方案详情
文
我们报告了一种新型的、有效的LLO表面改性方法,使用一种基于Zr的MOF前驱体来生产原位MOF衍生的二氧化锆涂层。
Zr基MOF前体,生产原位MOF衍生的ZrO2涂层。
TFBDC的强酸性特征诱导了H+-Li+交换。
在富含锂的层状氧化物颗粒的表面进行H+-Li+交换,从而促进了Zr-TFBDC的均匀涂覆。
这有利于Zr-TFBDC的均匀涂层,并在LLO微球上形成明显的多孔结构。
LLO微球。ZrO2很容易在LLO的表面形成,F被成功地掺入LLO颗粒中。
通过简单的一步式MOF辅助改性,成功地将F掺入到LLO颗粒中。在这项研究中,电化学性能,如
速率能力、循环能力和LLO的首次库仑效应。
在MOF衍生的ZrO2包覆的LLO(MDZ@LLO)中的电化学性能得到了明显的改善和显著提高。
方案详情

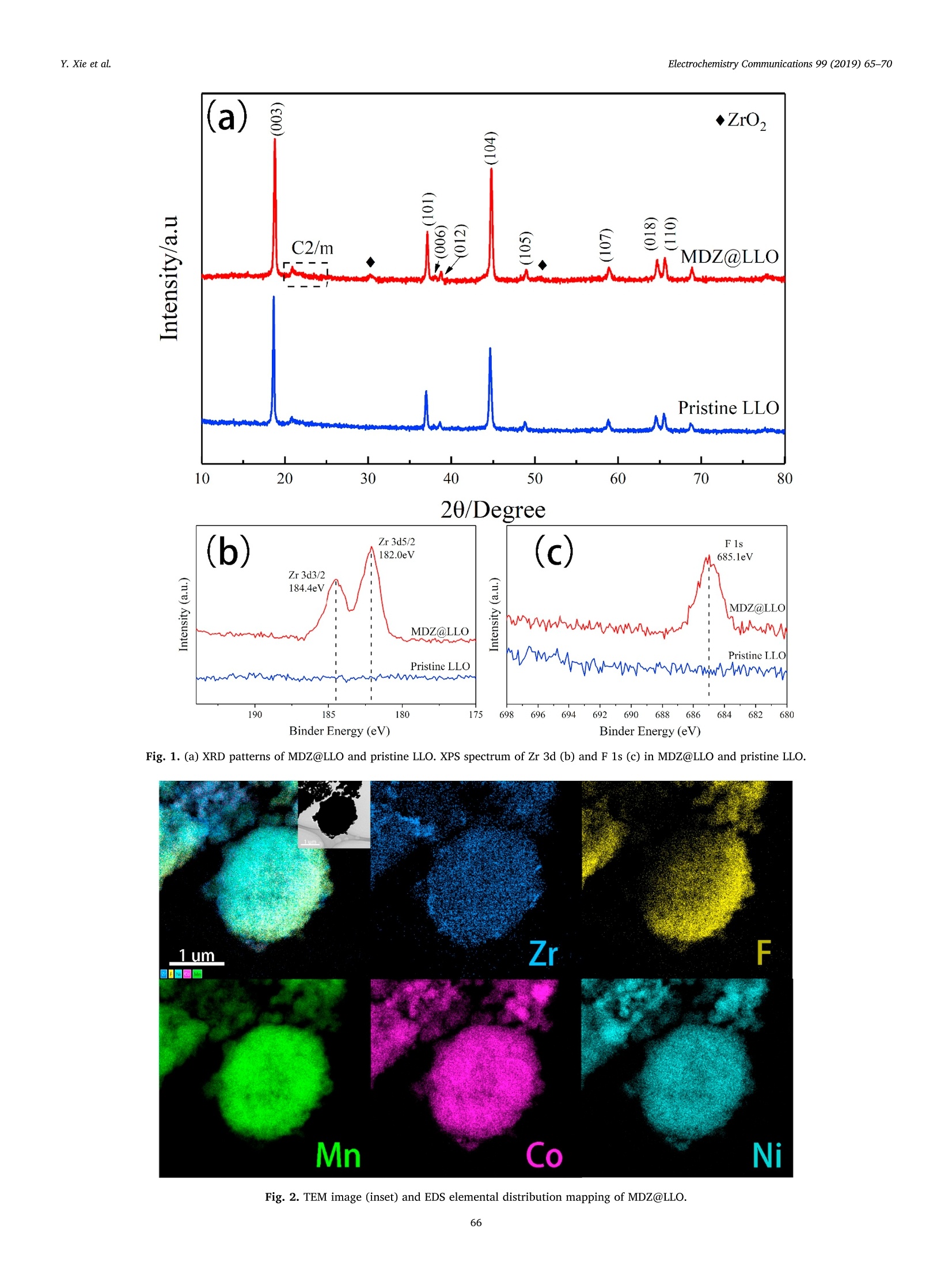
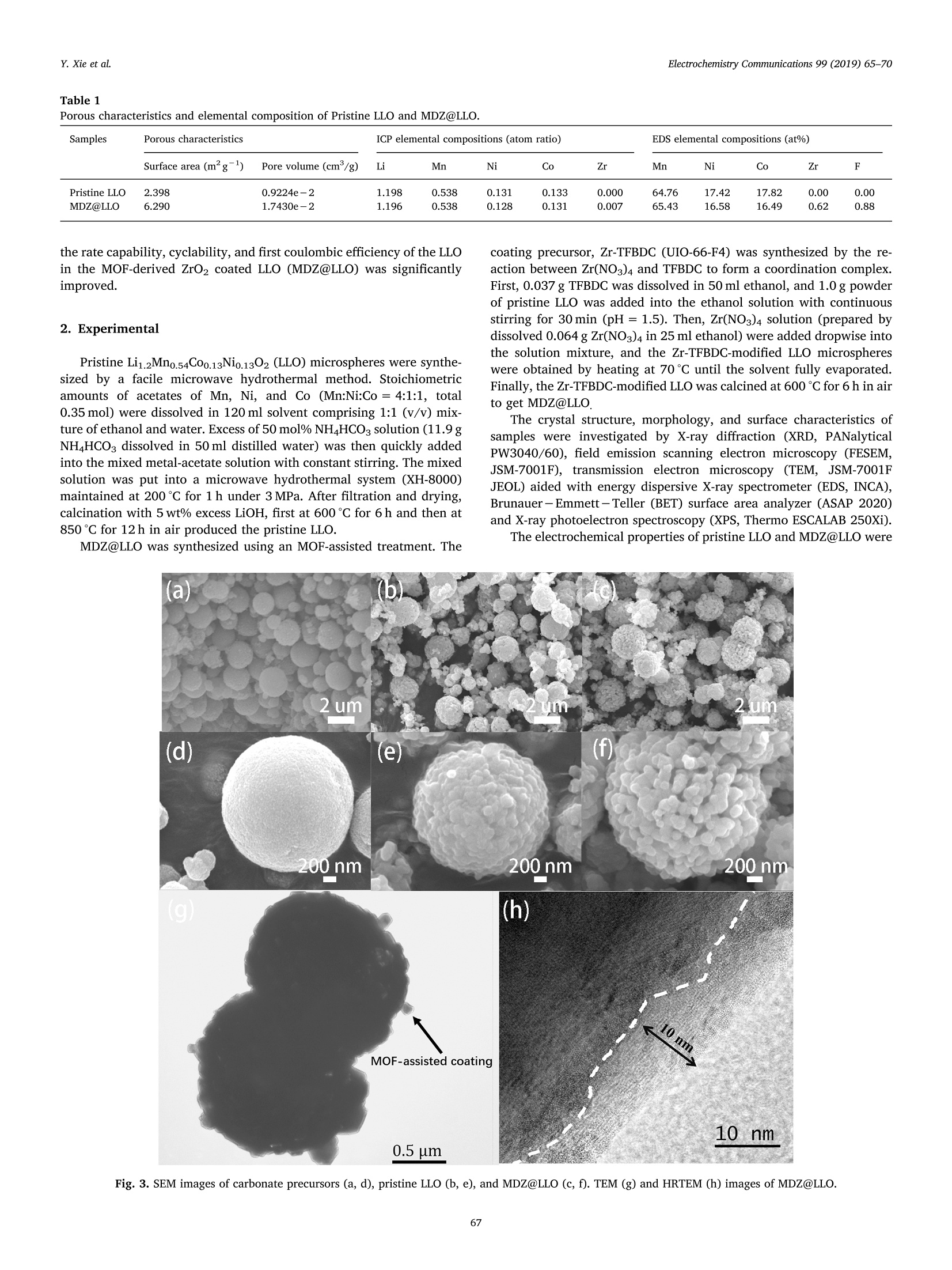
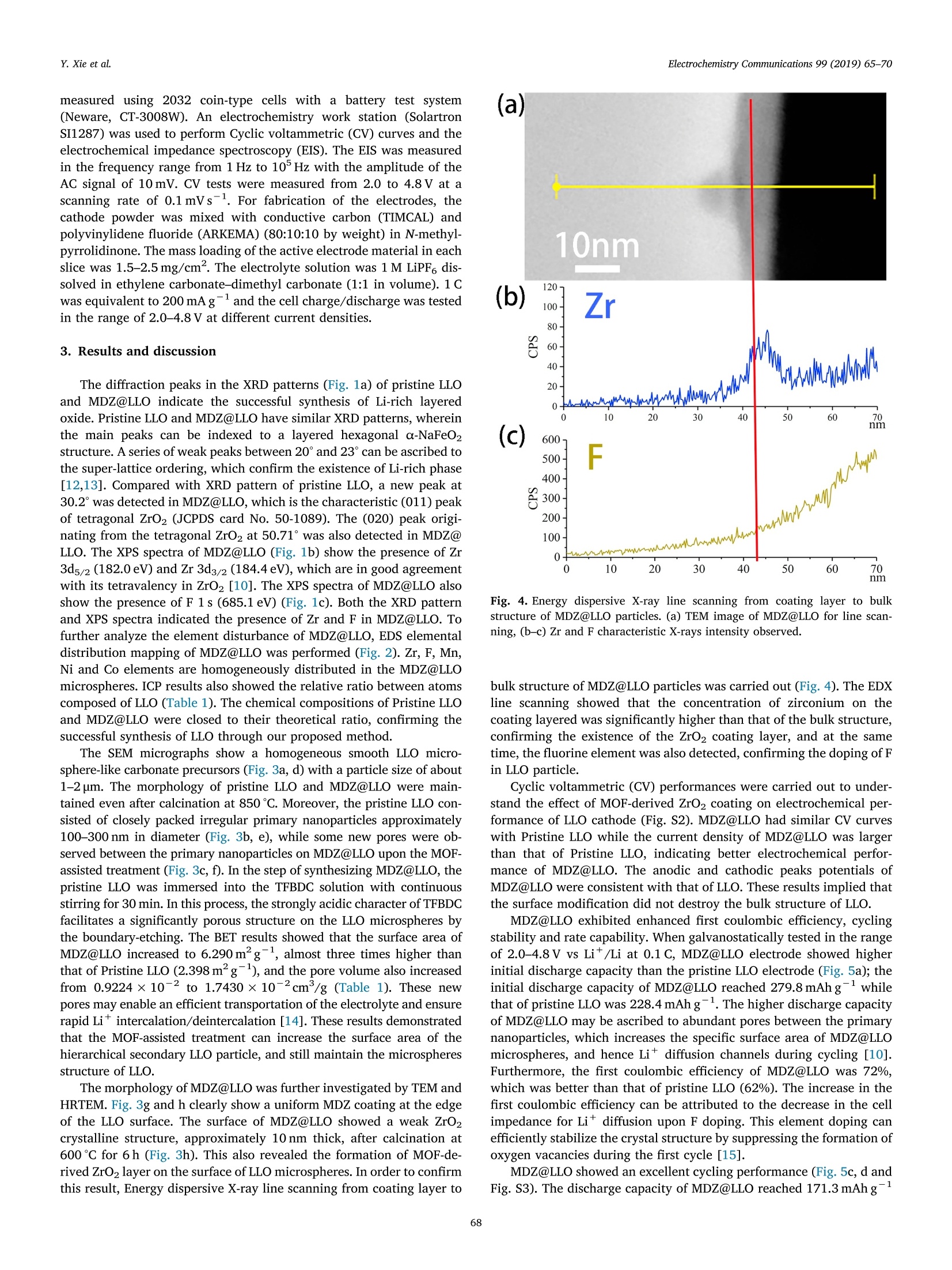
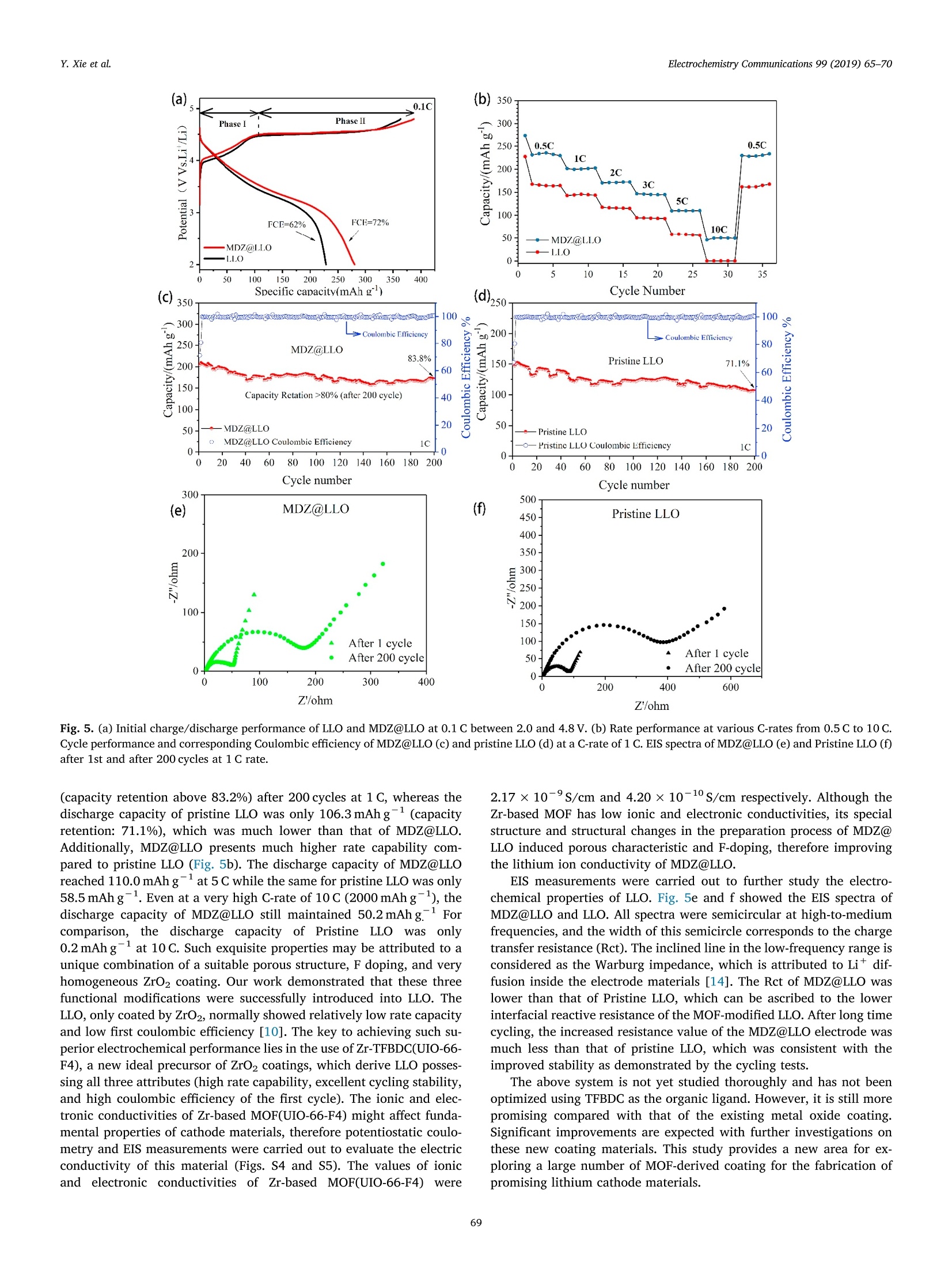
Electrochemistry Communications 99 (2019) 65-70 Contents lists available at ScienceDirect Electrochemistry Communications journal homepage:www.elsevier.com/locate/elecom Short communication Enhanced electrochemical performance of Li-rich layered oxide,Li1.2Mno.54Co0.13Nio.13O2, by surface modification derived from aMOF-assisted treatment Yuxiang Xie, Shengzhou Chen *, Zhuoying Lin, Wei Yang, Hanbo Zou",Raymond Wai-Yin Sun° School of Chemistry and Chemical Engineering, Guangzhou University, Guangzhou 510006, China 'Guangzhou Key Laboratory for New Energy and Green Catalysis, Guangzhou University, Guangzhou 510006, China ‘Guangzhou