
方案详情
文
造粒是一种能够形成平均直径为0.5 - 2.0 mm的球形珠或球团的技术。这些微丸最终可以被包衣,经常用于控释剂型。 造粒和球团的使用可改善细粉的流动性、外观和混合性能,从而避免过多的粉尘和减少分离,一般来说,消除不良性能,改善细粉的物理或化学性能。 球团的制备有不同的技术,如挤压和滚圆,旋转造粒,溶液,悬浮或粉末分层,喷雾干燥或喷雾凝结。 本文的目的是回顾一些一般方面的球团和球团化和一些常用的技术在制药工业。 目前正在开发几种替代方法,如:热熔挤压、冷冻造粒、乳液/溶剂蒸发、使用泡沫水粘合剂造粒等。
方案详情

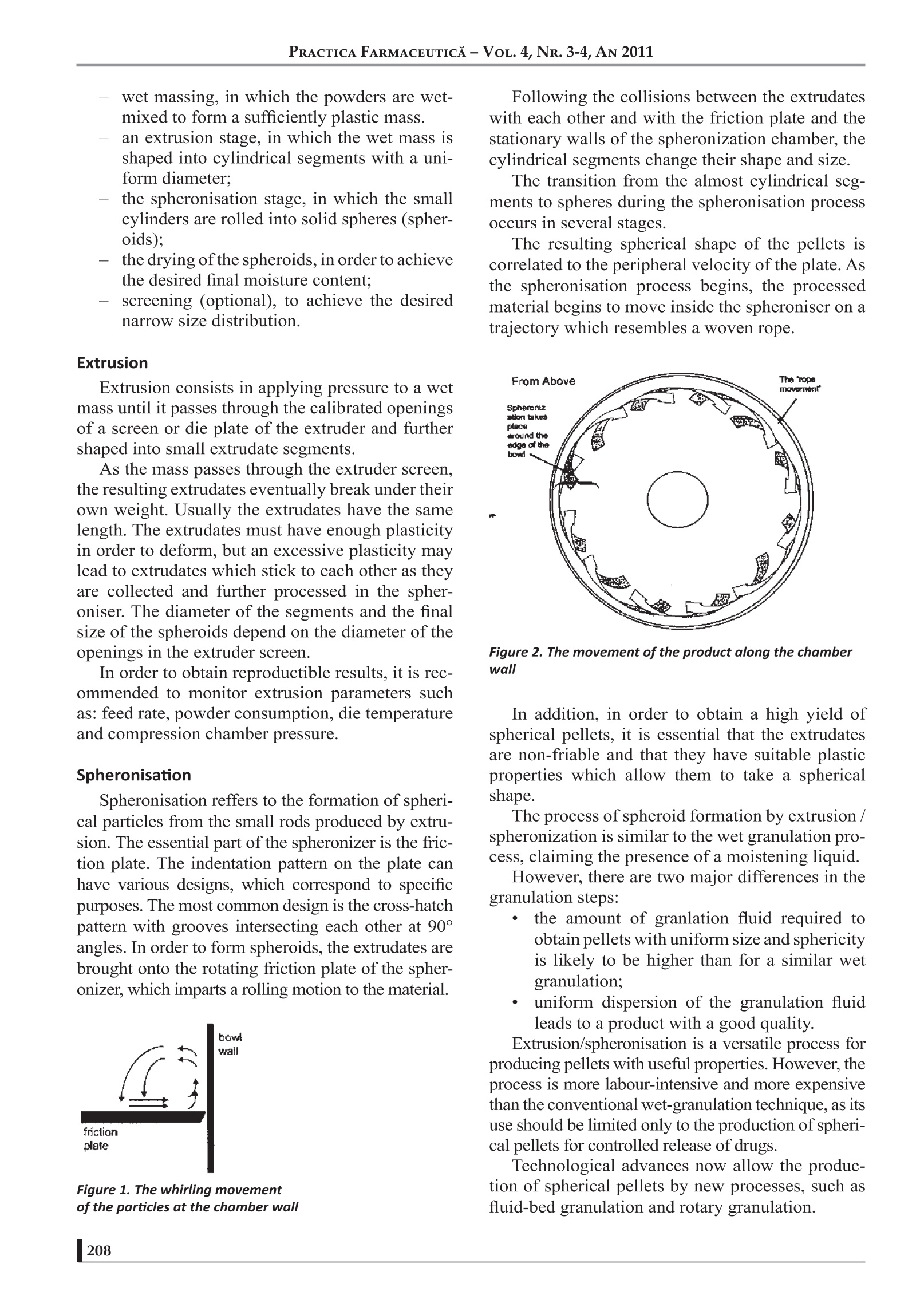
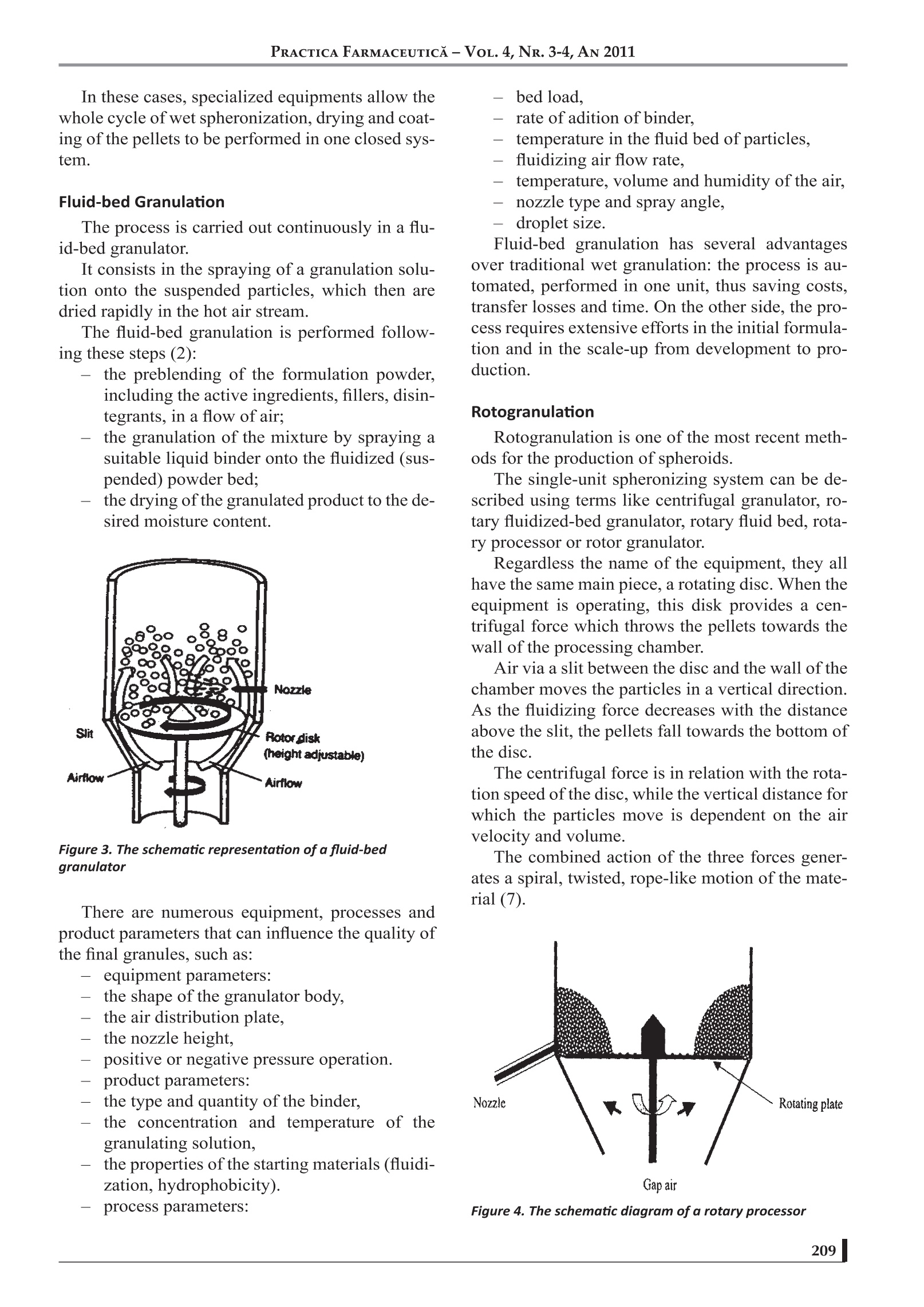
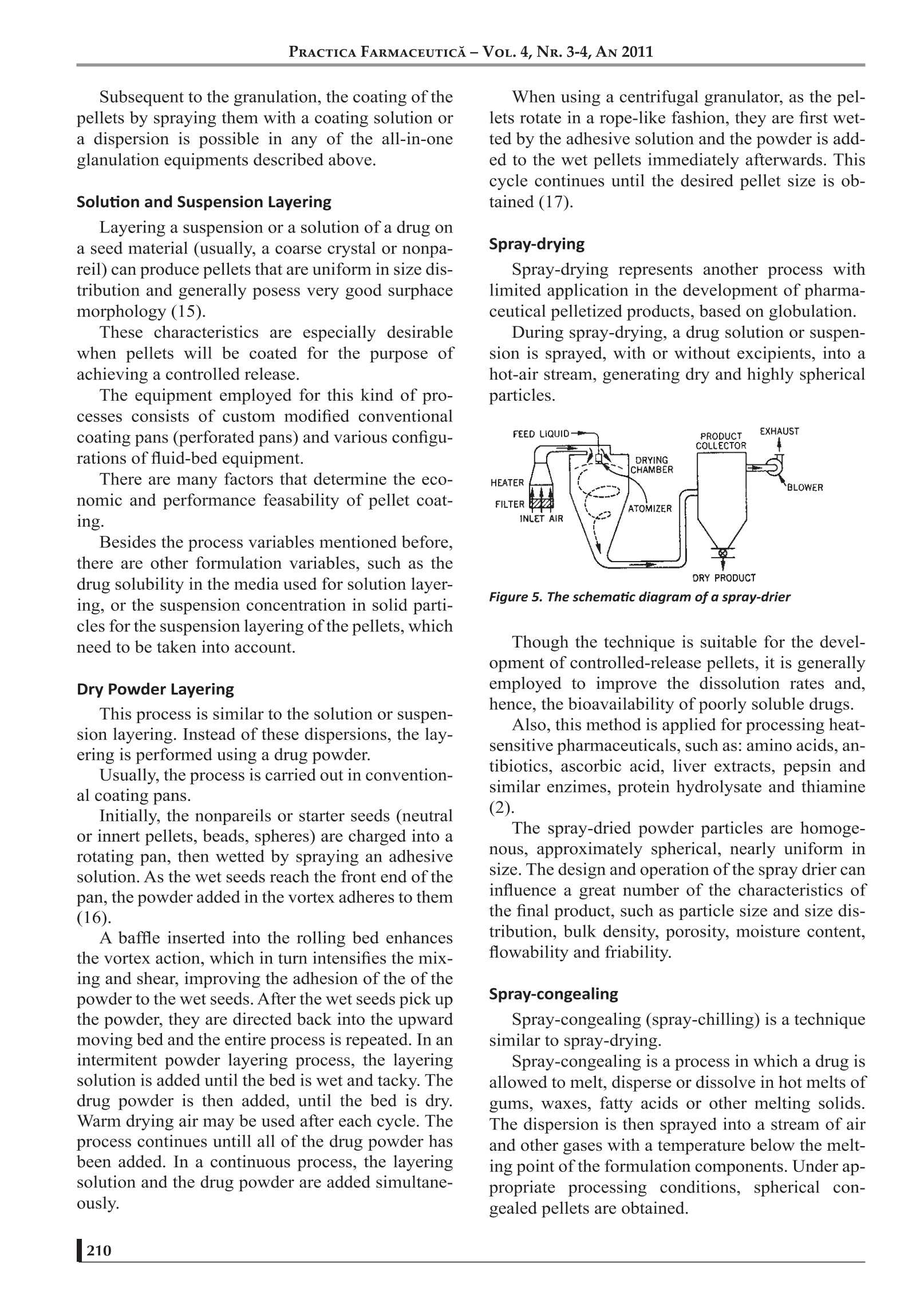
17 PRACTICA FARMACEUTICA-VOL.4, NR. 3-4, AN 2011 PELLETIZATION TECHNIQUES USEDIN PHARMACEUTICALFIELDS Mircea Hirjau, MD, Anca Cecilia Nicoara, MD, Victoria Hirjau, MD, PhD, D.Lupuleasa,MD, PhD Department of Pharmaceutical Technology and Biopharmacy, Faculty of Pharmacy, University of Medicine and Pharmacy “Carol Davila”, Bucharest, Romania -ABSTRACT—— Pelletization is a technique that enables the formation of spherical beads or pellets with a mean diameter usuallyranging from 0.5 to 2.0 mm. These pellets can evantually be coated and very often used in controlled-release dosageforms. The use of pelletization and pellets leads to an improvement in the flowability, appearance and mixing properties,thus avoiding excessive dust and reducing segregation and, generally, eliminating undesirable properties and improvingthe physical or chemical properties of fine powders. Pellets are prepared by different techniques, such as extrusion andspheronisation, rotogranulation, solution, suspension or powder layering, spray-drying or spray-congealing. The aim ofthis paper is to review some general aspects about pellets and pelletization and some common techniques used in thepharmaceutical industry. Several alternative methods are currently being developed, such as: hot-melt extrusion, freezepelletization, emulsion / solvent evaporation,granulation using foamed aqueous binders, etc. (1-7). INTRODUCTION Pelletization can be defined as an agglomeration(size-enlargment) process that converts fine pow-ders or particles of bulk drugs and excipients intosmall, free-flowing, more or less spherical units,called pellets (7). Granulation is also known as pelletization, ag-glomeration or spheronization, and the units ob-tained are referred to as granules, pellets, agglomer-ates or spheroids (8). The general terms“granulation”and“pelletiza-tion” are sometimes used synonymously and noclear distinction is made between them. Generally, if a size-enlargement process produc-es agglomerates of a size distribution within therange of 0.1 to 2.0 mm and a high porosity (about20-50%), the process may be called“granulation”,and the resulting agglomerates are called “granu-lates”. “Pelletization”is often referred to as a size-en-largement process that involves the manufacture ofagglomerates with a relatively narrow size range,usually with mean size from 0.5 to 2.0 mm, named“pellets". Pellets