方案详情
文
Biotage推出PFAS检测方案,助力生活饮用水检验新标准
方案详情

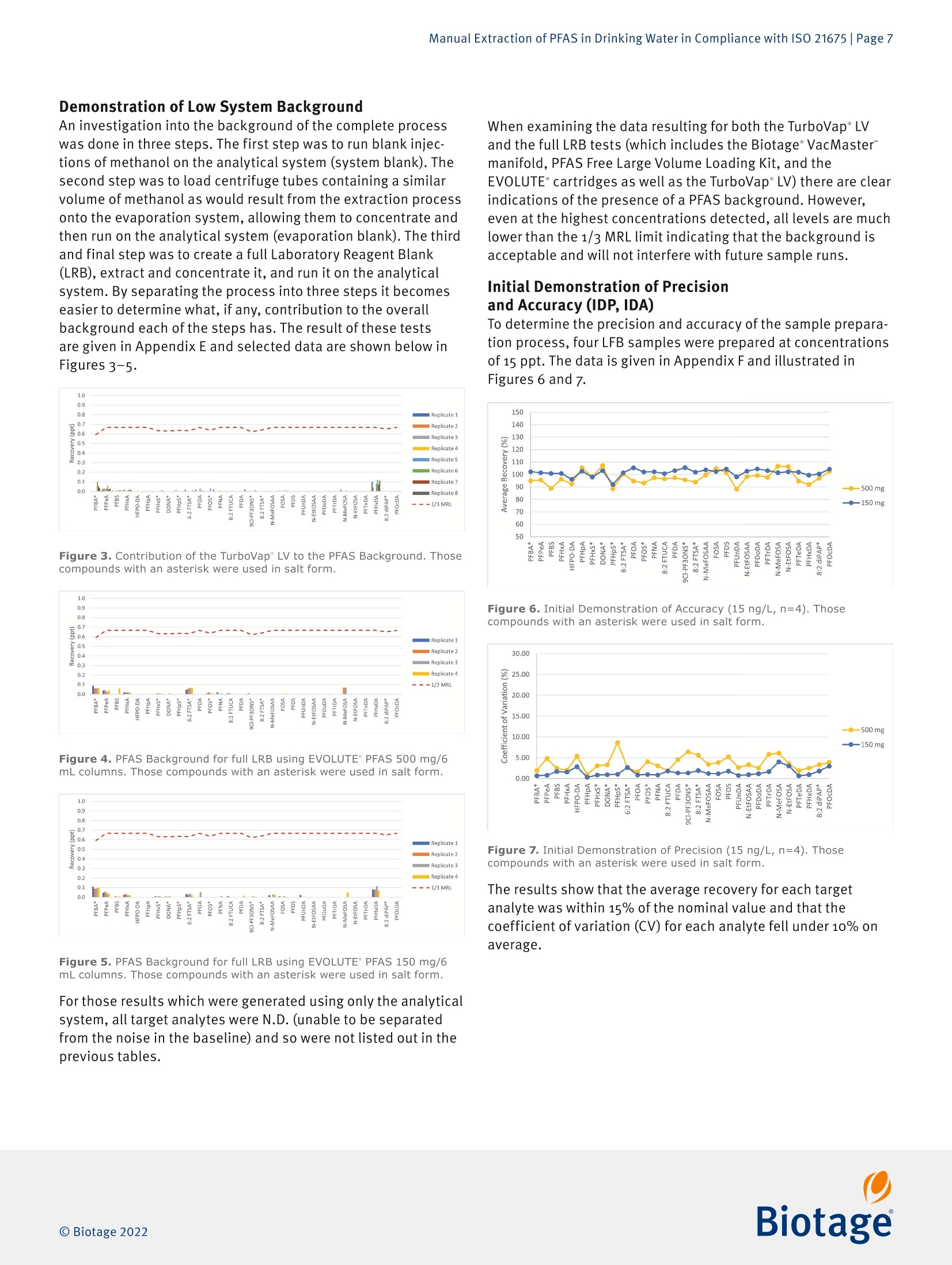
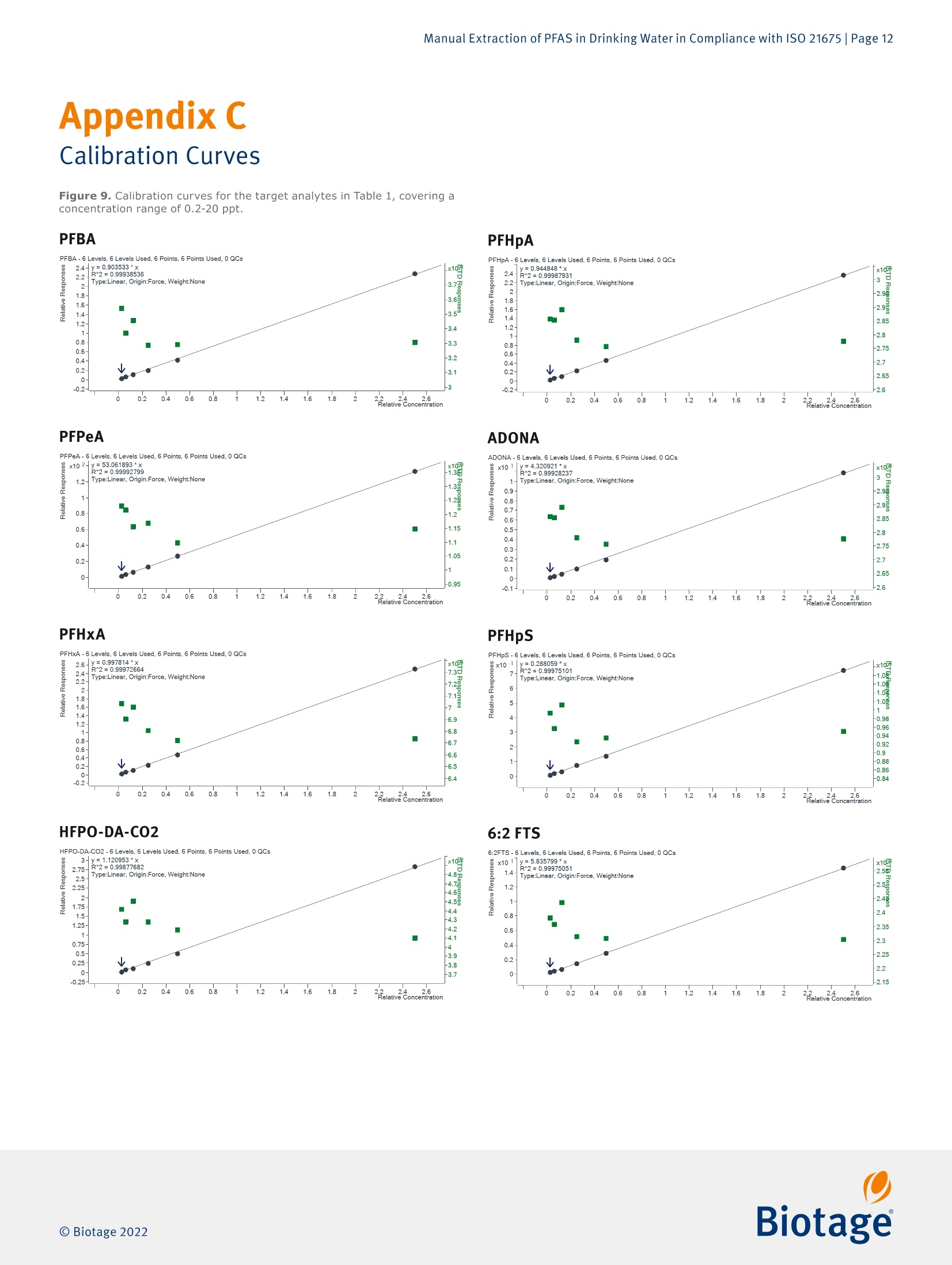
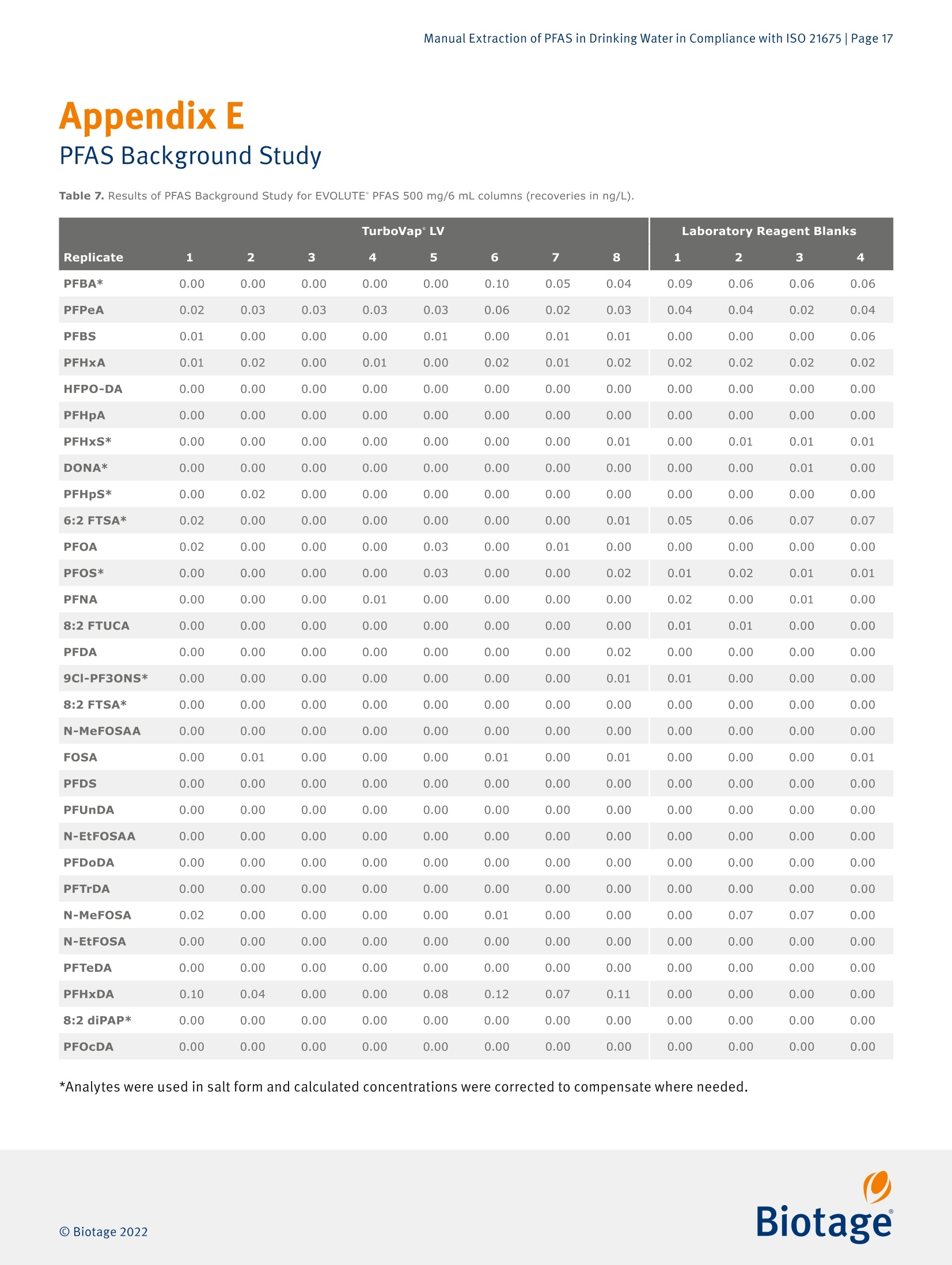
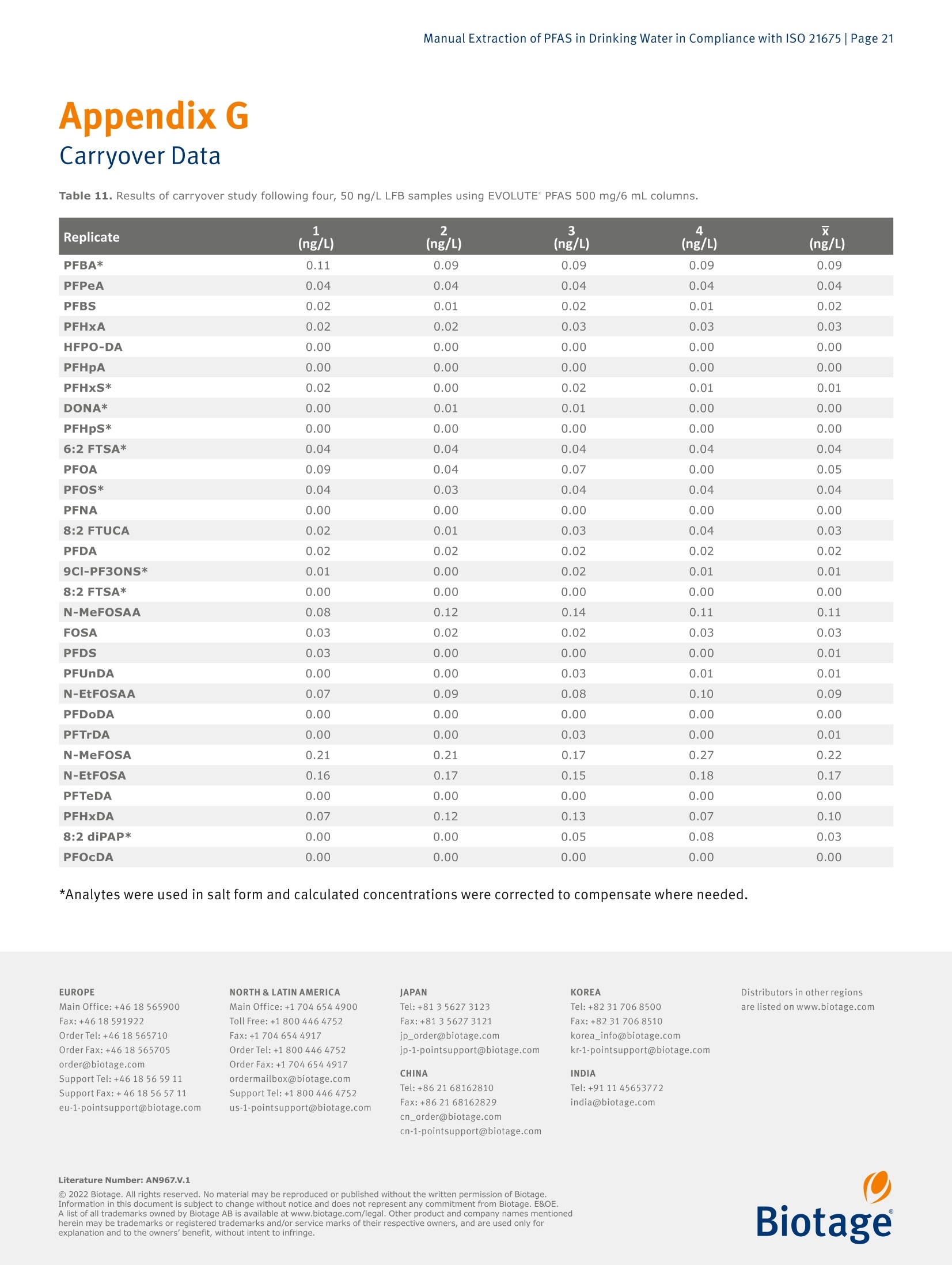
最新发布并即将实施的GB/T 5750-2023 《生活饮用水标准检验方法》系列标准新增了PFAS(Per- and Polyfluoroalkyl Fubstances,即全氟和多氟烷基物质)残留量的测定。由于其广泛使用及环境持久性,检测过程中避免背景干扰带来的影响就显得更加困难和尤为重要。为应对这一挑战,Biotage推出完整的饮用水中PFAS检测方案,其中包含用于PFAS提取的EVOLUTE® PFAS固相萃取柱和低背景干扰的TurboVap® LV全自动样品浓缩仪。EVOLUTE® PFAS固相萃取柱针对从环境基质中提取PFAS进行了优化。它包含高纯度WAX填料和成分,可提供分析物的高回收率,同时不会污染提取物,适用于GB、EPA、 ISO、DIN 和其他标准方法。图1. Biotage® EVOLUTE® PFAS固相萃取柱一台洁净、可靠的样品浓缩系统对PFAS检测实验室是不可或缺的。我们的验证结果表明,采用EVOLUTE® PFAS小柱进样提取,结合TurboVap® LV全自动样品浓缩仪进行样品浓缩,不会带来背景干扰,满足PFAS检测的背景水平要求,可用于合规的PFAS数据的产生。图2. TurboVap® LV全自动样品浓缩仪EVOLUTE® PFAS和TurboVap® LV的低背景水平验证整个背景水平验证分三步进行。 第一步是在分析系统(系统空白)上进行甲醇空白进样。 第二步是将含有与提取过程产生的相近体积甲醇的样品管放置到TurboVap® LV上进行浓缩,然后在分析系统(浓缩空白)上运行。 第三步也是最后一步是创建一个完整的实验室试剂空白,将其提取和浓缩,然后在分析系统上运行。 通过将流程分为三个步骤,可以更轻松地确定每个步骤对整体背景的贡献(如果有的话)。测试的结果如下图3和图4。图3. TurboVap® LV 的 PFAS 背景水平图4. 使用 EVOLUTE® PFAS 150 mg/6 mL 的实验室试剂空白的背景水平图5. TurboVap® LV和使用EVOLUTE® PFAS 150 mg/6 mL的实验室试剂空白的PFAS背景值以上数据表明,即使在检测到的最高浓度下,所有的背景水平都远低于MRL (最低报出限,此处MRL为2.0 ng/L,小于GB/T 5750-2023中最低检测浓度3.0 ng/L)的三分之一,这表明背景水平是可接受的并且不会干扰样品分析。结论针对 PFAS 的检测中,找到可靠的符合标准要求的实验耗材和设备至关重要。实验结果表明, EVOLUTE® PFAS固相萃取柱和TurboVap® LV可轻松满足并超出方法的要求。Manual Extraction of PFAS in Drinking Water in Compliance with ISO 21675 |Page 1Application Note AN967.V.1 Manual Extraction of PFAS in Drinkin g Water in Compliance with ISO 21675 Authors William Jones, Biotage, Salem, NH, USA Matthew Harden , Biota ge , Salem, NH, USA Per- and polyfluorinated alkyl substances (PFAS) have been used ab u ndantly sinc e their inception in the twentieth century and have become a cl os e ly m o nitored c l ass of compounds within environmental testing. This application n o t e ou tl ines a proce- dure for those seeking to follow ISO 21675. The dat a presented was gen e rated us i ng a Biotage° VacMaster" vacuum manifold with a PFAS free Large Volume Extraction (LVE) kit in conjunction with EVOLUTE° PFAS SPE columns and a T urboVap° LV system. Equipment and Materials Used Biotage 》 Biotage °VacMaster~ 20 Sample Processing Station With 15 mm Rack, p/n 121-2015ML, fitted with polypropylene (PFAS free) stopcocks (p/n 121-0009-PP) 》 Biotage ° VacMaster " LVE Ki t (PFAS) for 1,3, 6 mL SPE Columns (p/n 121-2190) 》EVOLUTE° PFAS 500 mg/6 mL SPE Columns, p/n 614-0050-CP 》EVOLUTE* PFAS 150 mg/6 mL SPE Columns, p/n 614-0015-CP 》 TurboVap"LV Automated Solvent Evaporation System, p/n 415000》 TurboVap° LV Multi Rack (48 Positions,10-20 mm Tubes),p/n 414964 Wellington Laboratories 》 ISO 21675:2019 Labelled Stock Solution,1.2 mL, p/n ISO 21675-LSS 》 ISO 21675:2019 Nat i ve Stock Solution, 1.2 mL, p/n ISO 21675-NSS Agilent 》Inf i nityLab PFC Delay Column, 4.6x30 mm,p/n 5062-8100》ZORBAX RRHD Eclipse Plus C18,95 A,2.1 x 50 mm, 1.8 pm, p/n 959757-902 Sigma-Aldrich 》 Ammonium Acetate, ACS Reagent Grade ≥ 97%, p/n 238074-25G 》 Acetic Acid , Glacial,ReagentPlus *, ≥99% p/n A6283 Honeywell 》Water, ACS Cer t ified, HPLC Grade,p/n AH365-4》Methanol, Burdick & Jackson", LC-MS Grade, p/n LC230-4 VWR 》 15 mL Polypropylene Centrifuge Tubes with Caps, p/n 21008-670 》 250 mL Polypropylene Wide Mouth Bottles, p/n 414004-125 Analytes Table 1. Listing of T arget Analytes and I nterna l Standards. Target Analyte Acronym CAS Perfluoro-n-butanesulfonic acid PFBS 375-73-5 Perfluoro-n-hexanesulfonic acid PFHxS 355-46-4 Perfluoro-n-heptanesulfonic acid PFHpS 375-92-8 Perfluoro-n-octanesulfonic acid PFOS 1763-23-1 Perfluoro-n-decanesulfonic acid PFDS 335-77-3 Perfluorooctanesulfonamide FOSA 754-91-6 N-methyl perfluoroctanesulfonamide N-MeFOSA 31506-32-8 N-ethyl perfluorooctanesulfonamide N-EtFOSA 4151-50-2 N-methyl perfluorooctanesulfonamidoacetic acid N-MeFOSAA 2355-31-9 N-ethyl perfluorooctanesulfonamidoacetic acid N-EtFOSAA 2991-50-6 6:2 Fluorotelomer sulfonic acid 6:2 FTSA 27619-97-2 8:2 Fluorotelomer sulfonic acid 8:2 FTSA 39108-34-4 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid 9CI-PF3ONS 73606-19-6 Perfluoro-n-butanoic acid PFBA 375-22-4 Perfluoro-n-pentanoic acid PFPeA 2706-90-3 Perfluoro-n-hexanoic acid PFHxA 307-24-4 Perfluoro-n-heptanoic acid PFHpA 375-85-9 Perfluoro-n-octanoic acid PFOA 335-67-1 Perfluoro-n-nonanoic acid PFNA 375-95-1 Perfluoro-n-decanoic acid PFDA 335-76-2 Perfluoro-n-undecanoic acid PFUnDA 2058-94-8 Perfluoro-n-dodecanoic acid PFDoDA 307-55-1 Perfluoro-n-tridecanoic acid PFTrDA 72629-94-8 Perfluoro-n-tetradecanoic acid PFTeDA 376-06-7 Perfluoro-n-hexadecanoic acid PFHxDA 67905-19-5 Perfluoro-n-octadecanoic acid PFOcDA 16517-11-6 8:2 Fluorotelomer unsaturated carboxylic acid 8:2 FTUCA 70887-84-2 8:2 Polyfluoroalkyl phosphate diester 8:2 diPAP 678-41-1 Hexafluoropropylene oxide dimer acid HFPO-DA 13252-13-6 4,8-Dioxa-3H-perfluorononanoic acid DONA 919005-14-4 Target Analyte Acronym CAS Internal Standard Sodium perfluoro-1-[2,3,4-13C3]butanesulfonate 13C3-PFBS Sodium perfluoro-1-[1,2,3-13Cs]hexanesulfonate 13C3-PFHxS Sodium perfluoro-1-[1,2,3-18O2]hexanesulfonate 18O2-PFHxS Sodium perfluoro-1-[1,2,3,4-13C4]octanesulfonate 13C4-PFOS Sodium perfluoro-1-[1,2,3,4-13Cs]octanesulfonate 13C8-PFOS Perfluoro-1-[3Cs]octanesulfonamide 13C:-FOSA N-methyl-d3-perfluoro-1-octanesulfonamide d3-N-MeFOSA N-ethyl-d5-perfluoro-1-octanesulfonamide