b ND Orn 7.25§ 1.41c ND 6.71§ 1.38c ND 14.26§ 7.73b ND Lys 66.01§ 7.66c 1.32 56.96§ 13.33c 1.14 563.31§ 94.42b 11.27 NH4 15.19§ 2.02c ND 15.08§ 2.19c ND 39.87§ 4.68b ND Arg 46.68§ 3.30c 0.93 37.30§ 6.23c 0.75 793.31§ 199.35b 15.87 Hypro 5.59§ 2.11b,c ND 2.90§ 2.34c ND 13.72§ 11.39b ND Pro 84.86§ 2.95c 986.54§ 88.27c 0.28 66.40§ 8.94b 797.99§ 176.65c 0.22 179.49§21.79 a 7757.31§1419.77b 0.60 Total *Control A: nonenzymatically treated samples without 85°C/15min treatment. **Control B: nonenzymatically treated samples with 85°C/15min treatment. abcDifferent letters in the same row indicate signiycant differences at P <0.05. Values are themean § standard deviation of 5 independent replicates(n = 5). which has a stronger activity of amidogen NH2 com-paredwiththe high molecularweight fractions(1−3 kDa and >3 kDa) (Liu et al., 2015). It was also found that lowmolecular weight peptides (<0.5 kDa) were the mainly contributors to the generation of volatile in this study contained various lower molecular weight peptides (1−5 kDa, 0.5−1 kDa and <0.5 kDa), which are likely taste peptides and potentiallavor precursors. CONCLUSIONS This study was theirst to investigate the effectiveness of 5 commercial proteases in hydrolyzing minced chicken carcasses, with the enzyme complex PB02 displaying the highest DH (43.95 § 1.24%), in a more economical way. The inal optimal conditions using RSM for the animal composite protease PB02 were found to be: enzyme/sub-strate ratio of 3.00:100 (w/w), temperature of 51.20°C, natural pH (6.62 § 0.05), substrate/water ratio of 1:1(w/v), and time of hydrolysis at 4 h, which together yielded a maximum DH of 45.44%. The chicken carcass hydrolysate rich in taste-active free amino acids and pep-tides has a high potential for processing into food lavor-ings and/or nutrient additives or a nitrogen-rich fermentation medium. Furthermore, the peptides distri-bution showed that the hydrolysate consisted primarily of the lowmolecular weight peptides (1−5 kDa, 0.5−1 kDa, and <0.5 kDa), which are potential taste peptides andla-vor precursors. Therefore, this study presents a potential route for further effective valorization of chicken car-casses. More broadly, this study may provide a guidance on the valorization of other animal by-products. ACKNOWLEDGMENTS This research did not receive any speciic grant from funding agencies in the public, commercial, or not-for-proit sectors. Authors’ Contributions: Xing Zhang, Xinzhi Li, and Shao-Quan Liu designed the research. Xing Zhang was responsible for the execution of the study, data collec-tion, and analysis. XZ and SL interpreted the data. Xing Zhang, Xinzhi Li, and Shao-Quan Liu playedmajor role in drafting, writing and revising the manuscript. All authors have known and agreed to thisinalmanuscript. All persons who meet authorship criteria are listed as authors, and all authors certify that they have partici-pated suficiently in the work to take public responsibil-ity for the content, including participation in the concept, design, analysis, writing, or revision of theman-uscript. Furthermore, each author certiees that this material or similarmaterial has not been and will not be submitted to or published in any other publication. Availability of Data andMaterial: All data generated or analyzed during this study will be made available from the corresponding author on reasonable request(and its supplementary informationiles). DISCLOSURES The authors have no afiliation with any organization with a direct or indirectinancial interest in the subject matter discussed in themanuscript. This Authorship &Conlicts of Interest Statement is agreed by all the authors listed in the manuscript to indicate agreement that the above information is true and correct. SUPPLEMENTARYMATERIALS Supplementary material associated with this article can be found in the online version at doi:10.1016/j. psj.2023.102791. REFERENCES Alao, B. O., A. B. Falowo, A. Chulayo, and V.Muchenje. 