have free-flowing properties and alow porosity (about 10%). The term“spheronization”is more specific, usu-ally associated with spherical units formed by asize-enlargement process that includes a spheroni-zation step where extrudates or agglomerates arerounded as they tumble on a rotating frictional baseplate, being named“spheroids”(9). A SHORT HISTORY OF PELLETS Although various industries have routinely uti-lized pelletization processes since the turn of theXXth century in order to manufacture particles withdefined sizes and shapes, it was only in the early1950's, in response to a desire to sustain the release ( Prof.Dr. Victoria Hirjau, Faculty of Pharmacy, University of Medicine and Pharmacy "Carol Davila", Traian Vuia S treet, No 6, District 2, Bucharest ) e-mail: victoriahirjau@yahoo.com of drugs over an extended period of time, that thepharmaceutical industry developed a keen interestin the technology. In 1949, pharmaceutical scientists at Smith Kline& French (SKF) realised the potential of candyseeds in developing sustained-release preparationsand began the development of tiny drug pellets thatcould be loaded into capsules. In time, extensive research was conducted to de-velop pelletization techniques and major ressourceswere allocated towards exploring methods that werefaster, cheaper and more efficient, both in terms offormulation and processing equipment (8). The trend is expected to continue in the forsee-able future. Also,the role of pellets, especially of spheroids,in oral dosage form design and development has in-creased substantially during recent decades. Currently, pellets containing the active ingredi-ents are administered in the form of suspensions,capsules or tablets, a great number of this kinds ofpharmaceutical products being available on themarket. Also, pelletization is used in various industries,such as agriculture (fertilizers and herbicides), min-eral processing (iron ore pelletization), food anddetergent industry. REASONS FOR PELLETIZATION The pharmaceutical industry has developed agreat interest in pelletization due to a variety of rea-sons (10): -1prevention of segregation of co-agglomeratedcomponents, resulting in an improvement ofthe uniformity of the content; prevention of dust formation, resulting in animprovement of the process safety, as fine pow-ders can cause dust explosions and the respira-tion of fines can cause health problems; increasing bulk density and decreasing bulkvolume: -tthe defined shape and weight improves theappearance of the product; -1improvement of the handling properties, dueto the free-flowing properties; -1improvement of the hardness and friability ofpellets; (controlled release application of pellets dueto the ideal low surface area-to-volume ratiothat provides