方案详情
文
景观树的落叶,作为一种新兴的生物质废物,利用常规水热碳化(HC)和微波辅助水热碳化(MHC)预处理,并比较表征为理化性质和热降解动力学。结果表明MHC 优于传统的 HC 操作,因为在 200℃,MHC 过程不仅提供更高的水炭产量(45.09对39.47 wt%),同时显着降低能耗(0.63 对 2.74 MJ g-1),而且在去除 K 和 Si 方面也更有效。对于等转化动力学分析,FWO 方法提供比 KAS 方法更好的结果,因为后者未能拟合树叶样本(R2 < 0.9)。高温(>400℃)下的热降解动力学表明从 MHC 工艺获得的水炭具有较低的平均活化能量比传统的 HC 工艺(~260 MJ kg-1)高约 190 MJ kg-1。这研究揭示了通过 MHC 对景观树木废物进行增值的潜力过程。
方案详情

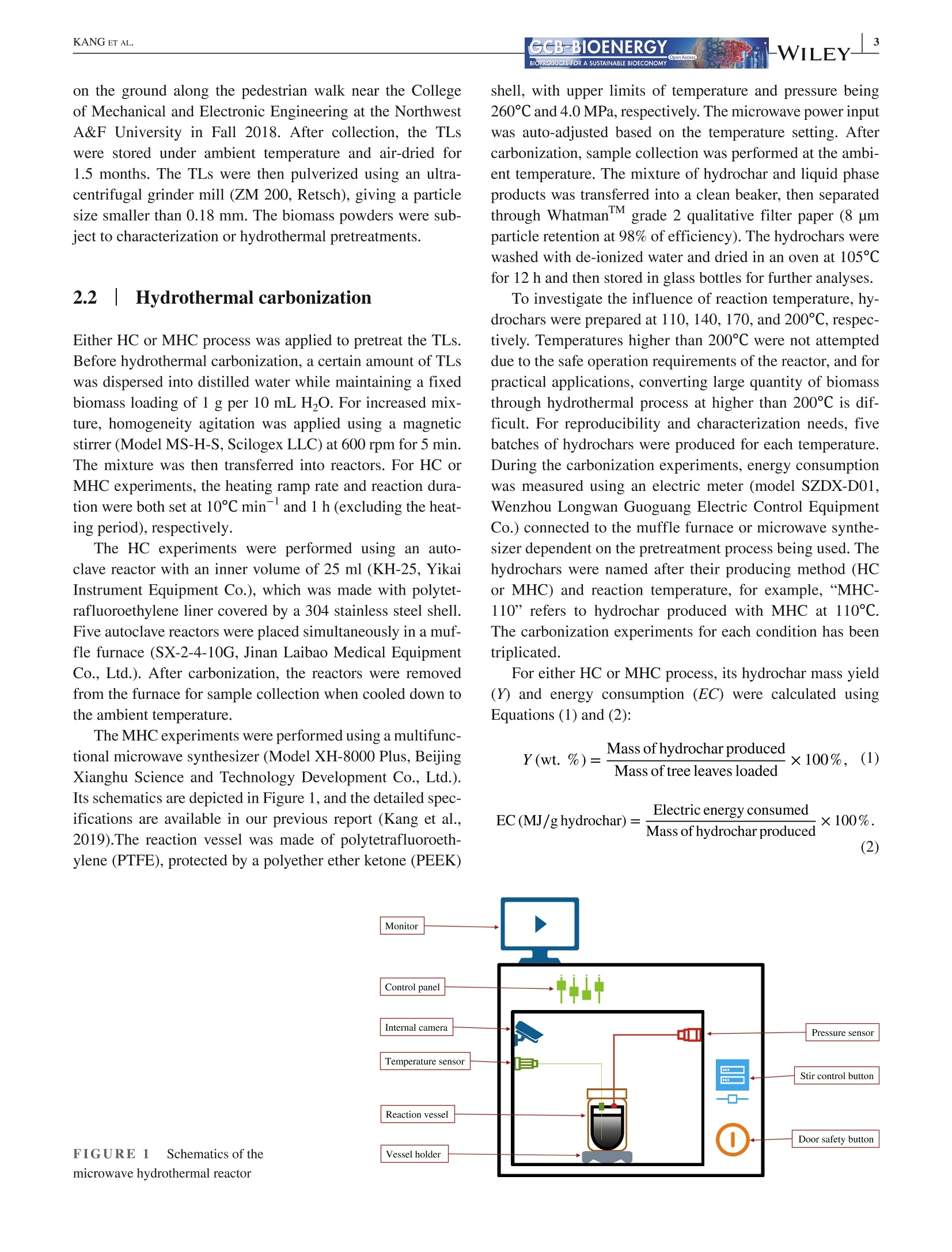
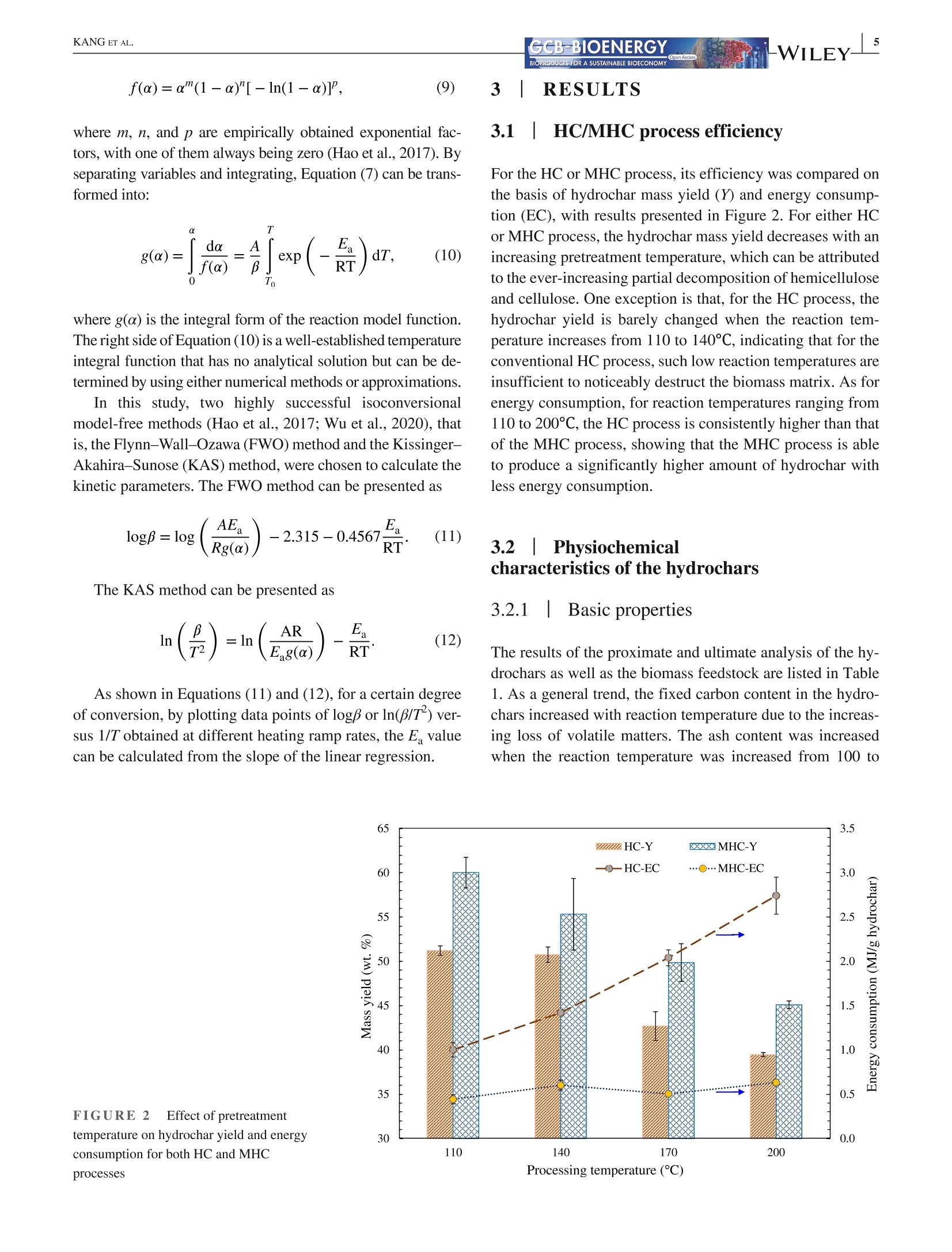
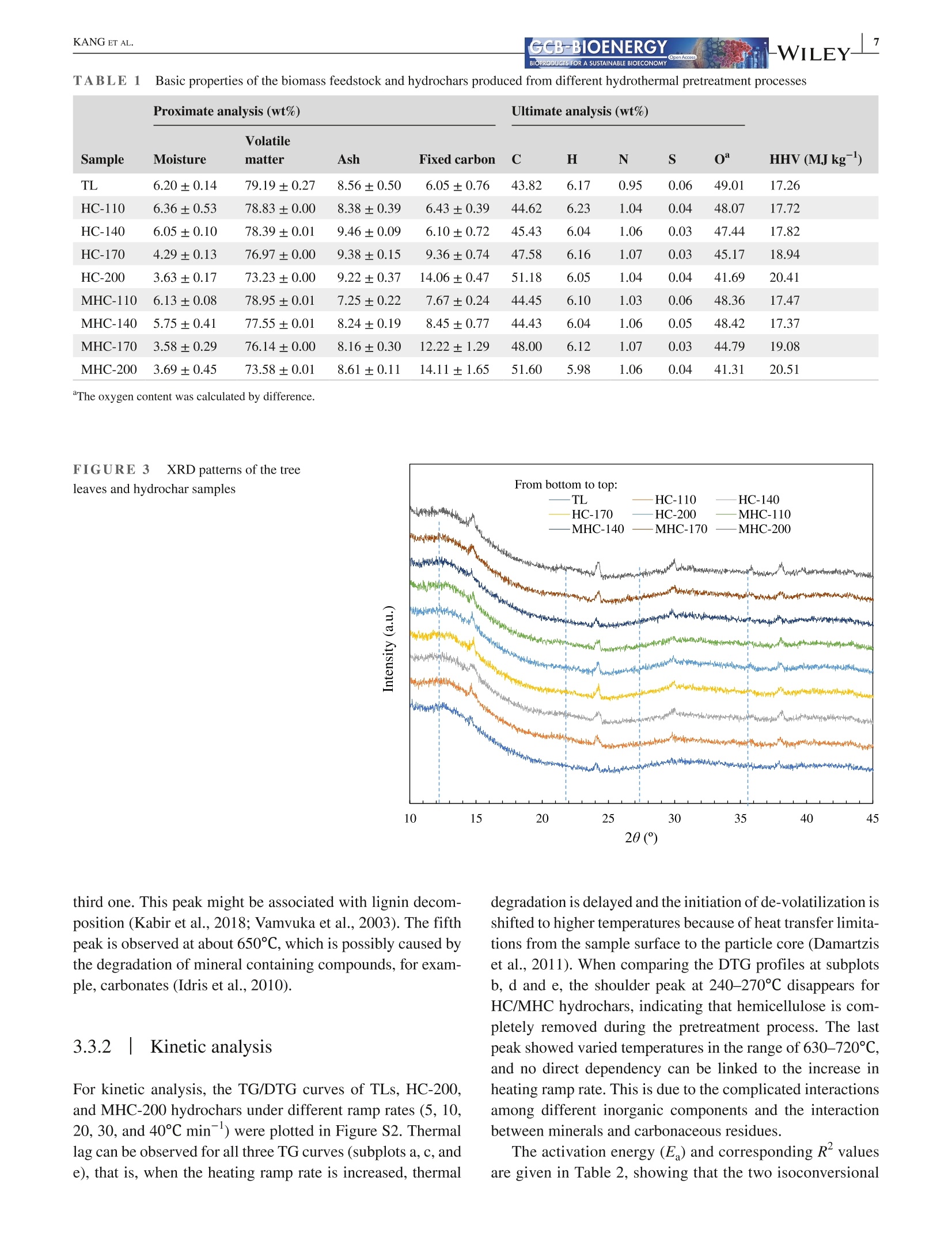
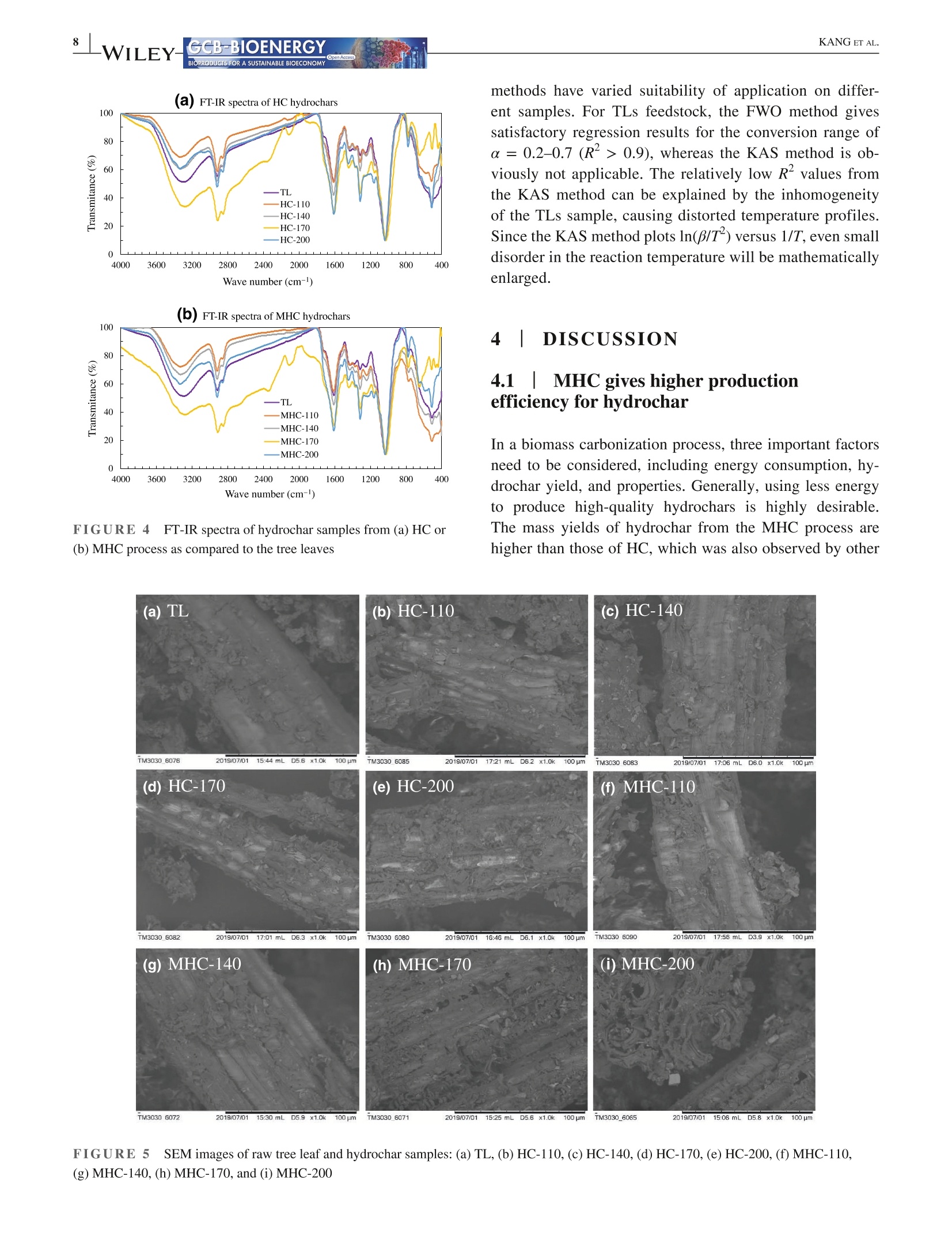
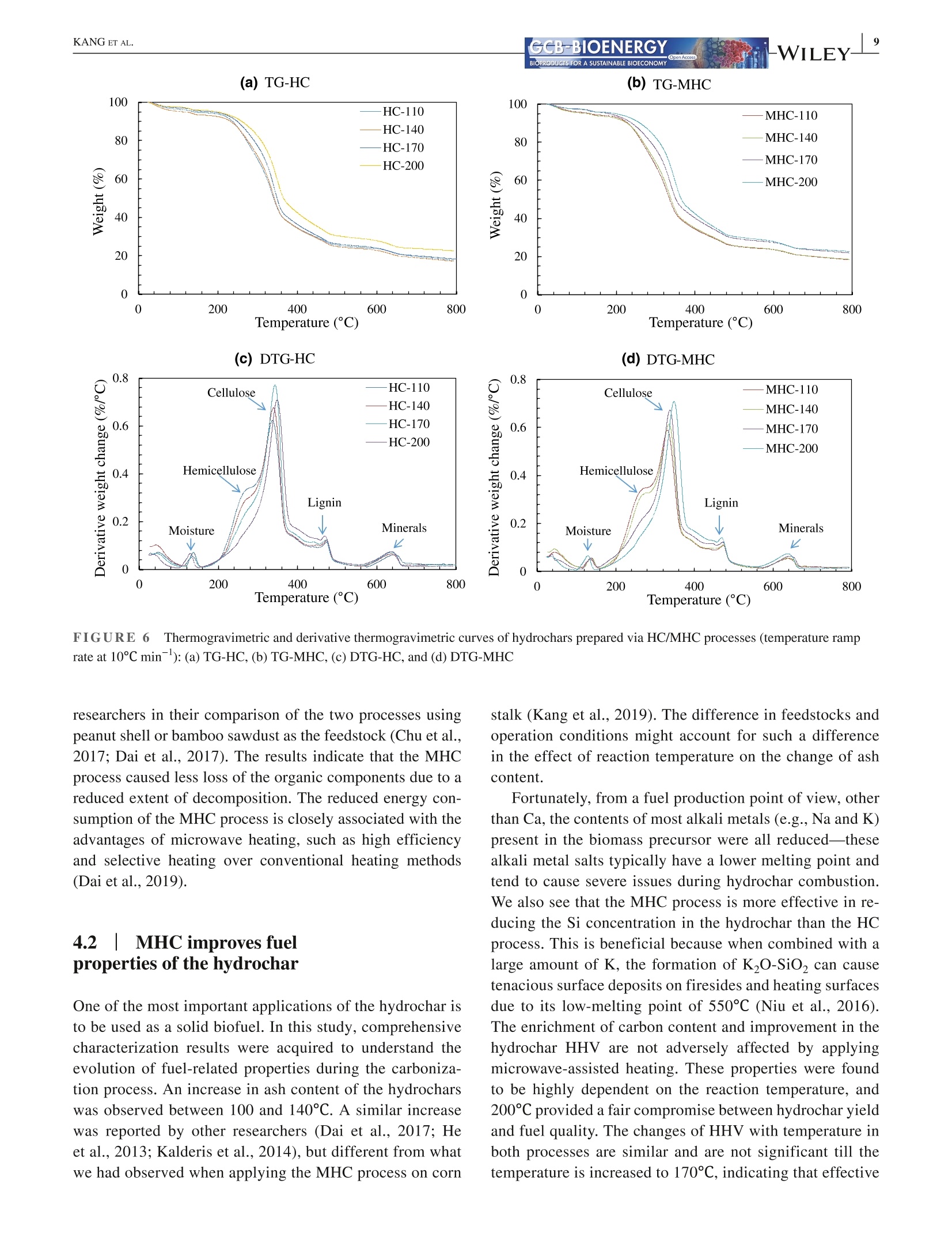
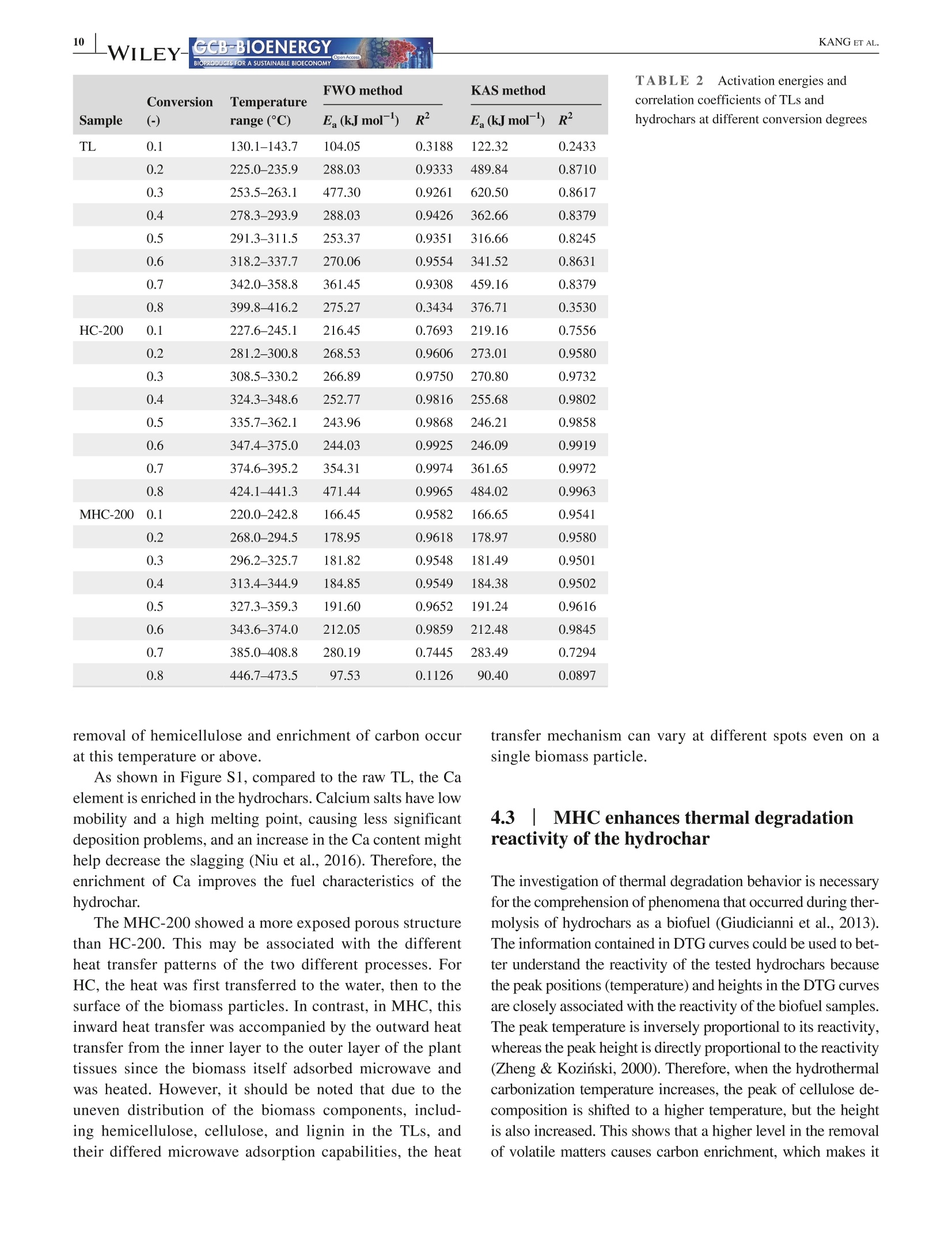
Received: 3 May 2021 Accepted: 27 June 2021DOI: 10.1111/gcbb.12882GCB-BIOENERGYBIOPRODUCTS FOR A SUSTAINABLE BIOECONOMY WILEYORIGINALRESEARCH KANG ETAL.GCB-BIOENERGYBIOPRODUCTS FOR A SUSTAINABLE BIOECONOMY 1GCB Bioenergy. 2021;00:1-14.wileyonlinelibrary.com/journal/gcbb Valorization of tree leaves waste using microwave-assistedhydrothermal carbonization process Kang Kang12Tianle Zhang'|Guotao Sun|Mingqiang Zhu’|Kankan Li’Dongbing Lit4:D College of Mechanical and Electronic Engineering, Northwest A&F University, Yangling, Shaanxi, China Institute for Chemicals and Fuels from Alternative Resources (ICFAR), Western University, London, ON, Canada College of Landscape Architecture and Art, Northwest A&F University, Yangling, Shaanxi, China "Key Comprehensive Laboratory of Forestry, College of Forestry, Northwest A&F University, Yangling, Shaanxi, China Correspondence Dongbing Li, Rm 311 - Office Building,College of Forestry, Northwest A&FUniversity, 3 Taicheng Rd., YanglingDistrict, Xianyang, Shaanxi Province712100, China. Email: lid@nwafu.edu.cn Funding informationShaanxi Provincial Department ofScience and Technology, Grant/Award Number: 2018ZDXM2-01 and2020QFY10-03; Natural Science BasicResearch Program of Shaanxi, Grant/Award Number: 2020JQ-243; Start-up Fund from the Northwest A&FUniversity; Ministry of Agricultureand Rural Affairs, China, Grant/Award Number: KLERUAR2018-02:Key Laboratory of Energy ResourceUtilization from Agriculture Residue,National Key Research and DevelopmentProgram of China, Grant/Award Number:2018YFE0127000; Key Research andDevelopment Program of Shaanxi,Grant/Award Number: 2020NY-105;Fundamental Research Funds for theCentral Universities, Grant/Award Number: 2452017299 Abstract Fallen leaves of landscape trees, as an emerging biomass waste, were valorized usingconventional hydrothermal carbonization (HC) and microwave-assisted hydrother-mal carbonization (MHC) pretreatments, and were comparatively characterized forphysicochemical properties and thermal degradation kinetics. The results show thatMHC is superior to conventional HC operation, because at 200°℃, the MHC processnot only gives a higher hydrochar yield (45.09 vs. 39.47 wt%) with significantly re-duced energy consumption (0.63 vs. 2.74 MJ g), but also is more effective in re-moving K and Si. For isoconversional kinetic analysis, the FWO method providesbetter results than the KAS method as the latter failed to fit the tree leaves sample(R<0.9). The thermal degradation kinetics at high temperatures (>400℃) showedthat the hydrochar obtained from the MHC process has a lower average activationenergy of ~190 MJ kgthan the conventional HC process (~260 MJ kg ). Thisstudy reveals the potential for valorization of the landscape tree wastes via the MHCprocess. KEYWORDS biomass, hydrochar, hydrothermal carbonization, isoconversional kinetic analysis, urban fallenleaves,valorization Biomass energy can dramatically contribute to the mitigationof greenhouse gas emissions from heavily carbon-dependent ------- sectors (Reid et al., 2020). Among the wide variety of bio-mass feedstocks, agricultural wastes and forestry residuesare the most studied (Williams et al.,2016). However, fallenleaves from landscape trees cultivated in the urban area are a ( This is an open a ccess article under the t erms of the Creative Commons Attr i bution License, which permi t s use, distribution and reproduction in any med i um, provided the original work is properly cited. ) C 2021 The Authors. GCB Bioenergy published by John Wiley & Sons Ltd. novel alternative source of biomass, which, however,are oftenunderestimated and disposed of together with other munici-pal solid wastes. Specifically, with rapid urbanization, manylandscape trees are planted for greening, decoration, noise re-duction, and air purification purposes (Trowbridge & Bassuk,2004). Fallen leaves from these trees accumulate rapidly to-ward large quantities. However, different from those in theforest field, they are not subject to the natural microbiologicaldigestion process as the ground in the urban area is often cov-ered by non-soil materials such as brick, concrete, cement, orasphalt. Currently, for quick disposal, fallen leaves are oftenburnt, resulting in significant air pollution. The surged accu-mulation of fallen leaves calls for efficient valorization. Although lignocellulosic biomass is often readily avail-able, its pretreatment remains one of the most criticalchallenges to biomass utilization due to its complex hierar-chical structure and recalcitrant nature (Hassan et al., 2018).Energy-efficient, environmentally friendly biomass valoriza-tion technologies are highly desirable. Without denying theadvantages of biological or chemical processes, given thecolossal quantity of fallen leaves a city can accumulate, ther-mochemical pretreatment seems to be more promising for en-ergy recovery because it is fast, environmentally benign, andeasily scalable (Adams et al., 2018; Patel et al.,2016). Hydrothermal carbonization is a highly efficient ther-mochemical pretreatment technology, converting biomassfeedstocks in the presence of subcritical water (Alper et al.,2020). After biomass pretreatment, the energy density, grind-ability, hydrophobicity, and ease of storage will be greatlyimproved (Dai et al., 2019; Wang et al., 2020). The hydro-chars derived from the hydrothermal carbonization processare being studied for biofuels (Sharma et al., 2020), sorp-tion of pollutants (Liu et al., 2021), soil amendment (Wanget al., 2020), soil C sequestration (Baronti et al., 2017), andits potential in climate change mitigation (Owsianiak et al.,2018). Based on the different heating methods, conventionalhydrothermal carbonization (HC) and microwave-assistedhydrothermal carbonization (MHC) are under development.When compared to the widely studied HC process, the MHCprocess emerges as an attractive technology thanks to itsunique benefits (Gao et al., 2021). First,microwave heatingis less energy demanding and more cost-effective in produc-ing various products such as hydrochars (Nizamuddin et al.,2018). Second, the MHC process reduces processing timebecause of its rapid volumetric heating. Third, it improvesthe processing capacity due to relatively lower residence timein converting the raw biomass into desired products (Afolabiet al.,2017). In addition, microwave can promote new path-ways during biomass valorization dependent on the uniqueproperties of the feedstock being used (Lei et al., 2021; Liu,Zhong, et al.,2021). Only a handful of reports give a direct comparison ofhydrochars obtained from the HC and MHC processes.Dai et al. (2017) produced hydrochars from bamboo sawdustvia the HC and MHC processes and compared the proper-ties and pyrolysis behaviors of these hydrochars. They foundthat the HC hydrochars had higher thermal stability. Elaigwuand Greenway (2016) compared the chemical and structuralproperties of hydrochars produced from Prosopis africanashell. They concluded that the MHC process is faster in car-bonization than HC, manifested by a higher level of conver-sion within a shorter time. The internal moisture content ofthe feedstock is favorable