Lee & Man Technology Company Limited, Guangzhou 510000, China A RTICLEINF O ABSTRACT Keywords:Lithium-ion batteryLithium-rich layered oxide materialSurface modificationFluorine doping We developed a promising cathode material prepared by effective surface modification of Li-rich layered oxide(LLO) materials, using a Zr-based MOF (UIO-66-F4), as a precursor to produce in-situ MOF-derived ZrO2 (MDZ)coatings. A new method for F doping on LLO was also proposed. The MOF-assisted treatment renders a uniformnanoscale coating of ZrO2 and a porous structure of Li1.2Mno.54Co0.13Nio.13O2 hierarchical secondary micro-sphere. The rate capability, cycling stability, and first coulombic efficiency of LLO were significantly improvedby the MOF-assisted treatment. The discharge capacity of the MOF-derived ZrO coated LLO (MDZ@LLO) ma-terial was 279 and 110.0 mAhgat 0.1 C and 5C, respectively. The capacity retention increased from 71.1% to83.8% after 200 cycles at 1C while the coulombic efficiency increased from 62% to 72% during the first cycle. Li-rich layered oxides (LLO), either as a solid solution or as a na-nocomposite of layered LiMnO and Li(M)02(M=Mn, Ni, Co), haveattracted much attention as one of the most promising candidates forcathode material in lithium ion batteries. They have high energy den-sity of over 800 Wh gand are low cost and less toxic (composed of asmall amount of Co and Ni) [1-4]. However, to obtain a high specificcapacity, the potential of Li-rich layered oxides must be charged up to4.5 V platform in the first cycle, which induces the partial extraction ofLi*as Li2O. Moreover, the continuous layered-to-spinel transformationof the surface microstructure during charge/discharge process leads tolow rate capability and capacity fading of LLO [5-7]. The low ratecapacity, poor coulombic efficiency of the first cycle, and low cyclingstability hinder the commercial application of LLO. It has been verified that surface coating can greatly improve thecycling stability of LLO. For example, the thin layers of Al203, SiO2, andTiO2 coated on LLO can protect the surface of LLO from electrolytecorrosion and suppress the side reactions and decelerate the oxygenrelease from LLO at high potential [8,9]. Although these coatings caneffectively enhance the cycle performance of LLO, they