an ideal shape for the applica-tion of film coatings. All these aspects can be considered as techno-logical advantages of pelletization. Aditionally, the production of controlled-releasemultiparticulate oral dosage forms using spheroids,designed to deliver drugs at a specific site withinthe gastrointestinal tract or over an extended periodof time, leads to a series of therapeutic advantagesover conventional oral dosage forms (tablets orcapsules) (11), such as: pellets can disperse freely throughout an areaof the gastrointestinal tract after administra-tion and consequently the drug absorbtion ismaximized as a large gastrointestinal surfacecan be involved in this process; peak plasma level of the drug can be reducedby the use of spherical particles with differentrelease rates; potential side effects are mini-mized without markedly lowering drug bio-availability; the wide distribution of spherical particles inthe gastrointestinaltract limitslocalizedbuild-up of the drug, avoiding the irritand ef-fect of some drugs on the gastric mucosa; modified-release1multiparticulate dceliverysystems are less susceptible to dose dumpingthan single-unit dosage forms. But pellets also present some disadvantages:often pellets can not be pressed into tabletsbecause they are too rigid. In that case, pelletshave to be encapsulated into capsules. - the production of pellets is often an expen-sive process and / or requires highly special-ised equipment. the control of the production process is diffi-cult (e.g. the amount of water to be added iscritical for the quality of the pellets and over-wetting can occur very easily). METHODS OF PELLETIZATION Pelletization methods used in the pharmaceuti-cal industry can be grouped by various criteria, e.g.by the type of equipment used, the intensity of themechanical forces involved or the techniques em-ployed for the production of pellets. The success of these methods depends on thecomplex relations between the equipment, the for-mulation and process variables (12-14). Extrusion/ Spheronisation Extrusion / spheronisation is a multistage pro-cess for obtaining pellets with uniform size fromwet granulates (extrudates). The method involves the following main steps: the dry mixing of the ingredients, in order toachieve homogenous powder dispersions; wet massing, in which the powders are wet-mixed to form a sufficiently plastic mass. an extrusion stage, in which the wet mass isshaped into cylindrical segments with a uni-form diameter: -tthe spheronisation stage, in which the smallcylinders are rolled into solid spheres (spher-91dsi -tthedrying ofthe spheroids, in order to achievethe desired final moisture content: screening (optional), to achieve the desirednarrow size distribution. Extrusion Extrusion consists