ds-N-EtFOSA N-deuteriomethylperfluoro-1-octanesulfonamidoacetic acid d3-N-MeFOSAA N-deuterioethylperfluoro-1-octanesulfonamidoacetic acid ds-N-EtFOSAA Sodium 1H,1H,2H,2H-perfluoro-1-[1,2-13C2]-octane sulfonate 13C2-6:2 FTSA Sodium 1H,1H,2H,2H-perfluoro-1-[1,2-13Cz]-decane sulfonate 13C2-8:2 FTSA Perfluoro-n-[1,2,3,4-13C4]butanoic acid 13C4-PFBA Perfluoro-n-[1,2,3,4,5-13Cs]pentanoic acid 13Cs-PFPeA Perfluoro-n-[1,2-13C2]hexanoic acid 13C2-PFHxA Perfluoro-n-[1,2-13Cs]hexanoic acid 13C5-PFHxA Perfluoro-n-[1,2,3,4-13C4]heptanoic acid 13C4-PFHpA Perfluoro-[1,2-13C4]octanoic acid 13C4-PFOA Perfluoro-[1,2-13Cs]octanoic acid 13C8-PFOA Perfluoro-n-[Cs]nonanoic acid 13C5-PFNA Perfluoro-n-[1Cg]nonanoic acid 13C9-PFNA Perfluoro-n-[1,2-13C2]decanoic acid 13C2-PFDA Perfluoro-n-[1,2-13Co]decanoic acid 13C6-PFDA Perfluoro-n-[1,2,3,4,5,6,7-13Cz]undecanoic acid 13Cz-PFUnDA Perfluoro-n-[1,2,3,4,5,6,7-13C7]undecanoic acid 13C7-PFUnDA Perfluoro-n-[1,2-13C2]dodecanoic acid 13C2-PFDoDA Perfluoro-n-[1,2-13C2]tetradecanoic acid 13C2-PFTeDA Perfluoro-n-[1,2-13C2]hexadecanoic acid 13C2-PFHxDA 2H-Perfluoro-[1,2-13C2]-2-decenoic acid 13C2-8:2 FTUCA Sodium bis(1H,1H,2H,2H-[1,2-13C2]perfluorodecyl)- 13C4-8:2 diPAP phosphate Tetrafluoro-2-heptafluoropropoxy-13Cs-propanoic acid 13C3-HFPO-DA Solution Preparation Ammonia/Methanol Solution 1.Add 400 pL of NH OH for every 10o mL of methano l to a clean beaker. Ag i tate to homogenize. Prepare new solution daily. Acetate Buffer 1.Measure out 499.5 mL of reagent water in a clean beaker. 2. Add 0.193 g of NHAc. 3.Sonicate the solution for5 minutes unti l the sal t is fully dissolved. 4.Add 570 pL of glacial acetic acid. 5. Agitate to homogenize the solution. Working Spiking Solution 1.Dilute 1oo pL of the native stock solution with goo pL of methanol to achieve a 1o ppt solution. Summary of SPE method SPE Column Format EVOLUTE ° PFAS 500 mg/6 mL or EVOLUTE ° PFAS 150 mg/6 mL Sample Pre-Treatment Adjust the pH of each sample to 3 using glacia l acetic acid. Add targets and internal standards. Conditioning Cond i tion each column with o.1% NH OH in methanol (10 mL)followed by methanol (10 mL). Equilibration Equilibrate each column with reagent water (10 mL). Sample Loading Load sample at a flow rate of 5 mL/m i n. Wash Rinse the sample container with acetate buffer solution (10 mL)and load onto the column. Repeat using reagent water (10 mL). Dry Dry the column for 5 minutes at a flow rate of 5 mL/min. Elution Rinse the sample container with methanol (5 mL) and use to elute the analytes from the column at a flow rate of 2 mL/min.Repeat using o.1% NH OH i n methanol (5mL). Post Extraction Concentrate the extract to a volume of 1 mL and analyze. Sample Preparation Procedure 1.Clean al l parts of the Biotage" VacMaster"system per the procedure given in Appendix A. 2.Set up and fill new sample containers with of water;250-500 mL are typical for this method. 3. Add glacial acetic acid to each of the sample containers to reduce the pH to 3 (approximately 10o pL for 250 mL sample volumes and 2o0 uL for 500 mL sample volumes). 4.Verify the pH of the sample is 3 using pH paper. To reduce the possibi l ity of contamination, a duplicate volume was collected and adjusted to the appropriate pH and the same volume of acid was added to the sample container. 5·Prepare for the determination of the i nitial sample volume by either marking the level of the sample on the container or by weighing the sample container. 6. Add 2o pL of the undiluted Labeled Stock Solution to each of the sample containers. I f desired, fortify a sample using target analytes: the addition of 125 uL or 37.5 uL of the native stock solution will yield either 50 ppt or 15 ppt concentra-tions respectively, while the addition of 50 uL of the working spiking solut i on will yield a 2 ppt concentration. I f the mixes used were different than the ones outlined in this note,adjus t the concentration or spiking amounts as needed. 7. Load the desired EVOLUTE° PFAS columns onto the Biotage °VacMaster". Seal any unused positions us i ng VacMaster Port Sealing Plugs (p/n 121-0005) 8.Rinse each column with 10 mL of 0.1% NHOH in methanol and apply vacuum at 10 mL/min to pull it to waste. Do not allow the sorbent to go dry. 9.Rinse each column with 10 mL of methanol and apply vacuum at 10 mL/min to pull i t to waste. Do not allow the sorbent to go dry. 10. Rinse each column with 10 mL of reagent water and apply vacuum at 10 mL/min to send i t to waste. Do not allow the water leve l to drop below the top of the packing. 11. Using the BiotageVacMaster" LVE Kit, place one end of the cleaned tubing into the bottom of each o f the sample containers, and secure in position using the clips provided. 12. Load the samples onto the columns using a flow rate of 5mL/m i n. 13. Once the sample has been fully loaded, rinse the sample containers using 1o mL of acetate buffer solution, s wirl to ensure the full r i nsing of the container, and load the aliquot onto the column at a rate of 5 mL/min. 14. Rinse the sample containers using 10 mL of reagent water,swirl to ensure the full rinsing of the container, and load the aliquot onto the column at a rate of 5 mL/min. 15. Dry the column for 5 minutes at a rate of 5 mL/min. 16. Load 15 mL centrifuge tubes into the rack corresponding to each of the column positions and load into the Biotage"VacMaster". 