as the water molecules could cou-ple with the electromagnetic field and promote microwavedielectric heating (Kannan et al., 2017). Previous studiesshowed that hydrochars could be produced from both pro-cesses and that, depending on the preparation conditions, thehydrochar properties might see significant variations. Thestructural change caused by microwave heating might haveprofound impact on thermal degradation behaviors of the hy-drochars, which needs further elucidation. Furthermore, todetermine the feasibility of using hydrochars as biofuels, theoverall process efficiency and the thermochemical-kineticproperties need to be carefully assessed. To the best of our knowledge, converting landscape treeleaves into biofuel via hydrothermal pretreatment has rarelybeen studied. The effect of heating methods and carboniza-tion temperature on yield and physicochemical properties offallen leaves hydrochar has not yet been discussed. Moreover,the impact of different heating methods on hydrochar ther-mochemical kinetics is not compared for the HC and MHCprocesses. The kinetic analysis will provide insight into thehydrochar thermal degradation mechanism, allowing selec-tion and optimization of the subsequent conversion process,and providing a valuable reference for subsequent reactor de-sign (Cai et al., 2019; Damartzis et al., 2011). Therefore, this study aims to (1) comparatively study theyield and physiochemical properties of hydrochars producedfrom tree leaves using two different heating methods, eachwith four different reaction temperatures,(2) to compareenergy consumption of the HC and MHC processes for thepretreatment of tree leaves, and (3) to compare the thermaldegradation kinetics of hydrochars produced by different hy-drothermal processes using different isoconversional analysismethods. Results reported in this study will provide import-ant reference for how to valorize fallen tree leaves via the dif-ferent carbonization pretreatments, and facilitate the designof subsequent hydrochar utilization process. 2 EXPERIMENTAL PROCEDURES 2.1 Materials Tree leaves (TLs) of Fraxinus chinensis Roxb, a widelycultivated landscape tree species in China, were collected on the ground along the pedestrian walk near the Collegeof Mechanical and Electronic Engineering at the NorthwestA&F University in Fall 2018. After collection, the TLswere stored under ambient temperature and air-dried for1.5 months. The TLs were then pulverized using an ultra-centrifugal grinder mill (ZM 200, Retsch), giving a particlesize smaller than 0.18 mm. The biomass powders were sub-ject to characterization or hydrothermal pretreatments. 2.2 Hydrothermal carbonization Either HC or MHC process was applied to pretreat the TLs.Before hydrothermal carbonization, a certain amount of TLswas dispersed into distilled water while maintaining a fixedbiomass loading of 1 g per 10 mL HO. For increased mix-ture, homogeneity agitation was applied using a magneticstirrer (Model MS-H-S, Scilogex LLC) at 600 rpm for 5 min.The mixture was then transferred into reactors. For HC orMHC experiments, the heating ramp rate and reaction dura-tion were both set at 10℃ min- and 1 h (excluding the heat-ing period), respectively. The HC experiments were performed using an auto-clave reactor with an inner volume of 25 ml (KH-25, YikaiInstrument Equipment Co.),which was made with polytet-rafluoroethylene liner covered by a 304 stainless steel shell.Five autoclave reactors were placed simultaneously in a muf-fle furnace (SX-2-4-10G, Jinan Laibao Medical EquipmentCo., Ltd.). After carbonization, the reactors were removedfrom the furnace for sample collection when cooled down tothe ambient temperature. The MHC experiments were performed using a multifunc-tional microwave synthesizer (Model XH-8000 Plus,BeijingXianghu Science and Technology Development Co., Ltd.).Its schematics are depicted in Figure 1, and the detailed spec-ifications are available in our previous report (Kang et al.,2019).The reaction vessel was made of polytetrafluoroeth-ylene (PTFE), protected by a polyether ether ketone (PEEK) shell, with upper limits of temperature and pressure being260℃ and4.0 MPa, respectively. The microwave power inputwas auto-adjusted based on the temperature setting. Aftercarbonization, sample collection was performed at the ambi-ent temperature. The mixture of hydrochar and liquid phaseproducts was transferred into a clean beaker, then separatedthrough WhatmanM grade 2 qualitative filter paper (8umparticle retention at 98% of efficiency). The hydrochars werewashed with de-ionized water and dried in an oven at 105°℃for 12 h and then stored in glass bottles for further analyses. To investigate the influence of reaction temperature, hy-drochars were prepared at 110, 140, 170, and 200°℃, respec-tively. Temperatures higher than 200℃ were not attempteddue to the safe operation requirements of the reactor, and forpractical applications, converting large quantity of biomassthrough hydrothermal process at higher than 200℃ is dif-ficult. For reproducibility and characterization needs, fivebatches of hydrochars were produced for each temperature.During the carbonization experiments, energy consumptionwas measured using an electric meter (model SZDX-D01,Wenzhou Longwan Guoguang Electric Control EquipmentCo.) connected to the muffle furnace or microwave synthe-sizer dependent on the pretreatment process being used. Thehydrochars were named after their producing method (HCor MHC) and reaction temperature, for example,“MHC-110” refers to hydrochar produced with MHC at 110°C.The carbonization experiments for each condition has beentriplicated. For either HC or MHC process, its hydrochar mass yield(Y) and energy consumption (EC) were calculated usingEquations (1) and (2): FIGURE 1 Schematics of themicrowave hydrothermal reactor 2.3 Characterizations 2.3.1 Proximate and ultimate analyses The proximate analysis of TLs and hydrochars was performedfollowing the ASTM 1762-84 and 3173-87 standards. Theultimate analysis was conducted using an elemental ana-lyzer (vario Macro cube, Elementar). Based on the elementalcomposition, the HHV of hydrochar was estimated using theMendeleev formula (Krysanova et al., 2019): 2.3.2 X-ray diffraction (XRD) analysis The XRD analysis was performed with a D8 ADAVNCE X-ray diffractometer (Bruker) equipped with a Cu Ko radiationsource, using a scanning 20 range of 10-90°. 2.3.3 FTIR and SEM analyses The functional groups present in the TLs and hydrocharswere detected using a Nicolet iS10 FT-IR spectrophotom-eter (ThermoFisher Scientific). The powdered samples werescanned under transmittance mode in the range of 400-4000 cmat the resolution of 4cm, and 16 accumulationswere used to obtain each spectrum. Surface morphologi-cal characteristics of the samples were investigated using ascanning electron microscope (SEM) (TM 3030 TabletopMicroscope, Hitachi Ltd.). 2.3.4 Inorganic elemental analysis The inorganic elements present in the TLs and hydrochars weremeasured following the procedures described in the LY/T 1270-1999 standard (Zhang & Yang, 1999). The contents of metalswere determined using a flame atomic absorption spectrom-eter (Z-2000, Hitachi Ltd.). For each test, 0.5 g of dry samplepassing through a 0.5-mm sieve was placed in a glass tube andmixed with 10 ml of 4:1 nitric acid and perchloric acid mixture.The solution is stabilized for 8 h, then digested in a thermostaticdigestor (DTD-40, Changzhou Mai Kenuo Instrument Co.,Ltd.) with 5 ml of acid mixture added, and the digestion wascontinued until a transparent solution was obtained. The liquidsample was then transferred into a 25-ml volumetric flask andreplenished with de-ionized water to meet the graduation line.The diluted sample was filtered with filter paper, which wasthen washed with 5% nitric acid. The filtrate was collected ina clean glass bottle and was used to determine the K, Na, Ca,Mg, and Fe content. The residue on the filter paper was used todetermine the Si content by weighing. 