in applying pressure to a wetmass until it passes through the calibrated openingsof a screen or die plate of the extruder and furthershaped into small extrudate segments. As the mass passes through the extruder screen,the resulting extrudates eventually break under theirown weight. Usually the extrudates have the samelength. The extrudates must have enough plasticityin order to deform, but an excessive plasticity1.12 1ma.ylead to extrudates which stick to each other as theyare collected and further processed in the spher-oniser. The diameter of the segments and the finalsize of the spheroids depend on the diameter of theopenings in the extruder screen. In order to obtain reproductible results, it is rec-ommended to monitor extrusion parameters suchas: feed rate,powder consumption, die temperatureand compression chamber pressure. Spheronisation Spheronisation reffers to the formation of spheri-cal particles from the small rods produced by extru-sion. The essential part of the spheronizer is the fric-tion plate. The indentation pattern on the plate canhave various designs, which correspond to specificpurposes. The most common design is the cross-hatchpattern with grooves intersecting each other at 90°angles. In order to form spheroids, the extrudates arebrought onto the rotating friction plate of the spher-onizer, which imparts a rolling motion to the material. Figure 1. The whirling movementof the particles at the chamber wall Following the collisions between the extrudateswith each other and with the friction plate and thestationary walls of the spheronization chamber, thecylindrical segments change their shape and size. The transition from the almost cylindrical seg-ments to spheres during the spheronisation processoccurs in several stages. The resulting spherical shape of the pellets iscorrelated to the peripheral velocity of the plate. Asthe spheronisation process begins, the processedmaterial begins to move inside the spheroniser on atrajectory which resembles a woven rope. Figure 2. The movement ofthe product along the chamberwall In addition, in order to obtain a high yield ofspherical pellets, it is essential that the extrudatesare non-friable and that they have suitable plastic.1properties which allow them to take a sphericalshape. The process of spheroid formation by extrusion/spheronization is similar to the wet granulation pro-cess, claiming the presence of a moistening liquid. However, there are two major differences in thegranulation steps: the amount of granlation fluid required toobtain pellets with uniform size and sphericityis likely to be higher than for a similar wetgranulation; uniform dispersion of the granulation fluidleads to a product with a good quality. Extrusion/spheronisation is a versatile