17. Rinse each sample container using 5 mL of methanol and swirl to ensure the full rinsing of the container . Load the aliquot through the appropriate column and collect at a dropwise rate. 18. Rinse each sample container using 5 mL of 0.1% NHOH in methanol and swirl to ensure the full rinsing of the container. Load the aliquot through the appropriate column and collec t at a dropwise rate. 19. Determine the initial sample volume by either using a gradu-ated cylinder and fi ll ing the sample container to the original mark or by taking an additional weight of the container. 20. Transfer the centrifuge tubes to the TurboVap°LV system and concentrate the samples to just under 1 mL us i ng nitrogen according to the parameters in Table 2. 21. Bring the final extract to 1 mL and transfer to an autosampler vial. 22. Load the extrac t onto a cal i brated LC-MS/MS system and process using the conditions given in the below sections. Table 2. TurboVap" LV Concentration Protoco l . *The nozzle pos i tion was adjusted such that it was as far to the right as poss i ble to g i ve the user a clear view of the vortex withi n the tube. LC-MS/MS Conditions Agilent 1290 Infinity II LC System Columns InfinityLab PFC Delay Column, 4.6x30 mm,p/n 5062-8100ZORBAX RRHD Ec l ipse Plus C18, 95 A, 2.1 x50 mm, 1.8 um,p/n 959757-902 Mobile Phases A 20 mM Ammonium Acetate in Water Methanol Table 3. LC Grad i ent. Time (min) %A %B 0.50 95.00 5.00 3.00 60.00 40.00 16.00 20.00 80.00 18.00 20.00 80.00 20.00 5.00 95.00 20.50 0.00 100.00 25.00 0.00 100.00 26.00 5.00 95.00 Flow Rate: o.2 mL/min Injection Volume:5 uL Column Temperature: 50℃ Agilent 6470 MS/MS, G6470B Gas Temperature: 230℃ Gas Flow: 4 L/m i n Nebulizer: 20 psi Sheath Gas Temperature:375℃ Sheath Gas Flow: 12 L/min Capillary Voltage (Pos i tive): 3500 V Capillary Voltage (Negative): 3500V Nozzle Voltage (Positive): 500V Nozzle Voltage (Negative): o V For a complete l isting o f MRM Transitions, see Appendix B Results System Calibration For the work being done here, a total of six points were used i n the calibration cover i ng a range of o.2-20 ppt i n the sample.The lowest three points were below the calculated MRL. T he curve was forced through zero and achieved excellent l inearity across the calibration range. PFBS PFBS-6 Le v e l s , 6 Le ve ls U s ed , 6 Points, 6 Po in ts U ae d ,0 Q Cs Figure 1. Calibration curves for PFBS and PFHxS. Ca l ibration curves fo r the remai n ing target analytes i n Table 1 are shown in Appendix C. Determination of the Minimum Reporting Level (MRL) and Detection Limits (DL) A target MRL of 2 ng/L was selected and at least seven replicate laboratory fort i fied blanks (LFBs) were created and run at that concentration. Figure 2 below illustrates the results of this test for both the 150 mg and 500 mg columns using 250 mL sample volumes; al l compounds were recovered within 15% of the spiked amount and had less than 10% CV. Figure 2. MRL and DL Recoveries. Those compounds with an asterisk were used i n salt form. The data for individual compounds is shown in Append i x D. Demonstration of Low System Background An investigation into the background of the complete process was done in three steps. The f irst step was to run blank injec-tions of methanol on the analytical system (system blank). The second step was to load centrifuge tubes containing a similar volume of methanol as would resul t from the extraction process onto the evaporation system, allowing them to concentrate and then run on the analytical system (evaporation blank). The third and final step was to create a full Laboratory Reagent Blank (LRB), extract and concentrate it, and run it on the analytical system. By separating the process into th r ee steps it becomes easier to determ i ne what, if any, contribution to the overall background each o f the steps has. The result o f these tests are given in Appendix E and selected data are shown below in Figures 3-5. Figure 3. Contr i bution of the T urboVa p ' LV to the PFAS Background. Thos e compounds with an asterisk were used i n sal t f orm. Figure 4. PFAS Background for full LRB using EVOLUTE PFAS 500 mg/6mL columns. Those compounds with an asterisk were used i n salt form. F i gure 5. P FAS Background fo r f ul l LRB using EVOLUTE PFAS 150 mg/6mL columns. Those compounds with an asteris k were used in salt form. For those results which were generated using on l y the analytical system, al l target analytes were N.D. (unable to be separated from the noise in the baseline) and so were not l isted out in the previous tables. When examining the data resulting for both the TurboVap°LV and the full LRB tests (which includes the Biotage° VacMaster manifold, PFAS Free Large Volume Loading Kit, and the EVOLUTE ° car t ridges a s well as the TurboVap"LV) there are clear indications o f the presence of a PFAS background. However,even at the highest concentrations detected , all levels are much lower than the 1/3 MRL l i mit indicating that the background is acceptable and wi ll not interfere with future sample runs. Initial Demonstration of Precision and Accuracy (IDP, IDA) To determine the precision and accuracy of the sample prepara-tion process, four LFB samples were prepared at concentrations of 15 ppt . The data i s