2.3.5 TG/DTG analysis The thermogravimetric (TG) and derivative thermogravi-metric (DTG) analyses of TLs and hydrochars were per-formed using a simultaneous thermal analyzer (TGA 55, TAInstruments). For each run, powdered sample of 15-20 mgwas heated from room temperature to 800°℃ with a nitrogenflow of 20 ml min, and the tests were conducted at dif-ferent thermal ramp rates of 5, 10, 20, 30, and 40℃ min,respectively. Weight loss and weight loss rate were recordedcontinuously as a function of temperature. 2.4 Kinetic analysis The TG/DTG data provide essential information on phys-icochemical changes during the thermal degradation pro-cess and are widely used for kinetic analysis (Cai et al.,2018). For this purpose, the degree of conversion (a) isdefined as where wo, w,and W are sample weight at the initial stage,at time t, and at the completion of the reaction/process,respectively. The general kinetic equation is given as or where do/dt is the mass conversion rate, k(T) is the thermaldegradation rate constant, and f(a) is the differential formof the kinetic model function. The solid sample was heatedat a constant ramp rate of β (K/min). The temperature de-pendence of the rate constant k(T) is described using theArrhenius equation: where A is the pre-exponential factor, E, is the activation en-ergy (kJ mol-), and R is the gas constant, 8.3145 J mol-K-.Combining Equations (6) and (7) leads to the fundamental ex-pression to calculate kinetic parameters from the TG results: For solid-state reactions, f(a) can be generalized as where m, n, and p are empirically obtained exponential fac-tors, with one of them always being zero (Hao et al.,2017). Byseparating variables and integrating, Equation (7) can be trans-formed into: where g(a) is the integral form of the reaction model function.The right side of Equation (10) is a well-established temperatureintegral function that has no analytical solution but can be de-termined by using either numerical methods or approximations. In this study, two highly successful isoconversionalmodel-free methods (Hao et al., 2017; Wu et al., 2020), thatis, the Flynn-Wall-Ozawa (FWO) method and the Kissinger-Akahira-Sunose (KAS) method, were chosen to calculate thekinetic parameters. The FWO method can be presented as The KAS method can be presented as As shown in Equations (11) and (12), for a certain degreeof conversion, by plotting data points of logf or ln(B/T) ver-sus 1/T obtained at different heating ramp rates, the Ea valuecan be calculated from the slope of the linear regression. 3 RESULTS 3.1 HC/MHC process efficiency For the HC or MHC process, its efficiency was compared onthe basis of hydrochar mass yield (Y) and energy consump-tion (EC), with results presented in Figure 2. For either HCor MHC process, the hydrochar mass yield decreases with anincreasing pretreatment temperature, which can be attributedto the ever-increasing partial decomposition of hemicelluloseand cellulose. One exception is that, for the HC process, thehydrochar yield is barely changed when the reaction tem-perature increases from 110 to 140°℃, indicating that for theconventional HC process, such low reaction temperatures areinsufficient to noticeably destruct the biomass matrix. As forenergy consumption, for reaction temperatures ranging from110 to 200C, the HC process is consistently higher than thatof the MHC process, showing that the MHC process is ableto produce a significantly higher amount of hydrochar withless energy consumption. 3.21 Physiochemicalcharacteristics of the hydrochars 3.2.1 Basic properties The results of the proximate and ultimate analysis of the hy-drochars as well as the biomass feedstock are listed in Table1. As a general trend, the fixed carbon content in the hydro-chars increased with reaction temperature due to the increas-ing loss of volatile matters. The ash content was increasedwhen the reaction temperature was increased from 100 to Processing temperature(C) 140℃ and then stabilized for both HC and MHC samplesderived from the TLs. Furthermore, we analyzed the inorganics present in theTLs and hydrochars produced under 200C, and the contentsof select elements are plotted in Figure S1 of the supple-mentary data. As shown in the figure, some of the inor-ganic components might have dissolved and were leachedout during the hydrochar formation, resulting in reducedamounts of Mg, Fe, K, and Na in the hydrochar. However,the content of some other inorganic elements (e.g., Ca) inthe hydrochar increases mostly because of the removal ofvolatile matters. 3.2.2 Spectroscopic analyses The XRD patterns of the TLs and hydrochars are given inFigure 3. For the TLs feedstock, only two peaks at the 20of 24.2° and 38.0° are obviously visible, which peaks couldbe attributed to the cellulose structure (Farrow et al.,2015)and the mineral phase of CaCOs (Xiao et al., 2017), respec-tively. Two new peaks were observed at the 20 of 14.8° and29.8° on the spectra of hydrochars. The first peak at 14.8° isregarded as plane 101 of cellulose (Jiang et al., 2013), andthe second peak at 29.8° derives from calcite crystallization(Yuan et al., 2011). The intensification of the first peak sug-gests that with the increase in reaction temperature, harsherhydrothermal pretreatments cause more severe damage to thebiomass particle surface, and the crystal structure of cellulosegets more exposure. The increase in the intensity of the sec-ond peak confirms the increase in the Ca content of the hy-drochar, which is consistent with the ICP-MS results (FigureS1). Therefore, the enrichment of Ca might improve the fuelcharacteristics of the hydrochar. The FT-IR spectra of the HC and MHC hydrochars areshown in Figure 4a,b, respectively. Hydrochars producedfrom both HC and MHC processes are rich in functionalgroups. Based on the literature (Kim et al., 2011, 2013; Xiaoet al., 2017; Yuan et al., 2011), the multiple peaks presentedon the spectra may be assigned to O-H stretching vibrationof hydroxyl (3200-3400 cm-), C-H stretching vibration ofthe fatty chain structure (2800-3000 cm-l), C= C stretch-ing vibration of the ethylenic bond (1608-1630 cm-), C=Cstretching vibration of the aromatics (1450-1600cm-), C-Hbending vibration of aliphatic structure (1420-1480 cm-),O-H/C-H bending vibration of olefins, alkyd,acids, phenols,and alcohol (1360-1430 cm-), C-O stretching vibration ofunsaturated ether (1200-1300 cm), C-H bending vibrationof aromatics (1140-1200 cm-),skeletal ring stretches aswell as the stretch modes of glycosidic C-O bonds and alco-holic C-O bonds (950-1150 cm-), and torsion or bendingmodes of the six-membered ring concerning the glycosidicbonds (400-800 cm-). The SEM images of the TLs and hydrochar surfaces were taken at the magnification of 1000× and are shown in Figure5. For the TLs sample, the particle surface showed naturallyformed cracks without any pore structure exposed. For bothHC and MHC hydrochars, the change in surface structure isstrongly dependent on the reaction temperature. Specifically,at 110 and 140℃, the changes were not significant whencompared to the TLs, and a limited number of pores wereobserved on the surface of MHC-140 hydrochar, but notHC-140. Moreover, the pattern of particle surface destruc-tion is influenced by heating methods. For example, com-paring the images recorded for hydrochars prepared at 170and 200℃, all hydrochars showed an increased extent of sur-face destruction, but the MHC-200 showed a more exposedporous structure than HC-200. For the HC hydrochars, thepores underneath the particle surface start to emerge with theincrease in surface decomposition. For the MHC hydrochars,however, the fiber surface and part of the inner fraction of theplant tissue are degraded simultaneously. 