process forproducing pellets with useful properties. However, theprocess is more labour-intensive and more expensivethan the conventional wet-granulation technique, as itsuse should be limited only to the production of spheri-cal pellets for controlled release of drugs. Technological advances now allow the produc-tion of spherical pellets by new processes, such asfluid-bed granulation and rotary granulation. In these cases, specialized equipments allow thewhole cycle of wet spheronization, drying and coat-ing of the pellets to be performed in one closed sys-tem. Fluid-bed Granulation The process is carried out continuously in a flu-id-bed granulator. It consists in the spraying of a granulation solu-tion onto the suspended particles, which then aredried rapidly in the hot air stream. The fluid-bed granulation is performed follow-ing these steps (2): - the preblending of the formulation powder,including the active ingredients, fillers, disin-tegrants, in a flow of air; 1tthe granulation of the mixture by spraying asuitable liquid binder onto the fluidized (sus-pended) powder bed; the drying of the granulated product to the de-sired moisture content. Figure 3. The schematic representation ofa fluid-bedgranulator There are numerous equipment, processes andproduct parameters that can influence the quality ofthe final granules, such as: equipment parameters: the shape of the granulator body, the air distribution plate, -tthe nozzle height, positive or negative pressure operation. product parameters: the type and quantity of the binder, the concentration and temperature of thegranulating solution, —the properties of the starting materials (fluidi-zation, hydrophobicity). process parameters: bed load. rate of adition of binder. temperature in the fluid bed of particles, fluidizing air flow rate, temperature, volume and humidity of the air, nozzle type and spray angle, - droplet size. Fluid-bed granulation has several advantagesover traditional wet granulation: the process is au-tomated, performed in one unit, thus saving costs,transfer losses and time. On the other side, the pro-cess requires extensive efforts in the initial formula-tion and in the scale-up from development to pro-duction. Rotogranulation Rotogranulation is one of the most recent meth-ods for the production of spheroids. The single-unit spheronizing system can be de-scribed using terms like centrifugal granulator, ro-tary fluidized-bed granulator, rotary fluid bed, rota-ry processor or rotor granulator. Regardless the name of the equipment, they allhave the same main piece, a rotating disc. When theequipment is operating, this disk provides a cen-trifugal force which throws the pellets towards thewall of the processing chamber. Air via a slit between the disc and the