given i n Appendix F and illustrated i n F i gures 6 and 7. Figure 6. Init i al Demonstration of Accuracy (15 ng/L, n =4). T hose compounds with an aste r isk we r e used in salt form . Figure 7. Ini t ial Demonstration of Precisio n (15 ng/L, n=4). Those compounds with an asterisk were used in sal t form. The results show that the average recovery for each target analyte was within 15% of the nomina l value and that the coefficient of variation (CV) for each analyte fell under 10% on average. Examination of System Carryover To simulate an influent sample, four LFB samples were created with concentrations which were above the range of the calibra-tion curve. These samples were extracted, and the cleanup procedure given in Appendix A was run three t i mes. T o ensure that the system background was adequately reduced, a set of four LRB samples were extracted immed i ately afterwards and analyzed. The LRB data is presented in Appendix G and ill u s-trated in Figure 8. Figure 8. Results of carryove r study fol l owing f our, 50 ng/L LFB samples using EVOLUTE PFAS 500 mg/6 mL columns . Those compounds with an asterisk were used i n salt form. The graph shown in Figure 8 shows a clear indication that the cleaning procedure in Appendix A was successful in reducing the background of PFAS compounds to below the 1/3 MRL l imit .For further reductions in the background, additional cleaning steps could be employed. Conclusion With the scrutiny being given to the presence of PFAS compounds i n the environment, it is essential to find reliable products which can meet the requirements of ISO 21675. This application note has shown that the Biotage° VacMaster"vacuum manifold with PFAS free accessories,EVOLUTE °PFAS SPE columns and the TurboVap°LV can be used to easily meet and exceed the demands of the method . Ordering Information Part Number Description Qty 121-2015ML Biotage°VacMaster 20 Sample Processing Station With 15 mm Rack 121-2190 Biotage° VacMaster LVE Kit (PFAS) for 1, 3,6 mL SPE Columns 121-0009-PP Polypropylene (PFAS) Stopcocks 614-0050-CP EVOLUTE PFAS 500 mg/6 mL columns 614-0015-CP EVOLUTE° PFAS 150 mg/6 mL columns 415000 TurboVap° LV Automated Solvent Evaporation System 1 414964 TurboVap° LV Multi Rack (48 Positions, 10-20 mm Tubes) Appendix A Biotage VacMaster " Cleaning Procedure For the best results, i t is recommended that this procedure be completed before the use of the Biotage ° VacMaster "each day and at the end of each extraction prior to proceeding with the next set o f samples. 1. Ensure that a column and column adapter is installed onto each VacMaster ~ position slated to be cleaned. 2.Fil l a clean beaker with 50 mL of methanol and place no more than four of the LVE Kit lines into the beaker. 3.Apply vacuum to the manifold and pull the methanol through the pos i tions into the waste container. 4.Remove the column and discard. 5·Us i ng methanol in a squeeze bottle, clean the exterior of the LVE Kit's lines, the column adapters, the stopcock, and the metal cannula . Discard all rinsate. 6.Repeat this up to three times for all positions which require cleaning. Note: In situations where the previous sample was highly concentrated, the above cleaning procedure may need to be repeated multiple t imes. If there is concern regarding potential carryover contamination regardless of the clean i ng procedure,a laboratory reagent blank should be run in that posi t ion to ensure its cleanliness. Appendix B MRM Transitions Table 4. MRM Tr ansitions for Agi l ent 6470 MS/MS. Cpd Name ISTD? Prec Ion MS1 Res Prod Ion MS2 Res Frag (V) CE (V) Cell Acc (V) Ret Time (min) Ret Window Polarity 6:2FTS No 427 Unit/Enh (6490) 406.8 Unit/Enh (6490) 125 24 5 13.3 1.37 Negative 6:2FTS No 427 Unit/Enh (6490) 80.9 Unit/Enh (6490) 125 40 5 13.3 1.37 Negative 8:2 diPAP No 989 Unit/Enh (6490) 543 Unit/Enh (6490) 100 20 5 22.15 2.33 Negative 8:2 diPAP No 989 Unit/Enh (6490) 97 Unit/Enh (6490) 100 20 5 22.15 2.33 Negative 8:2 FTS No 527 Unit/Enh (6490) 506.8 Unit/Enh (6490) 170 28 5 16.2 1.64 Negative 8:2 FTS No 527 Unit/Enh (6490) 80.9 Unit/Enh (6490) 170 40 5 16.2 1.64 Negative 8:2 FTUCA NO 457 Unit/Enh (6490) 393 Unit/Enh (6490) 100 28 5 15.25 1.56 Negative 8:2 FTUCA No 457 Unit/Enh (6490) 343 Unit/Enh (6490) 100 28 5 15.25 1.56 Negative 9CI-PF30NS No 530.9 Unit/Enh (6490) 350.9 Unit/Enh (6490) 145 28 5 15.7 1.6 Negative 9CI-PF30NS No 530.9 Unit/Enh (6490) 83 Unit/Enh (6490) 145 32 5 15.7 1.6 Negative ADONA No 377 Unit/Enh (6490) 250.9 Unit/Enh (6490) 80 12 5 11.8 1.23 Negative ADONA No 377 Unit/Enh (6490) 85 Unit/Enh (6490) 80 36 5 11.8 1.23 Negative C2-6:2FTS No 429 Unit/Enh (6490) 81 Unit/Enh (6490) 125 24 5 13.3 1.37 Negative C2-8:2FTS No 529 Unit/Enh (6490) 81 Unit/Enh (6490) 170 28 5 16.2 1.64 Negative C2-8:2FTUCA NO 459 Unit/Enh (6490) 394 Unit/Enh (6490) 100 28 5 15.25 1.56 Negative C2-PFDoA No 615 Unit/Enh (6490) 570 Unit/Enh (6490) 79 5 5 18.2 1.84 Negative C2-PFHxDA No 815 Unit/Enh (6490) 770 Unit/Enh (6490) 100 20 5 21.5 2.19 Negative C2-PFTeDA No 715 Unit/Enh (6490) 670 Unit/Enh (6490) 79 5 5 19.9 2.03 Negative C3-HFPO-DA NO 287 Unit/Enh (6490) 169 Unit/Enh (6490) 155 4 5 10.3 1 Negative C3-PFBS No 302 Unit/Enh (6490) 80 Unit/Enh (6490) 100 45 5 7.9 1.61 Negative C3-PFHxS No 402 Unit/Enh (6490) 80 Unit/Enh (6490) 100 49 5 11.7 1.5 Negative