3.3Thermal degradationcharacteristics of the hydrochars 3.3.1 Thermal degradation behavior To understand the influence of hydrothermal carbonizationtemperature and the two different heating methods, TG/DTGcurves of the hydrochars were obtained at the heating ramprate of 10°C min-and plotted in Figure 6. On the TG curvesof both HC and MHC hydrochars, two general trends couldbe observed. First, the main weight loss stage is shifted tohigher temperatures when the pretreatment temperature is in-creased. Second, hydrochars prepared at 200°C exhibit higherweight retain rates at the analyzing temperature of 800°℃. More detailed interpretation may be illustrated using theDTG curves, that is, weight loss peaks occurred at differenttemperatures can be attributed to the decomposition of dif-ferent constituents of the hydrochar sample. In Figure 6c,d,the first peak occurred at around 130℃ may be assigned towater evaporation. The second one, which is a shoulder peaklocated at approximately 266C, is associated with the de-composition of hemicellulose (Naik et al., 2010). Regardlessof the heating method, this peak gradually disappears whenthe hydrothermal carbonization temperature reaches 170°℃,indicating that both the HC and MHC processes effectivelyremove or destroy the hemicellulose in the TLs. The thirdpeak is of the highest intensity and located at the temperaturerange of 332-351°℃, which can be attributed to cellulose de-composition (Shi et al., 2019). The fourth peak at 460-470°℃has the smallest area and cannot be well separated from the BIOPRODUCTS FOR A SUSTAINABLE BIOECONOMY TABLE1Basic properties of the biomass feedstock and hydrochars produced from different hydrothermal pretreatment processes Volatile Sample Moisture matter Ash Fixed carbon C H N S O HHV(MJkg) TL 6.20±0.14 79.19±0.27 8.56±0.50 6.05±0.76 43.82 6.17 0.95 0.06 49.01 17.26 HC-110 6.36±0.53 78.83±0.00 8.38±0.39 6.43±0.39 44.62 6.23 1.04 0.04 48.07 17.72 HC-140 6.05±0.10 78.39±0.01 9.46±0.09 6.10±0.72 45.43 6.04 1.06 0.03 47.44 17.82 HC-170 4.29±0.13 76.97±0.00 9.38±0.15 9.36±0.74 47.58 6.16 1.07 0.03 45.17 18.94 HC-200 3.63±0.17 73.23±0.00 9.22±0.37 14.06±0.47 51.18 6.05 1.04 0.04 41.69 20.41 MHC-110 6.13±0.08 78.95±0.01 7.25±0.22 7.67±0.24 44.45 6.10 1.03 0.06 48.36 17.47 MHC-140 5.75±0.41 77.55±0.01 8.24±0.19 8.45±0.77 44.43 6.04 1.06 0.05 48.42 17.37 MHC-170 3.58±0.29 76.14±0.00 8.16±0.30 12.22±1.29 48.00 6.12 1.07 0.03 44.79 19.08 MHC-200 3.69±0.45 73.58±0.01 8.61±0.11 14.11±1.65 51.60 5.98 1.06 0.04 41.31 20.51 "The oxygen content was calculated by difference. FIGURE 3XRD patterns of the treeleaves and hydrochar samples 20(°) third one. This peak might be associated with lignin decom-position (Kabir et al., 2018; Vamvuka et al.,2003). The fifthpeak is observed at about 650°C, which is possibly caused bythe degradation of mineral containing compounds, for exam-ple, carbonates (Idris et al.,2010). 3.3.2 Kinetic analysis For kinetic analysis, the TG/DTG curves of TLs, HC-200,and MHC-200 hydrochars under different ramp rates (5, 10,20, 30, and 40C min-) were plotted in Figure S2. Thermallag can be observed for all three TG curves (subplots a, c, ande), that is, when the heating ramp rate is increased, thermal degradation is delayed and the initiation of de-volatilization isshifted to higher temperatures because of heat transfer limita-tions from the sample surface to the particle core (Damartziset al., 2011). When comparing the DTG profiles at subplotsb, d and e, the shoulder peak at 240-270°℃ disappears forHC/MHC hydrochars, indicating that hemicellulose is com-pletely removed during the pretreatment process. The lastpeak showed varied temperatures in the range of 630-720°℃,and no direct dependency can be linked to the increase inheating ramp rate. This is due to the complicated interactionsamong different inorganic components and the interactionbetween minerals and carbonaceous residues. The activation energy (E) and corresponding R valuesare given in Table 2, showing that the two isoconversional &WILEY- Wave number (cm-) (b) FT-IR spectra of MHC hydrochars FIGURE4 FT-IRspectra of hydrochar samples from (a) HC or(b) MHC process as compared to the tree leaves methods have varied suitability of application on differ-ent samples. For TLs feedstock, the FWO method givessatisfactory regression results for the conversion range ofa=0.2-0.7 (R’> 0.9), whereas the KAS method is ob-viously not applicable. The relatively low Rvalues fromthe KAS method can be explained by the inhomogeneityof the TLs sample, causing distorted temperature profiles.Since the KAS method plots In(B/T) versus 1/T, even smalldisorder in the reaction temperature will be mathematicallyenlarged. 4 DISCUSSION 4.1|MHC gives higher productionefficiency for hydrochar In a biomass carbonization process, three important factorsneed to be considered, including energy consumption, hy-drochar yield, and properties. Generally, using less energyto produce high-quality hydrochars is highly desirable.The mass yields of hydrochar from the MHC process arehigher than those of HC, which was also observed by other (a) TL (b) HC-110_ (c)HC-140 TM3030 6072 2019/07/01 15:30 mL D5.9 x1.0k100pm TM3030 607 2019/07/0115:25 mL D5.6 x1.0k100 pmTM3030_6065 2019/07/01 15:06 mLD5.8 x1.0k 100 um FIGURE 5 SEM images of raw tree leaf and hydrochar samples: (a) TL, (b) HC-110, (c) HC-140, (d) HC-170, (e) HC-200, (f) MHC-110,(g) MHC-140, (h)MHC-170, and (i)MHC-200 FIGURE 6 Thermogravimetric and derivative thermogravimetric curves of hydrochars prepared via HC/MHC processes (temperature ramprate at 10℃ min-): (a) TG-HC, (b) TG-MHC, (c) DTG-HC, and (d) DTG-MHC researchers in their comparison of the two processes usingpeanut shell or bamboo sawdust as the feedstock (Chu et al.,2017; Dai et al.,2017). The results indicate that the MHCprocess caused less loss of the organic components due to areduced extent of decomposition. The reduced energy con-sumption of the MHC process is closely associated with theadvantages of microwave heating, such as high efficiencyand selective heating over conventional heating methods(Dai et al., 2019). 4.2 |MHC improves fuelproperties of the hydrochar One of the most important applications of the hydrochar isto be used as a solid biofuel. In this study, comprehensivecharacterization results were acquired to understand theevolution of fuel-related properties during the carboniza-tion process. An increase in ash content of the hydrocharswas observed between 100 and140°C. A similar increasewas reported by other researchers (Dai et al., 2017; Heet al., 2013; Kalderis et al., 2014), but different from whatwe had observed when applying the MHC process on corn stalk (Kang et al., 2019). The difference in feedstocks andoperation conditions might account for such a differencein the effect of reaction temperature on the change of ashcontent. Fortunately, from a fuel production point of view, otherthan Ca, the contents of most alkali metals (e.g., Na and K)present in the biomass precursor were all reduced-thesealkali metal salts typically have a lower melting point andtend to cause severe issues during hydrochar combustion.We also see that the MHC process is more effective in re-ducing the Si concentration in the hydrochar than the HCprocess. This is beneficial because when combined with alarge amount of K, the formation of KO-SiO, can causetenacious surface deposits on firesides and heating surfacesdue to its low-melting point of 550℃ (Niu et al., 2016).The enrichment of carbon content and improvement in thehydrochar HHV are not adversely affected by