wall of thechamber moves the particles in a vertical direction.As the fluidizing force decreases with the distanceabove the slit, the pellets fall towards the bottom ofthe disc. The centrifugal force is in relation with the rota-tion speed of the disc, while the vertical distance forwhich the particles move is dependent on the airvelo0cci1ty and volume. The combined action of the three forces gener-ates a spiral, twisted, rope-like motion of the mate-rial (7). Figure 4. The schematic diagram ofa rotary processor Subsequent to the granulation, the coating of thepellets by spraying them with a coating solution ora dispersion is possible in any of the all-in-oneglanulation equipments described above. Solution and Suspension Layering Layering a suspension or a solution of a drug ona seed material (usually, a coarse crystal or nonpa-reil) can produce pellets that are uniform in size dis-tribution and generally posess very good surphacemorphology (15). These characteristics are especially desirablewhen pellets will be coated for the purpose ofachieving a controlled release. The equipment employed for this kind of pro-cesses consists of custom modified conventionalcoating pans (perforated pans) and various configu-rations of fluid-bed equipment. There are many factors that determine the eco-nomic and performance feasability of pellet coat-ing. Besides the process variables mentioned before,there are other formulation variables, such as thedrug solubility in the media used for solution layer-ing, or the suspension concentration in solid parti-cles for the suspension layering of the pellets, whichneed to be taken into account. Dry Powder Layering This process is similar to the solution or suspen-sion layering. Instead of these dispersions, the lay-ering is performed using a drug powder. Usually, the process is carried out in convention-al coating pans. Initially, the nonpareils or starter seeds (neutralor innert pellets, beads, spheres) are charged into arotating pan, then wetted by spraying an adhesivesolution. As the wet seeds reach the front end of thepan, the powder added in the vortex adheres to them(16). A baffle inserted into the rolling bed enhancesthe vortex action, which in turn intensifies the mix-ing and shear, improving the adhesion of the of thepowder to the wet seeds. After the wet seeds pick upthe powder, they are directed back into the upwardmoving bed and the entire process is repeated. In anintermitent powder layering process, the layeringsolution is added until the bed is wet and tacky. Thedrug powder is then added, until the bed is dry.Warm drying air may be used after each cycle. Theprocess continues untill all of the drug powder hasbeen added. In a continuous process, the layeringsolution and the drug powder are