C4-8:2diPAP No 993 Unit/Enh (6490) 545 Unit/Enh (6490) 100 20 5 22.15 2.23 Negative C4-PFBA No 217 Unit/Enh (6490) 172 Unit/Enh (6490) 60 8 5 5.1 1.45 Negative C4-PFHpA No 367 Unit/Enh (6490) 322 Unit/Enh (6490) 72 0 5 11.6 1.2 Negative C5-PFHxA No 318 Unit/Enh (6490) 273 Unit/Enh (6490) 70 8 5 9.7 1.14 Negative C5-PFPeA No 268 Unit/Enh (6490) 223 Unit/Enh (6490) 60 20 5 7.4 1.55 Negative C6-PFDA NO 519 Unit/Enh (6490) 474 Unit/Enh (6490) 81 4 5 16.2 1.65 Negative C7-PFUdA No 570 Unit/Enh (6490) 525 Unit/Enh (6490) 100 20 5 17.2 1.75 Negative C8-FOSA No 506 Unit/Enh (6490) 78 Unit/Enh (6490) 150 20 5 16.8 1.71 Negative C8-PFOA No 421 Unit/Enh (6490) 376 Unit/Enh (6490) 80 8 5 13.4 1.38 Negative C8-PFOS NO 507 Unit/Enh (6490) 80 Unit/Enh (6490) 100 50 5 15 1.53 Negative C9-PFNA No 472 Unit/Enh (6490) 427 Unit/Enh (6490) 66 4 5 14.9 1.52 Negative d3-N-MeFOSA No 515 Unit/Enh (6490) 169 Unit/Enh (6490) 100 28 5 18.8 1.91 Negative d3-N-MeFOSAA NO 573 Unit/Enh (6490) 419 Unit/Enh (6490) 100 20 5 16.7 1.7 Negative d5-N-EtFOSA No 531 Unit/Enh (6490) 169 Unit/Enh (6490) 100 32 5 19.6 1.2 Negative d5-N-EtFOSAA No 589 Unit/Enh (6490) 419 Unit/Enh (6490) 100 20 5 17.25 1.75 Negative FOSA No 498 Unit/Enh (6490) 169 Unit/Enh (6490) 100 38 5 16.8 1.71 Negative FOSA No 498 Unit/Enh (6490) 78 Unit/Enh (6490) 150 38 5 16.8 1.71 Negative Cpd Name ISTD? Prec Ion MS1 Res Prod Ion MS2 Res Frag (V) CE (V) Cell Acc (V) Ret Time (min) Ret Window Polarity HFPO-DA-CO2 No 285 Unit/Enh (6490) 184.9 Unit/Enh (6490) 155 16 5 10.3 1.5 Negative HFPO-DA-CO2 No 285 Unit/Enh (6490) 168.9 Unit/Enh (6490) 155 4 5 10.3 1.5 Negative N-EtFOSA No 526 Unit/Enh (6490) 219 Unit/Enh (6490) 100 32 5 19.6 1.99 Negative N-EtFOSA NO 526 Unit/Enh (6490) 169 Unit/Enh (6490) 100 28 5 19.6 1.99 Negative N-EtFOSAA No 584 Unit/Enh (6490) 526 Unit/Enh (6490) 100 20 5 17.3 1.75 Negative N-EtFOSAA No 584 Unit/Enh (6490) 419 Unit/Enh (6490) 100 20 5 17.3 1.75 Negative N-MeFOSA No 512 Unit/Enh (6490) 219 Unit/Enh (6490) 100 28 5 18.8 1.91 Negative N-MeFOSA No 512 Unit/Enh (6490) 169 Unit/Enh (6490) 100 28 5 18.8 1.91 Negative N-MeFOSAA No 570 Unit/Enh (6490) 512 Unit/Enh (6490) 100 20 5 16.7 1.7 Negative N-MeFOSAA No 570 Unit/Enh (6490) 419 Unit/Enh (6490) 100 20 5 16.7 1.7 Negative PFBA No 213 Unit/Enh (6490) 168.9 Unit/Enh (6490) 60 8 5 5.1 1.48 Negative PFBS No 298.9 Unit/Enh (6490) 98.9 Unit/Enh (6490) 100 29 5 7.9 1.41 Negative PFBS No 298.9 Unit/Enh (6490) 80 Unit/Enh (6490) 100 45 5 7.9 1.41 Negative PFDA No 513 Unit/Enh (6490) 469 Unit/Enh (6490) 81 4 5 16.2 1.65 Negative PFDA No 513 Unit/Enh (6490) 218.7 Unit/Enh (6490) 100 16 5 16.2 1.65 Negative PFDoA NO 613 Unit/Enh (6490) 569 Unit/Enh (6490) 79 5 5 18.2 1.84 Negative PFDoA No 613 Unit/Enh (6490) 268.7 Unit/Enh (6490) 100 20 5 18.2 1.84 Negative PFDS No 599 Unit/Enh (6490) 99 Unit/Enh (6490) 100 40 5 17.15 1.75 Negative PFDS No 599 Unit/Enh (6490) 80 Unit/Enh (6490) 100 40 5 17.15 1.75 Negative PFHpA No 362.9 Unit/Enh (6490) 319 Unit/Enh (6490) 72 0 5 11.6 1.2 Negative PFHpA No 362.9 Unit/Enh (6490) 169 Unit/Enh (6490) 72 12 5 11.6 1.2 Negative PFHpS No 448.9 Unit/Enh (6490) 98.7 Unit/Enh (6490) 100 44 5 13.5 1.39 Negative PFHpS No 448.9 Unit/Enh (6490) 79.7 Unit/Enh (6490) 100 52 5 13.5 1.39 Negative PFHXA No 313 Unit/Enh (6490) 268.9 Unit/Enh (6490) 70 8 5 9.7 1.12 Negative PFHXA No 313 Unit/Enh (6490) 119 Unit/Enh (6490) 70 18 5 9.7 1.12 Negative PFHxDA No 813 Unit/Enh (6490) 769 Unit/Enh (6490) 79 5 5 21.5 2.19 Negative PFHxDA No 813 Unit/Enh (6490) 369 Unit/Enh (6490) 100 20 5 21.5 2.19 Negative PFHxS NO 398.9 Unit/Enh (6490) 99 Unit/Enh (6490) 100 45 5 11.7 1.5 Negative PFHxS No 398.9 Unit/Enh (6490) 80 Unit/Enh (6490) 100 49 5 11.7 1.5 Negative PFNA No 463 Unit/Enh (6490) 419 Unit/Enh (6490) 66 4 5 14.9 1.52 Negative PFNA No 463 Unit/Enh (6490) 219 Unit/Enh (6490) 66 17 5 14.9 1.52 Negative PFOA No 413 Unit/Enh (6490) 369 Unit/Enh (6490) 69 4 5 13.4 1.38 Negative PFOA No 413 Unit/Enh (6490) 169 Unit/Enh (6490) 69 12 5 13.4 1.38 Negative PFOcDA No 913 Unit/Enh (6490) 869 Unit/Enh (6490) 79 5 5 22.35 2.25 Negative PFOcDA No 913 Unit/Enh (6490) 369 Unit/Enh (6490) 100 20 5 22.35 2.25 Negative PFOS No 498.9 Unit/Enh (6490) 99 Unit/Enh (6490) 100 50 5 15 1.53 Negative PFOS No 498.9 Unit/Enh (6490) 80 Unit/Enh (6490) 100 50 5 15 1.53 Negative PFPeA No 263 Unit/Enh (6490) 218.9 Unit/Enh (6490) 60 8 5 7.4 1.77 Negative PFTeDA No 713 Unit/Enh (6490) 669 Unit/Enh (6490) 79 5 5 19.9 2.03 Negative PFTeDA No 713 Unit/Enh (6490) 369 Unit/Enh (6490) 100 20 5 19.9 2.03 Negative PFTrDA No 663 Unit/Enh (6490) 619 Unit/Enh (6490) 79 5 5 19 1.93 Negative PFTrDA Appendix C Calibration Curves F i gure 9. Cal i brat i on curves f or the target analytes i n Table 1, covering a concentration range of 0.2-20 ppt . PFBA PFHpA PFPeA P FP eA -6 L ev e l s , 6 Le v e ls U s e d , 6 P o i n ts , 6 Po in t s U sed ,0 Q C s x 10y =53.061893*X PFHpS P F H x A-6 Le v e l s , 5 Le ve ls Used , 6 Points , 6 Po i nts U se d ,0 Q Cs PF H pS-6 L e v ela , 6 Le v e ls Us e d, 6 Points , 6 Po i n ts U se d ,0 QC : 2.6 y=0.997814*X x 10 HFPO-DA-CO2 6:2 FTS H F PO -D A -CO 2-6 Le v e ls , 6 L e v els U s e d , 6 P o i n t s , 6 P o i nt s Use d ,0 QC : 6:2F T S -6 Le vel s, 6 Le ve ls U se d, 6 P o in t s , 6 P oi n t s U s e d,0 Q C : y =1.120953*x PFOA 9CI-PF30NS P F O A -6 