2017. The potential of animal by-products in food systems: production, pros-pects and challenges. Sustainability (Switzerland) 9:1–18. Ang, S. S., and M. R. Ismail −Fitry. 2019. Production of different mushroomprotein hydrolysates as potential lavourings in chicken soup using stem bromelain hydrolysis. Food Technol. Biotechnol.57(4):472. Begum, N., A. Raza, D. Shen, H. Song, Y. Zhang, L. Zhang, and P. Liu. 2020. Sensory attribute and antioxidant capacity ofMail-lard reaction products from enzymatic hydrolysate of bovine bone marrow extract. J. Food Sci. Technol. 57:1786–1797. Bhaskar, N., T. Benila, C. Radha, and R. G. Lalitha. 2008. Optimiza-tion of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla ) for preparing protein hydrolysate using a commercial protease. Bioresour. Technol. 99:335–343. Breternitz, N. R., H.M. A. Bolini, andM. D. Hubinger. 2017. Sensory acceptance evaluation of a new food lavoring produced bymicro-encapsulation of a mussel (Perna perna) protein hydrolysate. LWT-Food Sci. Technol. 83:141–149. Chen, Y. P.,M.Wang, I. Blank, J. Xu, and H. Y. Chung. 2021. Salti-ness-enhancing peptides isolated from the Chinese commercial fer-mented soybean curds with potential applications in salt reduction. J. Agric. Food Chem. 69:10272–10280. Chiang, J. H., M. T. Y. Yeo, D. S. M. Ong, and C. J. Henry. 2022. Comparison of themolecular properties and volatile compounds of Maillard reaction products derived from animal- and cereal-based protein hydrolysates. Food Chem. 383:132609. Cui, H., C. Jia, K. Hayat, J. Yu, S. Deng, E. Karangwa, and X. Zhang. 2017. Controlled formation of lavor compounds by preparation and application of Maillard reaction intermediate(MRI) derived from xylose and phenylalanine. RSC advances 7:45442–45451. Dang, Y., X. Gao, F. Ma, and X. Wu. 2015. Comparison of umami taste peptides in water-soluble extractions of Jinhua and Parma hams. LWT Food Sci. Technol. 60:1179–1186. Dong, X. B., X. Li, C. H. Zhang, J. Z.Wang, C. H. Tang, H.M. Sun, W. Jia, Y. Li, and L. L. Chen. 2014. Development of a novel method for hot-pressure extraction of protein from chicken bone and the effect of enzymatic hydrolysis on the extracts. Food Chem.157:339–346. Eric, K., L. V. Raymond, M. Huang, M. J. Cheserek, K. Hayat, N. D. Savio,M. Am es ee, and X. Zhang. 2013. Sensory attributes and antioxidant capacity of Maillard reaction products derived fromxylose, cysteine and sunlower protein hydrolysatemodel sys-tem. Food Res. Int. 54:1437–1447. FAO. 1991. Protein quality evaluation: Report of the Joint FAO/WHO Expert Consultation. Food and Agriculture Organization of the United Nations, Bethesda,Md., USA. Feng, X., C. Fu, and H. Yang. 2017. Gelatin addition improves the nutrient retention, texture andmass transfer of ish balls without altering their nanostructure during boiling. LWT Food Sci. Tech-nol. 77:142–151. Jia, J., Y. Zhou, J. Lu, A. Chen, Y. Li, and G. Zheng. 2010. Enzy-matic hydrolysis of alaska pollack (Theragra chalcogramma ) skin and antioxidant activity of the resulting hydrolysate. J. Sci. Food Agric. 90:635–640. Kong, Y.,L.-L. Zhang, J. Zhao, Y.-Y. Zhang, B.-G. Sun,and H.-T. Chen. 2019. Isolation and identiZcation of the umami pepti-des from shiitakemushroom by consecutive chromatography and LC-Q-TOF-MS. Food Res. Int. 121:463–470. Lasekan, A., F. Abu Bakar, and D. Hashim. 2013. Potential of chickenby-productsassourcesofusefulbiologicalresources. WasteManage 33:552–565. Li, X., P. R. Lee, F. Taniasuri, and S. Q. Liu. 2021. Biotransformation of pork trimmings into protein hydrolysate using microbial pro-teases aided by response surfacemethodology. J. Food Sci. Tech-nol. 1–10. Lin, H., X. Yu, J. Fang, Y. Lu, P. Liu, Y. Xing, Q.Wang, Z. Che, and Q. He. 2018. Flavor compounds in Pixian broad-bean paste: non-volatile organic acids and amino acids.Molecules 23:1299. Liu, J.,M. Liu, C. He, H. Song, and F. Chen. 2015. Effect of thermal treatment on thelavor generation fromMaillard reaction of xylose and chicken peptide. LWT Food Sci. Technol. 