applyingmicrowave-assisted heating. These properties were foundto be highly dependent on the reaction temperature, and200°℃ provided a fair compromise between hydrochar yieldand fuel quality. The changes of HHV with temperature inboth processes are similar and are not significant till thetemperature is increased to 170C, indicating that effective Conversion Temperature FWO method KAS method Sample (-) range (°C) E(kJ mol-) R Ea(kJ mol-) R’ TL 0.1 130.1-143.7 104.05 0.3188 122.32 0.2433 0.2 225.0-235.9 288.03 0.9333 489.84 0.8710 0.3 253.5-263.1 477.30 0.9261 620.50 0.8617 0.4 278.3-293.9 288.03 0.9426 362.66 0.8379 0.5 291.3-311.5 253.37 0.9351 316.66 0.8245 0.6 318.2-337.7 270.06 0.9554 341.52 0.8631 0.7 342.0-358.8 361.45 0.9308 459.16 0.8379 0.8 399.8-416.2 275.27 0.3434 376.71 0.3530 HC-200 0.1 227.6-245.1 216.45 0.7693 219.16 0.7556 0.2 281.2-300.8 268.53 0.9606 273.01 0.9580 0.3 308.5-330.2 266.89 0.9750 270.80 0.9732 0.4 324.3-348.6 252.77 0.9816 255.68 0.9802 0.5 335.7-362.1 243.96 0.9868 246.21 0.9858 0.6 347.4-375.0 244.03 0.9925 246.09 0.9919 0.7 374.6-395.2 354.31 0.9974 361.65 0.9972 0.8 424.1-441.3 471.44 0.9965 484.02 0.9963 MHC-200 0.1 220.0-242.8 166.45 0.9582 166.65 0.9541 0.2 268.0-294.5 178.95 0.9618 178.97 0.9580 0.3 296.2-325.7 181.82 0.9548 181.49 0.9501 0.4 313.4-344.9 184.85 0.9549 184.38 0.9502 0.5 327.3-359.3 191.60 0.9652 191.24 0.9616 0.6 343.6-374.0 212.05 0.9859 212.48 0.9845 0.7 385.0-408.8 280.19 0.7445 283.49 0.7294 0.8 446.7-473.5 97.53 0.1126 90.40 0.0897 TABLE 2 Activation energies andcorrelation coefficients of TLs andhydrochars at different conversion degrees removal of hemicellulose and enrichment of carbon occurat this temperature or above. As shown in Figure S1, compared to the raw TL,the Caelement is enriched in the hydrochars. Calcium salts have lowmobility and a high melting point, causing less significantdeposition problems, and an increase in the Ca content mighthelp decrease the slagging (Niu et al., 2016). Therefore, theenrichment of Ca improves the fuel characteristics of thehydrochar. The MHC-200 showed a more exposed porous structurethan HC-200. This may be associated with the differentheat transfer patterns of the two different processes. ForHC, the heat was first transferred to the water, then to thesurface of the biomass particles. In contrast, in MHC, thisinward heat transfer was accompanied by the outward heattransfer from the inner layer to the outer layer of the planttissues since the biomass itself adsorbed microwave andwas heated. However, it should be noted that due to theuneven distribution of the biomass components, includ-ing hemicellulose, cellulose, and lignin in the TLs, andtheir differed microwave adsorption capabilities, the heat transfer mechanism can vary at different spots even on asingle biomass particle. 4.3 MHC enhances thermal degradationreactivity of the hydrochar The investigation of thermal degradation behavior is necessaryfor the comprehension of phenomena that occurred during ther-molysis of hydrochars as a biofuel (Giudicianni et al., 2013).The information contained in DTG curves could be used to bet-ter understand the reactivity of the tested hydrochars becausethe peak positions (temperature) and heights in the DTG curvesare closely associated with the reactivity of the biofuel samples.The peak temperature is inversely proportional to its reactivity,whereas the peak height is directly proportional to the reactivity(Zheng & Kozinski, 2000). Therefore, when the hydrothermalcarbonization temperature increases, the peak of cellulose de-composition is shifted to a higher temperature, but the heightis also increased. This shows that a higher level in the removalof volatile matters causes carbon enrichment, which makes it less reactive at lower temperatures but can release heat moreintensively once it starts to decompose. To calculate the activation energy, linear regression plotsaccording to the FWO or KAS methods are presented inFigure S3, together with their equations and determinationcorrelation coefficients (R’). Telling from low R’values, theregressions using either method typically fail at conversion(a) lower than 0.1 or higher than 0.9. This phenomenon hasbeen reported by other researchers (Damartzis et al., 2011;Ma et al., 2019;Ren et al., 2013). At a low conversion (a) of0.1, the corresponding temperature range is 130.1-143.7℃for TLs under different heating ramp rates, which is mainlyfor moisture removal. This occurs at the temperature rangesof 227.6-245.1°C and 220-242.8°C for HC-200 and MHC-200 hydrochars, respectively. The greatly elevated tempera-ture of achieving 10% of conversion shows that moistureremoval has been accomplished during hydrochar formation,confirmed by results in Table 1. This is desirable for fuelutilization as less heat will be required for demoisturizationbefore ignition. At a high conversion (α) of 0.9, the corre-sponding temperatures are 508.8℃ for TLs, 532.8C for HC-200 hydrochar, and 608.6℃ for MHC-200 hydrochar at theheating ramp rate of 5℃ min. Under these temperatures,lignin, carbonaceous residues, and mineral phase of the TLsor hydrochars may undergo complicated reactions, affectingthe linearity of the apparent regression relation. In addition,the low R’values may result from the methods being used,as it has suggested that the accuracy of both FWO and KASmethods will decrease in predicting the biomass activationenergy when lignin decomposition occurs (Alvarez et al.,2016). This is because the lignin decomposition processcombines complicated exothermal and endothermal reactionswith competitive pathways, restricting the application ofmodel-free analysis methods (Kawamoto, 2017; Opfermannet al.,2002). Both FWO and KAS methods accurately described thethermal decomposition of the HC hydrochars in the conver-sion range of a=0.2-0.8, which is consistent with the trendsreported by a previous study (Ma et al., 2019). When com-pared to the TLs, generally higher Rvalues with hydrocharsalso indicate that the hydrothermal carbonization processconverts the thermochemically unstable raw material intomore homogenous fuel with higher predictability in terms ofthermal degradation behavior. Both methods also work well on the MHC data; however,the best-fitting range is shifted to lower conversions of 0.1-0.6, and regressions at the conversion of 0.7 and 0.8 give lowR’values. A close examination of the data reveals that theFWO and KAS methods performed the best in describingthe decomposition characteristics in the temperature rangeof 230-360°C for TLs, 280-440°C for HC-200 hydrochar,and 220-370℃ for MHC-200 hydrochar. Therefore, it is rea-sonable to deduce that the thermal degradation mechanism of MHC-200 hydrochar is the same as TLs in this specificrange, whereas for HC hydrochar, this shared decompositionbehavior is shifted to higher temperatures. This phenomenonalso shows that the HC process is more efficient than MHCin decomposing hemicellulose in the TLs. Based on the satisfactory regression results (α=0.2-0.6),the average activation energies and R values were calculated.Even in this select a range, the KAS method does not workwell for TLs, providing an average R of only 0.8516. Asmentioned before, the high inhomogeneity of TLs makes theKAS method ineffective in describing the thermal degrada-tion of this biomass feedstock (Ma et al., 2017). Moreover, theresults show that the average activation energy is decreasedin the hydrochar samples, suggesting that the hydrochars aremore active than the TLs during thermal degradation. Thisis because, to a different extent, the hydrothermal carboniza-tion process destroys the complex lignocellulosic matrix ofTLs, which enhances the thermochemical reactivity of thehydrochars. The MHC-200 hydrochar shows lower