Levels , 6 Le v e l s Uae d , 6 Po i nt s, 6 P oi n t s U s ed ,0 Q Cs y=0.962711*x 8:2 FTS P F OS -6 Le v e ls , 6 Lev e l s U s ed, 6 Po in ts , 6 Po i nt s U s ed ,0 QC s 8:2 F TS -6 L e v e l s , 6 L e v e ls U s ed , 6 P o i n t s, 6 Poi nt s U s e d .0 QC s 2.66-y =0.976114*x L x10 x 10l y=6.533891*× -1.0是 F 1.08 -1.02 -1 0.98 0.96 F 0.94 -0.92 Rel at iveC once n t r at i o r PFNA N-MeFOSAA x 10 FOSA R e l a t iv e C o n c e n tr at i o n PFDA PFDS P F D A -6 L e v e l s, 6 L e v e l s Used , 6 P o in ts, 6 Po i n t s U s ed, 0 Q Cs PFUnA N-EtFOSA PF UnA -6 L e ve l s,6 Levels Us e d, 6 Po i nt s , 6 P o ints U s e d ,0Q C 8:x 101y =4.037145*x Rolat i ve Concentratic cen t r a t i on N-EtFOSAA PFTeDA N -E t FOSAA -6 Le ve l s, 6 Le v e ls U sed , 6 P c ints, 6 Po in t s U sed ,0 Q C s PF Te DA -6 Levels, 6 Le v e l s Us e d , 6 P oints , 6 Paints Used,0Q C s y=1.091264*x 22.6-y =0.982224*x 2.75 PFDoA PFHxDA 8:2 diPAP 吸 PFOcDA N-Me FOS A -6 Lev el s , 6 L e v el s U sed, 6 P o i n t s , 6 P oints U s ed.0QCs PFOcD A -6 L e v els,5 Le ve l s Used. 5 Poi nt s, 6 P o i n ts U sed ,0QCs 10 Appendix D MRL and DL Data Table 5. MRL and DL Recoveries fo r EVOLUTE" PFAS 500 mg/6 mL columns. Conc. 1 2 3 4 5 6 7 S Cv DL (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (%) (ng/L) (%) (ng/L) PFBA* 1.77 1.67 1.71 1.67 1.68 1.81 1.60 1.80 1.71 96 0.08 4.43 0.24 PFPeA 2.00 1.85 1.83 1.92 1.97 1.88 1.75 1.91 1.87 94 0.07 3.81 0.22 PFBS 2.00 1.73 1.90 1.82 1.70 1.73 1.65 1.95 1.78 89 0.11 6.12 0.34 PFHXA 2.00 1.80 1.92 1.89 1.80 1.98 1.75 1.92 1.87 93 0.08 4.50 0.26 HFPO-DA 2.00 1.80 1.58 1.48 1.83 1.89 1.73 1.69 1.71 86 0.14 8.39 0.45 PFHpA 2.00 1.96 2.06 2.04 1.94 2.01 1.80 2.08 1.99 99 0.09 4.75 0.30 PFHxS* 1.90 1.85 1.78 1.77 1.70 1.82 1.73 1.77 1.77 94 0.05 2.84 0.16 DONA* 1.89 1.76 1.85 1.79 1.85 1.79 1.64 1.80 1.78 94 0.07 3.98 0.22 PFHpS* 1.91 1.90 1.90 1.86 1.65 1.92 1.51 1.73 1.78 93 0.16 8.79 0.49 6:2 FTSA* 1.90 1.80 1.87 1.88 1.86 1.87 1.68 1.86 1.83 96 0.07 4.02 0.23 PFOA 2.00 1.80 1.86 1.93 1.91 1.94 1.67 1.94 1.86 93 0.10 5.34 0.31 PFOS* 1.92 1.96 1.89 1.79 1.89 1.92 1.69 1.89 1.86 97 0.09 4.98 0.29 PFNA 2.00 1.88 1.92 1.95 1.90 1.94 1.79 2.07 1.92 96 0.08 4.30 0.26 8:2 FTUCA 2.00 1.93 1.87 1.90 1.95 2.05 1.89 1.97 1.94 97 0.06 3.15 0.19 PFDA 2.00 1.87 1.89 1.87 1.83 2.01 1.79 2.01 1.89 95 0.09 4.55 0.27 9CI-PF30NS* 1.87 1.75 1.71 1.76 1.73 1.74 1.53 1.75 1.71 92 0.08 4.72 0.25 8:2 FTSA* 1.92 1.73 1.83 1.72 1.77 1.90 1.70 2.10 1.82 95 0.14 7.93 0.45 N-MeFOSAA 2.00 1.83 1.90 1.92 1.81 1.94 1.76 2.02 1.88 94 0.09 4.80 0.28 FOSA 2.00 2.09 2.06 2.12 1.97 2.16 1.98 2.28 2.09 105 0.11 5.17 0.34 PFDS 2.00 2.10 2.06 1.95 2.14 1.70 1.64 1.93 1.93 97 0.19 9.98 0.61 PFUnDA 2.00 1.89 1.91 1.90 1.81 1.88 1.75 1.94 1.87 93 0.07 3.51 0.21 N-EtFOSAA 2.00 2.04 1.86 2.00 1.98 1.99 1.73 1.94 1.93 97 0.11 5.44 0.33 PFDoDA 2.00 1.85 1.96 1.96 1.81 2.00 1.75 1.96 1.90 95 0.10 5.04 0.30 PFTrDA 2.00 1.79 1.85 1.87 1.98 2.02 1.75 2.04 1.90 95 0.11 5.89 0.35 N-MeFOSA 2.00 2.17 2.14 2.01 1.94 2.36 2.04 2.34 2.14 107 0.16 7.50 0.51 N-EtFOSA 2.00 1.99 1.97 2.06 2.11 2.09 1.99 2.03 2.03 102 0.05 2.59 0.17 PFTeDA 2.00 1.79 1.82 1.91 1.85 1.89 1.83 1.86 1.85 93 0.04 2.12 0.12 PFHXDA 2.00 1.95 1.94 1.93 1.99 1.95 1.89 2.07 1.96 98 0.06 2.81 0.17 8:2 diPAP* 1.96 1.79 1.85 2.00 1.90 1.78 1.78 2.16 1.90 97 0.14 7.51 0.45 PFOcDA 2.00 1.71 1.88 2.07 1.60 1.71 1.64 1.75 1.77 88 0.16 9.18 0.51 *Analytes were used in salt form and calculated concentrations were corrected to compensate where needed. Table 6. MRL and DL Recoveries for EVOLUTE° PFAS 150 mg/6 mL co l umns . Conc. 1 2 3 4 5 6 7 8 又 叉 S Cv DL (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) (%) (ng/L) (%) (ng/L) PFBA* 1.77 1.88 1.83 1.87 1.94 1.83 1.86 1.79 1.83 1.85 104 0.05 2.56 0.14 PFPeA 2.00 1.96 1.92 1.96 2.05 1.97 1.93 1.86 1.96 1.95 98 0.05 2.79 0.16 PFBS 2.00 1.87 1.76 1.84 1.73 1.77 1.81 1.73 1.75 1.78 89 0.05 2.83 0.15 PFHxA 2.00 2.09 2.00 2.04 2.10 2.03 2.09 1.98 2.08 2.05 103 0.05 2.20 0.14 HFPO-DA 2.00 1.97 1.92 1.82 1.92 1.89 2.14 2.16 2.17 2.00 100 0.14 6.92 0.41 PFHpA 2.00 2.08 1.96 1.98 1.91 2.04 1.95 2.01 2.01 1.99 100 0.06 2.76 0.17 PFHxS* 1.90 1.75 1.83 1.80 1.85 1.66 1.74 1.77 1.74 1.77 93 0.06 3.34 0.18 DONA* 1.89 1.98 1.90 1.96 1.96 1.93 1.84 1.96 1.90 1.93 102 0.05 2.35 0.14 PFHpS* 1.91 1.77 1.78 1.85 1.79 1.64 1.66 1.69 1.64 1.73 91 0.08 4.69 0.24 6:2 FTSA* 1.90 1.91 1.84 1.97 1.92 2.05 2.06 2.01 2.11 1.98 104 0.09 4.58 0.27 PFOA 2.00 1.96 1.99 2.01 2.08 1.97 2.05 1.91 1.97 1.99 100 0.05 2.59 0.15 PFOS* 1.92 2.00 1.89 1.93 2.01 1.95 1.95 1.98 1.96 1.96 102 0.04 1.99 0.12 PFNA 2.00 2.08 1.94 2.02 2.01 2.04 2.08 2.11 2.02 2.03 102 0.05 2.59 0.16 8:2 FTUCA 2.00 1.93 1.92 1.97 1.95 1.94 1.98 1.95 1.98 1.95 98 0.02 1.17 0.07 PFDA 2.00 1.99 1.89 1.96 1.99 1.91 2.03 2.07 2.04 1.98 99 0.06 3.12 0.19 9CI-PF30NS* 1.87 1.96 1.92 1.91 1.97 1.86 1.84 1.83 1.92 1.90 102 0.05 2.68 0.15 8:2 FTSA* 1.92 1.92 1.93 2.01 1.99 1.90 1.84 1.88 1.97 1.93 101 0.06 3.06 0.18 N-MeFOSAA 2.00 1.85 1.84 1.84 1.85 1.91 2.00 2.03 2.06 1.92 96 0.09 4.87 0.28 FOSA 2.00 1.96 2.00 1.99 2.12 2.16 2.20 2.16 2.15 2.09 105 0.09 4.49 0.28 PFDS 2.00 1.92 2.08 1.81 2.04 2.01 1.93 2.04 1.90 1.97 98 0.09 4.61 0.27 PFUnDA 2.00 2.09 1.97 1.93 1.95 1.89 1.97 1.95 1.98 1.97 98 0.06 2.94 0.17 N-EtFOSAA 2.00 1.86 1.93 1.98 1.98 