64:316–325. Liu, P.,M. Huang, S. Song, K. Hayat, X. Zhang, S. Xia, and C. Jia. 2012. Sensory characteristics and antioxidant activities ofMaillard reaction products fromsoy protein hydrolysates with differentmolecular weight distribution. Food Bioprocess Technol. 5:1775–1789. Marculescu, C., and C. Stan. 2011. Poultry processing industry waste to energy conversion. Energy Procedia 6:550–557. Mei, F., J. Liu, J.Wu, Z. Duan,M. Chen, K.Meng, S. Chen, X. Shen, G. Xia, andM. Zhao. 2020. Collagen peptides isolated from Salmo salar and Tilapia nilotica skin accelerate wound healing by altering cutaneous microbiome colonization via upregulated NOD2 and BD14. J. Agric. Food Chem. 68:1621–1633. Nilsang, S., S. Lertsiri, M. Suphantharika, and A. Assavanig. 2005. Optimization of enzymatic hydrolysis of ish soluble concentrate by commercial proteases. J. Food Eng. 70:571–578. Nothling,M. D., Z. Xiao, A. Bhaskaran,M. T. Blyth, C.W. Bennett, M. L. Coote, and L. A. Connal. 2018. Synthetic catalysts inspired by hydrolytic enzymes. ACS Catal. 9:168–187. Ogasawara,M., T. Katsumata, andM. Egi. 2006. Taste properties of Maillard-reaction products prepared from1000 to 5000Da peptide. Food Chem. 99:600–604. Poltowicz, K., and J. Doktor. 2012. Effect of slaughter age on perfor-mance and meat Quality of slow-growing broiler chickens. Ann. Anim. Sci. 12:621–631. Shafee, T. (2014). Evolvability of a Viral Protease: Experimental Evolution of Catalysis, Robustness and Speciicity. Thesis, Univer-sity of Cambridge. Shankar, T. J., S. Sokhansanj, S. Bandyopadhyay, and A. S. Bawa. 2010. A case study on optimization of biomass low during single-screw extrusion cooking using genetic algorithm(GA) and response surfacemethod (RSM). Food Bioprocess Tech-nol. 3:498–510. Sun,F.,H.Cui,H.Zhan, M. Xu,K.Hayat, M. U.Tahir, S. Hussain, X. Zhang, and C. Ho. 2019. Aqueous preparation of Maillard reaction intermediate from glutathione and xylose and its volatile formation during thermal treatment. J. Food Sci. 84:3584–3593. Sun, H.-M., J.-Z. Wang, C.-H. Zhang, X. Li, X. Xu, X.-B. Dong, L. Hu, and C.-H. Li. 2014. Changes of lavor compounds of hydro-lyzed chicken bone extracts duringmaillard reaction. J. Food Sci.79:C2415–C2426. USDA. 2022. Livestock and Poultry:WorldMarkets and Trade. For-eign Agricultural Service, Ithaca, NY, USA. accessed June 15,2022. https://www.fas.usda.gov/data/livestock-and-poultry-world-markets-and-trade . accessed June 15, 2022. Van Boekel,M. A. J. S. 2006. Formation of lavour compounds in the Maillard reaction. Biotechnol. Adv. 24:230–233. You, X. Y., Y. Xu, Z. B. Huang, and Y. P. Li. 2010. Nonvolatile taste compounds of jiangluobo (a traditional chinese fermented food). J. Food Qual. 33:477–489. Zhang, Y., Y. Ma, Z. Ahmed, W. Geng, W. Tang, Y. Liu, H. Jin, F. Jiang, J.Wang, and Y.Wang. 2019. Puriication and identiica-tion of kokumi-enhancing peptides from chicken protein hydroly-sate. Int. J. Food Sci. Technol. 54:2151–2158. Zhao, J., T.Wang, J. Xie, Q. Xiao,W. Du, Y.Wang, J. Cheng, and S. Wang. 2019. Meat lavor generation from different composi-tion patterns of initial Maillard stage intermediates formed in heatedcysteine-xylose-glycinereactionsystems. Food Chem.274:79–88. Zhu, G. Y., X. Zhu, X. L.Wan, Q. Fan, Y. H.Ma, J. Qian, X. L. Liu, Y. J. Shen, and J. H. Jiang. 2010. Hydrolysis technology and kinet-ics of poultry waste to produce amino acids in subcritical water. J. Anal. Appl. Pyrolysis 88:187–191. Zhu,W., H. Luan, Y. Bu, J. Li, X. Li, and Y. Zhang. 2021. Changes in taste substances during fermentation of ish sauce and the correla-tion with protease activity. Food Res. Int. 144:110349.
确定






还剩8页未读,是否继续阅读?
中国格哈特为您提供《鸡胴体酶解制备蛋白水解物的蛋白质和总脂肪含量的检测》,该方案主要用于酱油中营养成分检测,参考标准《GB 5009.6 食品中脂肪的测定》,《鸡胴体酶解制备蛋白水解物的蛋白质和总脂肪含量的检测》用到的仪器有格哈特全自动超级总脂肪测定系统、格哈特快速干燥仪STL56、格哈特自动升降凯氏定氮电热消解仪KT-L 20s、格哈特带自动进样器全自动凯氏定氮仪VAP500C、格哈特维克松无水低能耗废气涤气系统VS、德国移液器MM、凯氏定氮催化剂5.0g K2SO4+0.5g CuSO4 x 5H2O
相关方案
更多
该厂商其他方案
更多
