added simultane-ously. When using a centrifugal granulator, as the pel-lets rotate in a rope-like fashion, they are first wet-ted by the adhesive solution and the powder is add-ed to the wet pellets immediately afterwards. Thiscycle continues until the desired pellet size is ob-tained (17). Spray-drying Spray-drying represents another process withlimited application in the development of pharma-ceutical pelletized products, based on globulation. During spray-drying, a drug solution or suspen-sion is sprayed, with or without excipients, into ahot-air stream, generating dry and highly sphericalparticles. Figure 5. The schematic diagram of a spray-drier Though the technique is suitable for the devel-opment of controlled-release pellets, it is generallyemployed to improve the dissolution rates and,hence, the bioavailability of poorly soluble drugs. Also, this method is applied for processing heat-sensitive pharmaceuticals, such as: amino acids, an-tibiotics, ascorbic acid, liver extracts, pepsin andsimilar enzimes, protein hydrolysate and thiamine(2). The spray-dried powder particles are homoge-nous, approximately spherical, nearly uniform insize. The design and operation of the spray drier caninfluence a great number of the characteristics ofthe final product, such as particle size and size dis-tribution, bulk density, porosity, moisture content,flowability and friability. Spray-congealing Spray-congealing (spray-chilling) is a techniquesimilar to spray-drying. Spray-congealing is a process in which a drug isallowed to melt, disperse or dissolve in hot melts ofgums, waxes, fatty acids or other melting solids.The dispersion is then sprayed into a stream of airand other gases with a temperature below the melt-ing point of the formulation components. Under ap-propriate processing conditions, spherical con-gealed pellets are obtained. The resultant material can be used for the pro-duction of prolonged-release dosage forms (2, 8). CONCLUSIONS In the later decades, pelletization has gained anincreased interest, especially due to the posibilitiesto use pellets in the development of modified-re-lease solid oral dosage forms. BIBLIOGRAFIE 1. Matei l. E., Pelete in luliana Popovici, D. Lupuleasa, TehnologieFarmaceutica, vol. 3, ed. Polirom, 2009, 355-381 2. Leucuta S. E., Tehnologie Farmaceutica Industriala, Ed. Dacia, ClujNapoca,2001, 269-288; 3.Summers M., Aulton M., Granulation, in Pharmaceutics: The science ofDosage Form Design, ed. by Aulton M., Churchill-Livingstone, 2002, 364-378: 14.Ke1arey M. C., Sheskey P. J., Drug Dev. And Ind. Pharm., vol. 30, No. 8,831-832,2004; 5.Cheboyina S., Walter G. C., Wyandt C. M., A Novel Freeze PelletizationTechnique for Preparing Matrix Pellets, Pharm. Technology, Oct., 2004,98: 6.Crowley M. M. at