1.96 2.16 2.10 2.02 2.00 100 0.09 4.68 0.28 PFDoDA 2.00 2.08 1.91 2.02 2.05 2.02 2.03 2.04 2.04 2.02 101 0.05 2.52 0.15 PFTrDA 2.00 2.01 1.93 1.85 1.99 1.94 2.06 1.98 1.99 1.97 98 0.06 3.21 0.19 N-MeFOSA 2.00 2.01 2.13 2.03 1.93 2.13 2.18 1.96 2.02 2.05 102 0.09 4.37 0.27 N-EtFOSA 2.00 1.86 2.10 1.88 2.27 1.91 2.16 2.05 2.13 2.04 102 0.15 7.30 0.45 PFTeDA 2.00 2.07 2.03 1.91 2.03 1.98 1.99 2.13 2.05 2.02 101 0.06 3.17 0.19 PFHxDA 2.00 2.01 1.91 1.81 2.08 2.05 1.95 2.02 2.03 1.98 99 0.09 4.43 0.26 8:2 diPAP* 1.96 2.05 2.03 2.03 2.06 2.05 2.11 1.78 1.94 2.01 103 0.10 5.12 0.31 PFOcDA 2.00 1.99 2.32 2.39 2.06 2.07 2.11 2.00 2.02 2.12 106 0.15 7.13 0.45 *Analytes were used in salt form and calculated concentrations were corrected to compensate where needed. Appendix E PFAS Background Stud y Tabl e 7. Resul t s of PFAS Backg r ound Study for EVOLU T E ° PFAS 500 mg/6 mL co l umns (recoveries in ng/L). TurboVap°LV Laboratory Reagent Blanks Replicate 1 2 3 4 5 6 7 8 1 2 3 4 PFBA* 0.00 0.00 0.00 0.00 0.00 0.10 0.05 0.04 0.09 0.06 0.06 0.06 PFPeA 0.02 0.03 0.03 0.03 0.03 0.06 0.02 0.03 0.04 0.04 0.02 0.04 PFBS 0.01 0.00 0.00 0.00 0.01 0.00 0.01 0.01 0.00 0.00 0.00 0.06 PFHXA 0.01 0.02 0.00 0.01 0.00 0.02 0.01 0.02 0.02 0.02 0.02 0.02 HFPO-DA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFHpA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFHxS* 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.01 0.00 0.01 0.01 0.01 DONA* 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.01 0.00 PFHpS* 0.00 0.02 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 6:2 FTSA* 0.02 0.00 0.00 0.00 0.00 0.00 0.00 0.01 0.05 0.06 0.07 0.07 PFOA 0.02 0.00 0.00 0.00 0.03 0.00 0.01 0.00 0.00 0.00 0.00 0.00 PFOS* 0.00 0.00 0.00 0.00 0.03 0.00 0.00 0.02 0.01 0.02 0.01 0.01 PFNA 0.00 0.00 0.00 0.01 0.00 0.00 0.00 0.00 0.02 0.00 0.01 0.00 8:2 FTUCA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.01 0.01 0.00 0.00 PFDA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.02 0.00 0.00 0.00 0.00 9CI-PF3ONS* 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.01 0.01 0.00 0.00 0.00 8:2 FTSA* 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 N-MeFOSAA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 FOSA 0.00 0.01 0.00 0.00 0.00 0.01 0.00 0.01 0.00 0.00 0.00 0.01 PFDS 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFUnDA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 N-EtFOSAA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFDoDA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFTrDA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 N-MeFOSA 0.02 0.00 0.00 0.00 0.00 0.01 0.00 0.00 0.00 0.07 0.07 0.00 N-EtFOSA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFTeDA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFHxDA 0.10 0.04 0.00 0.00 0.08 0.12 0.07 0.11 0.00 0.00 0.00 0.00 8:2 diPAP* 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFOcDA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 *Analytes were used in salt form and calculated concentrat i ons were corrected to compensate where needed. Table 8. Results of PFAS Background Study for EVOLUTE PFAS 150 mg/6 mL columns (recoveries in ng/L). TurboVap°LV Laboratory Reagent Blanks Replicate 1 2 3 4 1 2 3 4 PFBA* 0.06 0.02 0.01 0.00 0.11 0.09 0.09 0.10 PFPeA 0.03 0.03 0.03 0.02 0.05 0.06 0.04 0.04 PFBS 0.01 0.01 0.00 0.00 0.00 0.01 0.01 0.01 PFHXA 0.02 0.02 0.01 0.00 0.03 0.03 0.02 0.02 HFPO-DA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFHpA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFHxS* 0.01 0.01 0.00 0.00 0.01 0.01 0.01 0.01 DONA* 0.01 0.00 0.00 0.00 0.01 0.01 0.01 0.00 PFHpS* 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 6:2 FTSA* 0.01 0.00 0.00 0.00 0.03 0.03 0.04 0.02 PFOA 0.00 0.02 0.03 0.00 0.00 0.00 0.05 0.00 PFOS* 0.00 0.00 0.01 0.01 0.00 0.00 0.00 0.00 PFNA 0.00 0.01 0.00 0.00 0.00 0.00 0.01 0.00 8:2 FTUCA 0.01 0.00 0.00 0.01 0.01 0.01 0.00 0.00 PFDA 0.00 0.02 0.00 0.00 0.00 0.00 0.00 0.02 9CI-PF30NS* 0.00 0.00 0.00 0.00 0.00 0.00 0.01 0.01 8:2 FTSA* 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 N-MeFOSAA 0.00 0.00 0.00 0.00 0.02 0.00 0.03 0.02 FOSA 0.00 0.01 0.00 0.00 0.01 0.00 0.01 0.01 PFDS 0.00 0.00 0.01 0.00 0.00 0.00 0.00 0.00 PFUnDA 0.00 0.00 0.00 0.00 0.02 0.00 0.00 0.00 N-EtFOSAA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFDoDA 0.00 0.06 0.00 0.00 0.00 0.00 0.00 0.00 PFTrDA 0.00 0.05 0.00 0.00 0.00 0.00 0.00 0.00 N-MeFOSA 0.00 0.00 0.00 0.02 0.00 0.00 0.00 0.05 N-EtFOSA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFTeDA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 PFHXDA 0.07 0.08 0.08 0.06 0.08 0.08 0.12 0.07 8:2 diPAP* 0.00 0.01 0.00 0.00 0.00 0.00 0.00 0.00 PFOcDA 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 *Analytes were used in salt form and calculated concentrations were corrected to compensate where needed. Appendix F IDP and IDA Data Table 9. Resul t s of IDP and IDA fo r EVOLUTEPFAS 500 mg/6 m L columns (15 ng/L,n=4). Replicate 1(%) 2(%) 3(%) 4(%) x(%) s(%) Cv(%) PFBA* 93.97 93.52 97.66 94.56 94.93 1.87 1.97 PFPeA 91.10 99.84 99.34 92.16 95.61 4.62 4.83 PFBS 88.73 88.20 91.76 86.48 88.79 2.20 2.47 PFHxA 94.83 95.12 99.13 95.85 96.23 1.98 2.06 HFPO-DA 88.53 89.13 99.41 92.60 92.42 4.99 5.40 PFHpA 104.38 106.43 104.54 106.18 105.38 1.07 1.02 PFHxS* 95.20 99.75 101.57 95.96 