activationenergy than the HC-200 hydrochar (~190 kJ mol-vs. 260kJmol-). During pyrolysis, high activation energy means thatthe reaction requires more energy from the surroundings toproceed (Liu et al.,2004).Therefore, at the preparation tem-perature of 200℃, the MHC process performs better than theHC process in reducing the energy demand for subsequenthydrochar thermal degradation. To sum up, under tested conditions for carbonization pre-treatment of tree leaves waste, the MHC process is more ef-fective than HC in producing a significantly higher amountof hydrochar with less energy consumption. At the pretreat-ment temperature of 200C, the resulting MHC hydrocharhas improved fuel properties, giving an increased HHVof 20.51 MJ kg(17.26 MJ kg- for TLs feedstock and20.41 MJ kg-for the HC hydrochar), reduced K and Si con-tent, and reduced activation energy for subsequent thermaldegradation (~190 MJ kg-vs. ~260 MJ kg-for HC hydro-char). The isoconversional analysis also reveals that the FWOmethod is applicable to characterize the TLs feedstock andHC/MHC hydrochars, while the KAS method is not suitablefor the characterization of TLs due to its high inhomogeneity.These results reveal how the fuel properties and thermal deg-radation mechanism of hydrochars were influenced by theHC and MHC processes,therefore, allowing the optimizationof the valorization process of landscape tree leaves as an al-ternative biomass resource. ACKNOWLEDGMENTS This work was supported by the Natural Science BasicResearchhProgram offShaanxi [2020JQ-243], KeyLaboratory of Energy Resource Utilization from AgricultureResidue, Ministry of Agriculture and Rural Affairs, China[KLERUAR2018-02], the NationalKey Research andDevelopment Programn dof (China [2018YFE01270001. Key Researchhaand1.Development Program (of SShaanxi[2020NY-105], FundamentalalResearchFunds for theCentralilUniversities [2452017299], ShaanxiiProvincialDepartment of Science and Technology [2018ZDXM2-01,2020QFY10-03], and Start-up Fund from the NorthwestA&F University for Dr.Dongbing Li. AUTHOR CONTRIBUTIONS Kang Kang involved in conceptualization, methodology,formal analysis, data curation, writing-original draft, andwriting-reviewing and editing. Tianle Zhang involved ininvestigation, data curation, and software. Guotao Sun in-volved in data curation and writing-reviewing and editing.Mingqiang Zhu and Kankan Li involved in resources andvisualization. Dongbing Li involved in methodology, formalanalysis, writing-original draft, and writing—reviewingand editing. DATA AVAILABILITY STATEMENT The data that support the findings of this study are availablefrom the corresponding author upon reasonable request. ( ORCID Dongbing Lil io https://orcid.org/0000-0002-6528-2083 ) REFERENCES ( Adams, P., B ridgwater, T ., Lea-Langton, A ., Ross, A ., & W a tson,I. (2018). Chapter 8—Biomass conversion t e chnologies. I n P.Thornley & P. Adams (Eds.), G reenhouse g a s balances of bioen-ergy systems (pp. 1 07-139). Academic Press. ) ( Afolabi, O. O., Sohail, M., & T homas, C. (2017). Characterization of solid fuel chars recovered from microwave hydrothermal c a r- bonization of human b i owaste. Energy, 1 3 4, 74-89. h t tps://doi.org/10.1016/j.energy.2017.06.010 ) ( Alper, K., Tekin, K., Karagoz,S. , & Ragauskas,A.J.(2020). Sustainableenergy a nd f uels from biomass: A r eview f ocusing on hydrother- mal biomass processing. Sustainable Energy & Fuels,4(9),4390- 4414. h ttps://doi.org/10.1039/d0se00784f ) ( Alvarez, A., Pizarro, C., Garcia, R., Bueno, J. L., & Lavín, A. G. (2016). Determination of k inetic parameters for biomass combustion. Bioresource T echnology, 2 16, 3 6 -43. ht t ps://doi.org/10.1016/j. biortech.2016.05.039 ) ( B aronti, S. , Alberti, G., Camin, F. , Criscuoli, I ., Genesio,L . , Mass, R., Vaccari, F . P ., Ziller , L. , & M iglietta, F . ( 2017). Hydrochar en- hances growth of p o plar for bioenergy wh i le marginally contrib- uting to direct soil carbon sequestration. GCB B i oenergy, 9(11),1618-1626. https://doi.org/10.111 1 /gcbb.12450 ) ( Cai, H ., Liu, J. , Xi e , W., Kuo, J ., Buyukada, M . , & Ev r endilek, F. (2019). Pyrolytic kinetics, reactio n mechanisms and products of waste tea vi a TG-FTIR and Py-GC/MS. Energy Conversion and Management, 184. 4 36-447. https://doi.org/10.1016/j.enconman.2019.01.031 ) ( Cai,J., Xu,D.,Dong, Z., Yu, X., Yang, Y., Banks, S. W., & Bridgwater,A. V . (2018). P rocessing t h ermogravimetric a n alysis d a ta f o risoconversional k inetic analysis o f l i gnocellulosic b i omass py-rolysis: Case study o f c orn s talk. Renewable a n d Su s tainable ) ( Energy R e views, 82, 2705-2715. https://doi.org/10.1016/j.rser.2017. 09.113 ) ( Chu, G., Z hao, J., Chen, F ., Dong, X., Z hou, D., Liang, N., W u , M., Pan, B., & S teinberg, C. E. W. ( 2017). Physi-chemical a nd sorp- tion properties of biochars prepared from p e anut shell using thermal pyrolysis a nd m i crowave irradiation. EnvironmentalPollution, 2 27, 3 7 2-379. htt p s://doi.org/10.1016/j.envpol.2017. 04.067 ) ( Dai, L ., He, C . , W ang,Y. , Li u , Y. , Yu, z. , Zhou, Y., Fan, L., Duan, D., & Ruan,R . (2017). Comparative s tudy on microwave and conven-tional hydrothermal pretreatment of bamboo s a wdust: H y drochar properties and it s pyrolysi s behaviors. E nergy Conversion and Management, 146, 1-7. https://doi.org/10 . 1016/j.encon man.2017.05.007 ) ( Dai, L., Wang, Y., L iu, Y ., R uan, R., Yu, Z . , & Jiang, L. (2019). C o mparative study on C c haracteristics of t he e b io-oilfrom microwave-assisted pyrolysis of l i gnocellulose and t r iacylglyc- erol. Science of the Total Environment, 659,9 5 -100. https://doi.org/10.1016/j.scitotenv.2018.12.241 ) ( Damartzis, T ., Vamvuka, D . , Sfakiotakis, S ., & Z abaniotou, A. (2011 ) .Thermal degradation s t udies a nd k i netic m odeling o f cardoon(C y nara cardunculus) pyrolysis using thermogravimetric a n alysis ( T GA). Bioresource T echnology, 102(10), 6230-6238. https://doi.org/10.1016/j.biortech.201 1 .02.060 ) ( Elaigwu, S. E., & Greenway, G. M . ( 2016). Microwave-assisted andconventional hydrothermal carbonization of lignocellulosic wastematerial: comparison o f the chemical and structural properties ofthe hydrochars. Journal of Analytical and Applied Pyrolysis, 1 1 8, 1 -8. h ttps://doi.org/10.1016/j.jaap.2015.12.013 ) ( Farrow, T.S., Sun, C.,& Snape, C. E. (201 5 ). Impact of CO, on biomass pyrolysis, nitrogen partitioning , and char combustion i n a d roptube f urnace. Journal o f Analytical and Applied P y rolysis, 113,323-331. https://doi.org/10.1016/j.jaap.2015.02.013 ) ( Gao, Y., Remon, J., & M atharu, A. S. (2021).Microwave-assisted hy- drothermal t r eatments for biomass valorisation: A c ritical review. Green Chemistry, https://doi.org/10.1039/D1GC00623A ) ( Giudicianni, P ., Cardone, G., & R agucci, R. (2013). Cellulose, h emi-cellulose and lignin slow s team p yrolysis: T hermal decompo- sition of biomass c omponents mixtures. J o urnal of Analyticaland Applied Py r olysis, 10 0 , 21 3 -222. http s ://doi.org/10.1016/j. jaap.2012.12.026 ) ( Hao, Y .-H. , Huang, Z ., Y e, Q .-Q., W ang, J.-W., Y ang, X.-Y., Fa n, X .-Y., Li, Y.-L . , & Peng, Y.-W. (2017). A comparison study on non-isothermal decomposition kinetics of chitosan with di f ferent analysis methods.Journal of Thermal Analysis and Calorimetry, 128(2), 1077-1091. https://doi.org/10.1007/s10973-016-5972-y ) ( Hassan, S . S ., Williams, G. A ., & Jaiswal, A . K . (2018). E merging technologies for the pretreatment of lignocellulosic biomass. Bioresource Technology, 262,310 - 318. https://doi.org/10.1016/j. biortech.2018.04.099 ) ( He, C., Giannis, A., & Wang,J.-Y.