all., Pharmaceutical Applications of Hot-melt Extrusion:Part l., Drug Dev. Ind. Pharm., 33, 909-926,2007; 7. Palmieri F. G., Grifantini R., Di Martino P., Martelli S., Emulsion/Solventevaporation as an Alternative Technique in Pellet Preparation, Drug Dev.Ind. Pharm., 26 (11),1151-1158,2000; 8.Sovgren K., Pellet preparation, in Industrial Aspects of Pharmaceutics,Ed. Sandell E., Swedish Pharmaceutical Press, Stockholm, 1992,200-212: 9.Ghebre-Sellasie l., Pellets: a general view, in PharmaceuticalPelletization Technology; Ed.Marcel Dekker Inc., New York, 1989,1-13; Currently there are several pelletization meth-ods, the most widely used being etxrusion/ spher-onization. Due to the good technological, pharmacokineticand biopharmaceutic characteristics of the pelletsand the flexibility of the maufacturing processes in-volved, pellets are expected to continue to play amajor role in design and fabrication of solid dosageforms. 10.L. Gu, C. V. Liew, P. W. S. Heng, Wet Spheronization by RotaryProcessing- A Multistage Single-Pot Process for Producing Spheroids;Drug Dev. Ind. Pharm., vol. 30, No. 2., 111-123,2004; ( 11. * ** Caleva processing solutions; ) 12. Bechegaard H., Nielson G. H., Controlled Release Multiple Unit andSingle Unit Doses, Drug Dev. Ind. Pharm., 1978, 4, 53-67; ( 13. Kristensen H. G., Agglomeration of powder, Acta Pharm. Succ., 1988, 25,187 -2 04; ) ( 14. Schwartz J. B., Granulation, Drug Dev. Ind. Pharm., 1988, 14 (4), 2071 - 2090; ) 15. Kristensen H. G., Schaefer T., Granulations in Encyclopedia ofPharmaceutical Technology, Swabrick J., Boylan J. C., Eds. MarcelDekker Inc., New York, 1993, vol. 7, 121-160; 16. David M. Jones, Solution and Suspension Layering, in PharmaceuticalPelletization Technology, ed. by Ghebre-Sellasiel., Ed. Marcel DekkerInc., New York, 1989, 145-165; ( 17. Sinchaipanit N et all., Influences of Layering Process on Theophylline P ellet Characteristics , Pharm. Dev. Tech., 9 , 136 -1 70,2004; ) 18. Goodhart F. W., Jan S., Dry Powder Layering, in PharmaceuticalPelletization Technology, ed. by Ghebre-Sellasie I., Ed. Marcel DekkerInc., New York, 1989, 165-187. RACTICA FARMACEUTICA-VOL.,NR. -, AN 造粒是一种能够形成平均直径为0.5 - 2.0 mm的球形珠或球团的技术。这些微丸最终可以被包衣,经常用于控释剂型。 造粒和球团的使用可改善细粉的流动性、外观和混合性能,从而避免过多的粉尘和减少分离,一般来说,消除不良性能,改善细粉的物理或化学性能。 球团的制备有不同的技术,如挤压和滚圆,旋转造粒,溶液,悬浮或粉末分层,喷雾干燥或喷雾凝结。 本文的目的是回顾一些一般方面的球团和球团化和一些常用的技术在制药工业。 目前正在开发几种替代方法,如:热熔挤压、冷冻造粒、乳液/溶剂蒸发、使用泡沫水粘合剂造粒等。 在后来的几十年里,球团化得到了广泛的应用兴趣增加,特别是由于微丸在开发改性缓释固体口服剂型方面的可能性。目前有几种造粒方法,应用最广泛的是溶出/球化。由于微丸具有良好的技术、药代动力学和生物药剂学特性以及所涉及的制造工艺的灵活性,微丸有望继续在固体剂型的设计和制造中发挥重要作用。
确定



还剩4页未读,是否继续阅读?
嘉盛(香港)科技有限公司为您提供《微丸、包衣中医药检测方案(挤出机)》,该方案主要用于复合材料中医药检测,参考标准--,《微丸、包衣中医药检测方案(挤出机)》用到的仪器有实验室挤出滚圆机、湿法制粒挤出滚圆一体机、德国DIOSNA中试流化床(喷雾造粒干燥机) MidiLab 、实验型高速混合湿法造粒机、实验室超微量包衣机
相关方案
更多
该厂商其他方案
更多