98.12 3.04 3.10 DONA* 102.56 111.10 108.04 107.16 107.22 3.53 3.30 PFHpS* 89.31 87.18 98.22 79.57 88.57 7.67 8.66 6:2 FTSA* 96.43 102.56 100.83 101.53 100.34 2.70 2.69 PFOA 94.28 94.77 96.59 92.79 94.61 1.56 1.65 PFOS* 91.58 93.17 98.48 89.66 93.22 3.79 4.06 PFNA 93.76 98.27 100.96 96.89 97.47 2.99 3.07 8:2 FTUCA 95.54 99.14 95.99 95.27 96.49 1.79 1.86 PFDA 96.19 91.59 101.20 100.51 97.38 4.45 4.57 9CI-PF3ONS* 94.73 93.29 104.59 90.45 95.76 6.15 6.42 8:2 FTSA* 87.94 97.85 90.95 98.83 93.89 5.30 5.64 N-MeFOSAA 102.65 101.19 100.15 94.75 99.68 3.44 3.45 FOSA 106.21 102.48 110.06 100.99 104.94 4.06 3.87 PFDS 96.50 102.88 108.47 98.56 101.60 5.29 5.21 PFUnDA 88.89 85.05 87.96 90.74 88.16 2.37 2.69 N-EtFOSAA 97.35 101.86 99.64 94.11 98.24 3.31 3.37 PFDoDA 96.65 99.00 102.74 98.79 99.30 2.53 2.55 PFTrDA 104.99 91.30 96.05 98.68 97.76 5.71 5.84 N-MeFOSA 103.38 114.84 108.73 99.89 106.71 6.53 6.12 N-EtFOSA 103.01 111.52 107.20 103.88 106.40 3.86 3.63 PFTeDA 92.57 95.31 96.88 94.10 94.71 1.83 1.93 PFHxDA 90.55 89.61 92.53 94.89 91.89 2.34 2.54 8:2 diPAP* 92.57 99.94 98.71 96.49 96.93 3.24 3.34 PFOcDA 104.86 101.06 106.48 97.58 102.49 3.99 3.89 *Analytes were used in salt form and calculated concentrat i ons were corrected to compensate where needed. Table 10. Results of IDP and IDA for EVOLUTE" PFAS 150 mg/6 mL co l umns (15 ng/L, n=4). Replicate 1(%) 2(%) 3(%) 4(%) x(%) s (%) Cv(%) PFBA* 102.37 101.69 101.80 103.26 102.28 0.72 0.70 PFPeA 101.89 102.16 101.32 100.30 101.42 0.82 0.81 PFBS 99.30 102.15 99.56 102.63 100.91 1.72 1.71 PFHxA 100.58 103.25 99.56 100.27 100.92 1.61 1.60 HFPO-DA 96.01 95.99 99.76 93.05 96.20 2.75 2.86 PFHpA 102.78 102.88 102.64 102.10 102.60 0.35 0.34 PFHxS* 97.07 98.52 98.87 97.40 97.96 0.86 0.88 DONA* 102.74 104.41 102.11 103.16 103.11 0.97 0.94 PFHpS* 91.57 92.08 90.92 93.18 91.94 0.95 1.04 6:2 FTSA* 99.40 99.12 104.89 102.46 101.47 2.74 2.70 PFOA 104.83 105.70 104.59 106.65 105.44 0.94 0.89 PFOS* 101.18 103.31 101.24 102.55 102.07 1.04 1.02 PFNA 102.52 103.28 101.34 101.57 102.18 0.90 0.88 8:2 FTUCA 98.30 100.36 102.72 101.33 100.68 1.86 1.84 PFDA 102.85 104.63 101.29 103.65 103.10 1.41 1.37 9CI-PF3ONS* 106.62 106.89 104.87 103.91 105.57 1.42 1.35 8:2 FTSA* 100.77 102.18 104.64 100.22 101.95 1.97 1.93 N-MeFOSAA 103.10 103.15 103.09 105.71 103.76 1.30 1.25 FOSA 103.88 101.39 103.04 101.42 102.43 1.23 1.20 PFDS 105.46 102.18 103.54 106.38 104.39 1.89 1.81 PFUnDA 98.55 97.67 97.27 98.90 98.10 0.76 0.77 N-EtFOSAA 101.28 102.59 103.05 103.63 102.64 1.00 0.97 PFDoDA 105.74 103.29 103.54 105.36 104.48 1.25 1.19 PFTrDA 103.97 103.44 104.62 100.69 103.18 1.73 1.67 N-MeFOSA 95.71 101.62 105.13 103.48 101.48 4.11 4.05 N-EtFOSA 100.27 105.18 104.36 98.73 102.14 3.12 3.06 PFTeDA 102.22 102.79 101.51 101.23 101.94 0.71 0.69 PFHxDA 98.11 100.02 100.23 99.48 99.46 0.95 0.96 8:2 diPAP* 102.35 99.99 98.16 101.37 100.47 1.82 1.81 PFOcDA 106.66 107.30 101.64 101.58 104.30 3.11 2.99 *Analytes were used in salt form and calculated concentrat i ons were corrected to compensate where needed. Appendix G Carryover Dat a Tabl e 11. Results of carryover study f ol l owing four, 50 ng/L LFB samp l es using EVOLUTE " PFAS 500 mg/6 mL columns. 1 2 3 4 灭 Replicate (ng/L) (ng/L) (ng/L) (ng/L) (ng/L) PFBA* 0.11 0.09 0.09 0.09 0.09 PFPeA 0.04 0.04 0.04 0.04 0.04 PFBS 0.02 0.01 0.02 0.01 0.02 PFHxA 0.02 0.02 0.03 0.03 0.03 HFPO-DA 0.00 0.00 0.00 0.00 0.00 PFHpA 0.00 0.00 0.00 0.00 0.00 PFHxS* 0.02 0.00 0.02 0.01 0.01 DONA* 0.00 0.01 0.01 0.00 0.00 PFHpS* 0.00 0.00 0.00 0.00 0.00 6:2 FTSA* 0.04 0.04 0.04 0.04 0.04 PFOA 0.09 0.04 0.07 0.00 0.05 PFOS* 0.04 0.03 0.04 0.04 0.04 PFNA 0.00 0.00 0.00 0.00 0.00 8:2 FTUCA 0.02 0.01 0.03 0.04 0.03 PFDA 0.02 0.02 0.02 0.02 0.02 9CI-PF30NS* 0.01 0.00 0.02 0.01 0.01 8:2 FTSA* 0.00 0.00 0.00 0.00 0.00 N-MeFOSAA 0.08 0.12 0.14 0.11 0.11 FOSA 0.03 0.02 0.02 0.03 0.03 PFDS 0.03 0.00 0.00 0.00 0.01 PFUnDA 0.00 0.00 0.03 0.01 0.01 N-EtFOSAA 0.07 0.09 0.08 0.10 0.09 PFDoDA 0.00 0.00 0.00 0.00 0.00 PFTrDA 0.00 0.00 0.03 0.00 0.01 N-MeFOSA 0.21 0.21 0.17 0.27 0.22 N-EtFOSA 0.16 0.17 0.15 0.18 0.17 PFTeDA 0.00 0.00 0.00 0.00 0.00 PFHxDA 0.07 0.12 0.13 0.07 0.10 8:2 diPAP* 0.00 0.00 0.05 0.08 0.03 PFOcDA 0.00 0.00 0.00 0.00 0.00 *Analytes were used in salt form and calculated concentrations were corrected to compensate where needed EUROPE Main Office: +46 18 565900 are l isted on www.biotage.com Fax:+46 18591922 OrderTel:+46 18 565710 OrderFax:+4618565705 order@biotage.com Supp ort Tel : +46 1856 5911 Support Fax:+46 18 5657 11 eu-1-pointsupport@biotage .com C 2022 Biotage . Al l rights reserved. No mater i al may be reproduced or publ i shed without the written permission of Biotage. Information in this document is subject to change without notice and does no OF t represent any commitmen t from BioA li st of a l l trademarks owned by Biotage AB is available at www.biotage.com/legal . Other product and company natmagees . mEe&nOtEio.ned herein may be trademarks or reg i stered trademarks and/or service marks of their respective owne r s , and a r e used only for explanation and to the owners’benefit , without intent to i nfringe .
确定

















还剩19页未读,是否继续阅读?
南京新飞达光电科学技术有限公司为您提供《Biotage推出PFAS检测方案,助力生活饮用水检验新标准》,该方案主要用于包装饮用水中环境污染物检测,参考标准--,《Biotage推出PFAS检测方案,助力生活饮用水检验新标准》用到的仪器有BIOTAGE全自动样品浓缩仪、BIOTAGE全自动样品前处理系统