(2013). Conversion of s ewage sludgeto clean solid fuel using hydrothermal carbonization: Hydrochar f uel characteristics and combustion behavior. Applied Energy,111 , 257-266. h t tps://doi.org/10.1016/j.apenergy.2013.04.084 ) ( Idris, S. S. , Rahman, N. A ., I smail, K., Alias, A. B., R ashid, Z. A., &Aris, M.J. (2010). Investigation on thermochemical behaviour of l ow rank Malaysian coal, o il palm biomass and their blends during pyrolysis via t hermogravimetric a nalysis ( T G A). BioresourceTechnology, 1 0 1(12), 45 8 4-4592. http s ://doi.org/10.1016/j.biort ech.2010.01.059 ) ( Jiang, L.-Q., Fang, Z ., Li, X.-K., & Luo, J.(2 0 13). Pr o duction of 2 ,3- b utanediol from cellulose a n d Jatropha hu l ls after ionic l i quid pretreatment a nd dilute-acid hydrolysis. A MB Express, 3(1), 48. https://doi.org/10.1186/2191-0855-3-48 ) ( Kabir, A. S., Yuan,Z., K uboki, T., & Xu, C. (2018). De-polymerizationof i ndustrial lignins to improve t h e t h ermo-oxidative sta b ility ofpolyolefins. Industrial Crops and Products, 1 20, 2 38-249. https:// doi.org/10.1016/j.indcrop.2018.04.072 ) ( Kalderis , D., Kotti , M. , Mendez , A., & 及 G asc6, G . (2014).Characterization of hydrochars produced b y hydrothermal c a r- bonization o f r ice husk. S olid E arth, 5 (1), 477-483. https://doi.org/10.5194/se-5-477- 2 014 ) ( Kang, K ., Nanda, S ., Sun, G., Qiu, L., Gu, Y., Zhang, T., Zhu, M., & S un,R . (2019). Microwave-assisted hydrothermal carbonization of corn stalk f or solid biofuel production: Optimization of process pa - rameters and characterization o f hydrochar. E nergy, 186,11 5 795.https://doi.org/10.1016/j.energy.2019.07.125 ) ( Kannan, S. , Gariepy, Y., & R aghavan, G. S. V . ( 2017). Optimization and characterization of hydrochar p roduced from microwave hy-drothermal carbonization of fis h waste. Waste Management, 65, 159-168. https://doi.org/10.1016/j.wasman.2017.04.016 ) ( Kawamoto, H. ( 2 017). Lignin pyrolysis re ac tions. Jo u rnal l of Wood S c ie n ce, 6 3( 2), 117- 1 32. http s ://doi.org/10.1007/s1008 6-016-1606-z ) ( Kim, P., Johnson, A., Edmunds, C. W., Radosevich, M. , Vogt, F., Rials, T . G. , &L a bbe, N. (2011). Su r face functionality and car b on struc- tures in lignocellulosic-derived biochars produced by fast pyroly-sis. Energy & Fuels, 25(10), 4693-4703. https://doi.org/10.1021/ef200915s ) ( Kim, S. H . , Lee, C. M . , & Ka f le, K. (2013). Characterization of c ry s - talline cellulose in t biomass: I B asic p r inciples, a p plications, and limitations o f XRD, NMR, IR, Raman, and S FG. Korean Journal of Chemical Engineering,30(12), 2127-214 1 .https://doi. org/10.1007/s11814-013-0162-0 ) ( Krysanova, K., K rylova, A . , & Za i chenko, V. (2 0 19). Pr op erties of b iochar o btained b y hydrothermal c a rbonization a n d to r re- faction of p eat. F uel, 256, 1 15929. ht t ps://doi.org/10.1016/j. fuel.2019. 1 15929 ) ( Lei, Q., Kannan, S ., & R aghavan, V. ( 2 021). Uncatalyzed a n d acid- aided microwave hydrothermal carbonization of orange peel waste. Waste M anagement, ,126, . 1 06-118. https://doi.org/10.1016/j. wasman.2021.02.058 ) ( Liu, J., Zhong, F., Niu, W. , Zhao, Y., Su, J., Feng , Y . , & Meng, H.(2021). Effects of temperature a nd catalytic methods on the phys- i cochemical p roperties of microwave-assisted h ydrothermal prod- ucts of crop residues. Journal of Cleaner Production, 279,123512.https://doi.org/10.1016/j.jclepro.2020.123512 ) ( Liu, Q., Hu, H . , Zhou, Q., Z hu, S., & Chen, G. ( 2 004). E f fect o f in- organic matter o n reactivity a nd kinetics o f coal p yrolysis. Fuel,83(6),713-718.ht t ps://doi.org/10.1016/j.fuel.2003.08.017. ) ( Liu, Z., Wang, Z., C hen, H., Cai, T ., & Liu, Z. ( 2021). H ydrochar and pyrochar f o r sorption of pollutants in wastewater and exhaust ga s :A cri t ical review. Environmental Pollution, 268, 11 5 910. htt p s:// doi.org/10.1016/j.envpol.2020.115910 ) ( Ma,J.,Luo,H.,Li, Y., Liu, Z., Li,D.,Gai, C., & Jiao, W.(2019). Pyrolysis kinetics and thermodynamic parameters of th e hy d rochars derivedfrom co-hydrothermal carbonization of sawdust and sewage sludgeusing thermogravimetric a n alysis. B ioresource T e chnology, 282, 1 3 3-141. https://doi.org/10.1016/j.biortech.2019.03.007 ) ( M a, P., Y ang, J . , X ing, X., Weihrich, S., Fan, F. , & Zh a ng, X. (2017). I soconversional kinetics and c haracteristic s of combustion on hydrothermally treated biomass . Renewable Energy, 114, 1069-1076. https://doi.org/10.1016/j.renene.2017.07.115 ) ( Naik, S., Goud, V . V ., Rout, P. K., Jacobson, K., & Dalai, A. K. (2010). Characterization of C anadian b i omass for al t ernative r en ew- able biofuel. Renewable E n ergy, 35(8), 16 2 4-1631. htt p s://doi.org/10.1016/j.renene.2009.08.033 ) ( Niu, Y., Tan, H., Hui, S. (2016).Ash-related issues during biomass com- bustion: Alkali-induced s lagging, s ilicate melt-induced slagging(ash f usion), a gglomeration, co r rosion, ash utilization, and rel a tedcountermeasures. P rogress in Energy and Combustion Science,52, 1-61.https://doi.org/10.1016/j.pecs.2015.09.003 ) ( Nizamuddin, S. , Baloch, H. A., Siddiqui, M.,Mubarak, N ., Tunio, M., B hutto, A., Jatoi, A. S . , Griffi n ,G., & Srinivasan , M. (2018). Anoverview o f microwave hydrothermal carbonization and m icro- wave pyrolysis of biomass. Reviews in Environmental Science andBio/Technology, 17 (4), 813-837. ht tp s://doi.org/10.1007/s1115 7-018-9476-z ) ( Opfermann, J. R . , Kaisersberger, E., & Flammersheim, H. J. ( 2002).Model-free a nalysis o f t h ermoanalytical data-advantages an d limitations . Thermochimica A cta, 3 91(1),11 9 - 127. ht t ps://doi.org/10.1016/S0040-6031(02)00169-7 ) ( Owsianiak, M . , B r ooks, J., Renz, M., & Laurent,A. (2018). Evaluating climate change mitigation potential of hydrochar s : Compoundinginsights from three different indicators. G CB Bi o energy, 10(4),230-245. https://doi.org/10.1111/gcbb.12484 ) ( Patel, M., Zhang, X., & K umar, A . (2016). T e chno-economic and lifecycle a ssessment o n l i gnocellulosic biomass thermochemicalconversion technologies: A review. Renewable and Sustainable Energy R eviews, 53. 1486-1499. h ttps://doi.org/ 1 0.1016/j. rser.2015.09.070 ) ( Reid, W. V., Ali, M. K., & F ield, C. B. (2020). The future of bioenergy. Global Change Bi o logy, 26(1), 27 4 -286. https://doi.org/10.1111/gcb.14883 ) ( Ren, S ., Lei, H. , Wang, L., Bu, Q., Chen , S., & Wu, J. (2013) . Thermal behaviour and kinetic study for woody biomass torrefaction and tor- refied biomass pyrolysis by T GA. Biosystems Engineering, 1 16(4), 420-426. h t tps://doi.org/10.1016/j.biosystemseng.2013.10.003 ) ( Sharma, H. B. , S armah, A. K., & Dubey, B. (2020). H y drothermal car- bonization of renewable waste b iomass for solid biofuel produc-tion: A discussion o n process mechanism, th e in f luence of pr o -cess parameters, environmental p erformance and fuel p r operties of h ydrochar. R enewable and Su s tainable En e rgy Reviews, 123,109761.h t tps://doi.org/10.1016/j.rser.2020 . 109761 ) ( Shi, K., Oladejo,J. M ., Yan, J., & Wu, T. (2019). Investigation o n the interactions among lignocellulosic c onstituents and m inerals ofbiomass and t h eir influences on co-firing. E nergy, 179, 129-137.https://doi.org/10.1016/j.energy.2019.05.008 ) ( T rowbridge, P. J., & Bassuk,N. L. (2004) . Trees in the urban landscape:site assessment, design, and installation. John Wiley & Sons. ) ( Vamvuka, D . , K a karas, E., K a stanaki, E . , & Gr a mmelis, P. ( 2003). Pyrolysis characteristics and kinetics of biomass residuals mixtures with l ignite. F uel, 8 2(15), 1 9 49-1960. https://doi.org/10.1016/S0016-2361(03)00153-4 ) ( Wang, B .,F u , H., H an,L.,Xie, H., Xue, L.,Feng, Y.,& X i ng, B. (2020).Physicochemical properties of aged hydrochar in a r ice-wheat ro- tation system: A 1 6-month observation. Environmental Pollution, 272, 11 6037. https://doi.org/10.1016/j.envpol.2020.116037 ) ( Williams, C . L., Westover, T . L ., Emerson, R. M., T u muluru, J. S . , & Li, C. (2016). Sources of biomass feedstock variability and the po- tential impact on biofuels production. BioEnergy Research, 9 (1), 1-14. https://doi.org/10.1007/s12155-015-9694-y ) ( Wu, L . , Jiang, X., Lv, G . , L i , X . , & Yan, J. (2 0 20). Interactive effect of the sorted components of solid recovered f uel manufactured from municipal s olid w aste b y t h ermogravimetric an d kin e tic analy-sis. Waste M anagement, 102, 270-280. http s ://doi.org/10.1016/j. wasman.2019.10.054 ) Xiao, Y., Xue, Y., Gao, F., & Mosa, A. (2017). Sorption of heavymetal ions onto crayfish shell biochar: Effect of pyrolysis tem-perature, pH and ionic strength. Journal ofthe Taiwan Instituteof Chemical Engineers, 80, 114-121. https://doi.org/10.1016/j.itice.2017.08.035 ( Yuan, J .-H., Xu , R.-K., & Zhang, H. ( 2011 ) . The forms of alkalis inthe biochar produced f r om c r op residues at d i fferent t empera- tures. Bioresource Technology, 102(3), 3 488-3497. h t tps://doi.org/10.1016/j.biortech.2010.11.018 ) ( Zhang, W., & Yang, G. (1999). LY/T 1270-1999 Determination of total nitrogen, phosphorus, potassium, s odium, c alcium, m agnesium, ) iron, zinc, manganese, copper and sulphur in forest plant and forestfloor. In: Beijing. Zheng, G.,& Kozinski, J. A. (2000). Thermal events occurring duringthe combustion of biomass residue. Fuel, 79(2), 181-192. https://doi.org/10.1016/S0016-2361(99)00130-1 SUPPORTING INFORMATION Additional supporting information may be found online inthe Supporting Information section. How to cite this article: Kang, K., Zhang, T., Sun,G., Zhu, M., Li, K., & Li, D. (2021). Valorization oftree leaves waste using microwave-assistedhydrothermal carbonization process. GCB Bioenergy,00, 1-14. https://doi.org/10.1111/gcbb.12882 利用微波辅助的水热碳化工艺对树叶废料进行价值化处理水热碳化过程01前言:景观树的落叶,作为一种新兴的生物质废物,利用常规水热碳化(HC)和微波辅助水热碳化(MHC)预处理,并比较表征为理化性质和热降解动力学。结果表明MHC 优于传统的 HC 操作,因为在 200℃,MHC 过程不仅提供更高的水炭产量(45.09对39.47 wt%),同时显着降低能耗(0.63 对 2.74 MJ g-1),而且在去除 K 和 Si 方面也更有效。对于等转化动力学分析,FWO 方法提供比 KAS 方法更好的结果,因为后者未能拟合树叶样本(R2 < 0.9)。高温(>400℃)下的热降解动力学表明从 MHC 工艺获得的水炭具有较低的平均活化能量比传统的 HC 工艺(~260 MJ kg-1)高约 190 MJ kg-1。这研究揭示了通过 MHC 对景观树木废物进行增值的潜力过程。02简介: 生物质能源可以极大地促进缓解严重依赖碳的部门的温室气体排放。(Reid et al., 2020)。在种类繁多的生物质原料中,农业废弃物和林业残余物是研究最多的(Williams等人,2016)。然而在城市地区栽培的景观树的落叶是一种新的生物质替代来源,然而,这通常是被低估并与其他城市固体废物一起处置。 具体而言,随着城市化进程的加快,许多种植景观树用于绿化、装饰、降噪和空气净化(Trowbridge& Bassuk ,2004)。 这些树上的落叶迅速大量积累。然而,与那些在林场,它们不受天然微生物的影响由于城市地区的地面通常被砖、混凝土、水泥或沥青覆盖。 目前,为了快速处理,落叶往往燃烧,造成严重的空气污染。落叶的激增需要有效的增值。 尽管木质纤维素生物质通常很容易获得,但其预处理仍然是最关键的处理之一,由于其复杂的层次结构和顽固的性质,生物质利用面临挑战(Hassan等人,2018 年)。节能、环保的生物质增值技术是非常可取的。考虑到生物或化学过程的优点,一个城市可以积累大量落叶,热化学预处理似乎更有希望用于能量回收,因为它速度快、环境友好,并且易于扩展(Adams 等人,2018 年;Patel 等人,2016 年)。 水热碳化是一种高效的热化学预处理技术,在亚临界水的作用下,将生物质原料转化为碳。在亚临界水的存在下转化生物质原料(Alper等人。2020). 经过生物质预处理后,其能量密度、可研磨性、疏水性和易储存性将得到极大改善(戴等人,2019;王等人,2020)。从水热碳化过程中得到的水焦油,正在研究用于生物燃料(Sharma等人,2020)、污染物吸附(Liu等人,2021)、土壤改良(Wang等人,2020年)、土壤固碳(Baronti等人,2017年)和它在减缓气候变化方面的潜力(Owsianiak等人。2018).基于不同的加热方法,传统水热碳化(HC)和微波辅助的水热碳化(MHC)正在开发中。与广泛研究的水热过程相比,MHC过程作为一项有吸引力的技术出现(Gao等人,2021)。首先,微波加热在生产各种产品(如水焦)时,对能源的需求较少,成本效益更高(Nizamuddin等人。2018).第二,微波加热过程减少了处理时间,因为其快速的体积加热。第三,由于停留时间相对较短,提高了处理能力,将原始生物质转化为所需产品(Afolabi等人,2017)。此外,在生物质价值化过程中,微波可以促进新的途径,这取决于所使用的原料的独特属性。被使用的原料的独特属性(Lei等人,2021年;Liu,Zhong,etal., 2021)。 只有少数几个报告直接比较了从HC和MHC工艺中获得的水焦。戴等人(2017年)通过HC和MHC工艺从竹子锯末中生产了水焦油,通过HC和MHC工艺生产水焦,并比较了这些水焦的特性和热解行为。他们发现,碳氢化合物的热稳定性更高。Elaigwu和Greenway(2016年)比较了从Prosychon和MHC生产的水焦的化学和结构特性。从非洲豹生产的水焦的化学和结构特性,他们得出结论,MHC过程的碳化速度比HC快,表现为在较短的时间有较高的转化率。原料的内部水分含量原料是有利的,因为水分子可以与电磁场耦合并促进微波介质加热加(Kannan等人,2017)。以前的研究显示,这两种工艺都可以产生水炭,而且根据制备条件的不同,水炭的特性可能会有很大的变化。微波加热引起的结构变化可能会对热降解产生深远影响,这需要进一步阐明。此外,为了确定使用水焦油作为生物燃料的可行性,需要对整体的工艺效率和热化学反应进行研究。就我们所知,通过水热预处理将景观树叶转化为生物燃料的研究还很少。加热方法和碳化温度对落叶水炭的产量和理化性质的影响还没有得到证实。此外。不同的加热方法对水炭热化学动力学的影响还没有比较HC和MHC,动力学分析将使人们深入了解水炭的热降解机制,从而可以选择和优化后续的转化过程。并为后续的反应器设计提供有价值的参考(Cai等人2019;Damartzis等人,2011)。 因此,本研究旨在(1)比较研究产量和理化性质。进行比较研究,采用两种不同的加热方法,每种方法都有四个不同的反应温度,(2)比较树叶预处理的HC和MHC工艺的能源消耗,以及比较树叶的热降解动力学,(3)使用不同的等效分析方法比较不同水热工艺生产的水焦的热降解动力学方法。本研究报告的结果将为如何通过不同的碳化预处理方法使落下的树叶增值提供重要参考,并有助于设计后续水炭利用过程的设计。03祥鹄仪器在此文献中的使用过程:MHC实验采用多功能微波合成仪(型号XH-8000 Plus,北京祥鹄科技发展有限公司)。其原理图见图1,详细规格见我们以前的报告(Kang et al,反应容器由聚四氟乙烯(PTFE)制成,由聚醚醚酮(PEEK)保护壳,温度和压力的上限为260℃和4MPa。微波输入功率根据温度设置自动调整。在炭化后,在环境温度下进行样品采集。水炭和液相的混合物被转移到一个干净的烧杯中,然后用WhatmanTM 2级定性过滤纸(8微米颗粒保留率为98%)。水炭被用去离子水洗净,在105℃的烘箱中干燥12小时,然后储存在玻璃瓶中以备进一步分析。04结论: 在生物质碳化过程中,三个重要因素需要考虑,包括能耗、水炭产量和性能。 通常,使用较少的能源生产高质量的水炭是非常可取的。MHC 过程中水炭的质量产率为高于 HC,其他研究人员在比较使用花生壳或竹锯末作为原料(Chu et al.,2017; 戴等人,2017)。 结果表明,MHC过程中有机成分的损失较少,因为分解程度降低。 MHC 工艺的能耗降低与微波加热的优点,例如效率高与传统加热方法相比,选择性加热(戴等人,2019)。 水炭最重要的应用之一作为一种固体生物燃料使用。在这项研究中,获得了全面的表征结果,以了解在碳化过程中与燃料有关的特性的演变。水炭的灰分含量在100-140℃之间有所增加。其他研究人员也报告了类似的增加(Dai等人,2017;He等人2013;Kalderis等人,2014),但与我们在应用MHC时观察到的情况不同。原料和操作条件的不同,可能是造成这种差异的原因,反应温度对灰分变化的影响。幸运的是,从燃料生产的角度来看,除了Ca之外,大多数碱金属(如Na和K)的含量在生物质前体中的含量都被还原了,这些碱金属盐通常有较低的熔点,并且在水炭燃烧过程中往往会造成严重的问题。我们还看到,MHC工艺在降低水炭中的Si浓度方面比HC工艺更有效,这是有好处的,因为当与大量的K结合在一起时,K2O-SiO2的形成会导致顽固的表面沉积在火炉边和加热表面上,由于其550℃的低熔点(Niu等人,2016)。碳含量的富集和水炭HHV的提高并不会对水炭产生不利影响。微波辅助加热不会对碳含量的富集和水炭HHV的提高产生不利影响。这些特性在很大程度上取决于反应温度。200℃在水炭产量和燃料质量之间提供了一个合理的折中点。在这两个过程中,HHV随温度的变化是相似的,并且在温度上升到170℃之前都不显著。这表明,在这个温度以上,半纤维素的有效去除和碳在这个温度或更高的温度下发生。如图S1所示,与原始TL相比,Ca元素在水焦油中被富集。钙盐具有低流动性和高熔点,导致较不明显的沉积问题,而增加钙的含量可能有助于减少结渣(Niu等人,2016)。因此,钙的富集改善了水炭的燃料特性。水炭MHC-200显示出比HC-200更多的暴露的多孔结构。比HC-200更多。这可能与两种不同工艺的传热模式有关。对于HC,热量首先被转移到水里,然后转移到生物质颗粒的表面。相反,在MHC中,这种向内的热传递伴随着向外的热传递。因为生物质本身吸附了微波并被加热。然而,应该指出的是,由于生物质成分,包括半纤维素、纤维素和木质素在TL中的不均匀分布,以及它们不同的微波吸附能力,传热机制在不同的点上也会有所不同。即使在一个单一的生物质颗粒上,不同点的传热机制也会不同。 对于理解作为生物燃料的水焦油热解过程中发生的现象是必要的(Giudicianni等人,2013)。DTG曲线中包含的信息可以用来更好地理解所测试的水焦油的反应性,因为DTG曲线中的峰值位置(温度)和高度与生物燃料样品的反应性密切相关。峰值温度与它的反应性成反比。而峰高则与反应性成正比(Zheng & Koziński, 2000)。因此,当水热碳化温度增加时,纤维素分解的峰值被转移到更高的温度,但其高度也会增加。这表明,挥发性物质的更高水平导致了碳的富集,这使得它在较低的温度下反应性较差,但一旦它开始分解,就会释放更多的热量。 为了计算活化能,根据FWO或KAS方法绘制的线性回归图见图S3,以及它们的方程式和确定的相关系数(R2)。从低的R2值可以看出,使用这两种方法的回归通常在转换率(α) 低于0.1或高于0.9时,回归失败。其他研究人员也报道过这种现象(Damartzis等,2011。Ma等人,2019;Ren等人,2013)。在低转换率(α)为0.1时,相应的温度范围为130.1-143.7℃。在不同的加热斜率下,TL的对应温度范围为130.1-143.7℃,这主要是为了为去除水分。这发生在温度范围HC-200和MHC-200的温度范围分别227.6-245.1℃ 和220-242.8℃。200水焦的温度范围。达到10%的转化率的温度大大升高,说明在水炭生产过程中已经完成了水分的去除。表1的结果证实了这一点。这对燃料的利用是可取的因为在点火前需要较少的热量来进行去湿。在高转化率(α)为0.9时,TLs的温度为508.8℃,HC-200的温度为532.8℃,MHC-200的温度为608.6℃。在这些温度下。木质素、碳质残留物和TLs的矿物相可能发生复杂的反应,影响到表观回归关系的线性。此外。低R2值可能是由所使用的方法造成的。因为它表明,FWO和KAS的准确度方法在预测木质素分解时的生物质活化能方面的准确性会下降(Álvarez等人。2016). 这是因为木质素的分解过程结合了复杂的外热和内热反应竞争途径,限制了无模型分析方法的应用。(Kawamoto, 2017; Opfermann等人,2002)。 FWO 和 KAS 方法都准确地描述了HC 水炭的热分解在 α = 0.2-0.8 的转换范围内,这与趋势一致,由先前的一项研究报告(Ma 等人,2019 年)。与 TL 相比,水炭的 R2 值通常较高,也表明水热碳化过程将热化学不稳定的原料转化为更均匀的燃料,具有更高的可预测性热降解行为。这两种方法也适用于 MHC 数据;然而,最佳拟合范围转移到 0.1– 的较低转换0.6,在 0.7 和 0.8 转换时的回归给出低R2 值。对数据的仔细检查表明,FWO 和 KAS 方法在描述方面表现最好温度范围内的分解特性TLs 为 230–360℃,HC-200 水炭为 280–440℃,MHC-200 水炭为 220–370℃。因此,可以合理推断热降解机理MHC-200水炭的TL与此特定的TL相同范围,而对于HC水炭,这种共享分解行为转移到更高的温度。这个现象还表明 HC 过程比 MHC 更有效在 TL 中分解半纤维素。基于令人满意的回归结果(α= 0.2-0.6),计算平均活化能和R2值。即使在这个选择α范围内,KAS 方法也不起作用对TL来说很好,提供的平均R2仅为0.8516。作为前面提到,TL 的高度不均匀性使得KAS 方法在描述这种生物质原料的热降解方面无效(Ma et al., 2017)。此外,结果表明平均活化能降低在水炭样品中,表明水炭是在热降解过程中比 TL 更活跃。这是因为,在不同程度上,水热碳化过程破坏了复杂的木质纤维素基质TLs,它增强了热化学反应性水炭。 MHC-200 水炭显示较低的活化能量高于 HC-200 水炭(~190 kJ mol-1 vs. 260 kJ摩尔-1)。在热解过程中,高活化能意味着该反应需要更多来自周围环境的能量继续(Liu 等人,2004 年)。因此,在 200℃的制备温度下,MHC 工艺的性能优于HC 工艺在减少后续能源需求水炭热降解。综上所述,在树叶废弃物碳化预处理的试验条件下,MHC 工艺比 HC 更有效地产生显着更高的量具有较少能量消耗的水炭。在200℃的预处理温度下,生成的MHC水炭改进了燃料特性,提高了 HHV20.51 MJ kg-1(17.26 MJ kg-1 用于 TL 原料和HC 水炭为 20.41 MJ kg-1),降低了 K 和 Si 含量,并降低了后续热的活化能降解(~190 MJ kg-1 与 ~260 MJ kg-1 用于 HC 水炭)。等转化分析还表明,FWO方法适用于表征 TL 原料和HC/MHC 水炭,而 KAS 方法不适用由于其高度不均匀性,用于表征 TL。这些结果揭示了水炭的燃料性质和热降解机制如何受到水炭的影响。因此,HC 和 MHC 过程允许优化景观树叶作为替代生物质资源的增值过程的研究。05文献原文:END
确定








还剩12页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《生物质废物中水热碳化(MHC)预处理检测方案(微波合成仪)》,该方案主要用于林产品中前处理检测,参考标准--,《生物质废物中水热碳化(MHC)预处理检测方案(微波合成仪)》用到的仪器有多用途微波化学合成仪、Nanocube 多功能微波水热平行合成仪