usually havelimited contribution in enhancing the rate performance and coulombic efficiency. Furthermore, a uniform coating of the metal oxide over theentire particle is difficult in an ex situ solvent evaporation method [10].So, it is very desirable to explore new surface coating method to furtherimprove properties in LLO. Metal-organic frameworks (MOFs), a class of materials with highsurface area and abundant Li* diffusion channels, are promising pre-cursors for functional hybrid materials with significant properties inelectrochemical applications [8]. Herein, we report a novel and effec-tive surface modification of LLO, using a Zr-based MOF (UIO-66-F4)precursor, to produce in situ MOF-derived ZrO2 (MDZ) coatings. TFBDC(2,3,5,6-tetrafluoro-1,4-benzenedicarboxylic acid) is a strong organicacid with four extremely strong electron withdrawing F substituents. Itsstrongly acidic character induces the H+-Liion exchange (protona-tion) on the surface of the Li-rich layered oxide particles, which facil-itate a significantly porous structure on the LLO microspheres [11].Additionally, TFBDC usually tends to aggregate near alkali metal oxideLLO and thereby forms the uniform coating of Zr-TFBDC. ZrO2 can beeasily produced on the surface of LLO through a facile heat treatment;this uniform coating protects the LLO from the direct contact with theelectrolyte, and does not hinder the transport of Lit. Meanwhile, F wassuccessfully doped into LLO particles by the facile one-step MOF-as-sisted modification. In this study, electrochemical performances such as ( E-mail address: sz c hen@ g zhu . edu . cn ( S. Chen). ) ( https://doi.org/ 1 0.1016/j. e lecom.2019.01.005 ) ( R eceived 1 3 November 2 0 18; Received in r evised form 3 January 2019; Accepted 9 January 2019 ) ( Available online 10 January 2019 ) ( 1388-2481/ C 2019 Pub l ished by Elsevier B.V. Thi s is an open access article under the CC BY- N C-ND license ) 20/Degree Binder Energy(eV) Fig. 1. (a) XRD patterns of MDZ@LLO and pristine LLO. XPS spectrum of Zr 3d (b) and F 1s (c) in MDZ@LLO and pristine LLO. Fig. 2. TEM image (inset) and EDS elemental distribution mapping of MDZ@LLO. Porous characteristics and elemental composition of Pristine LLO and MDZ@LLO. Samples Porous characteristics ICP elemental compositions (atom ratio) EDS elemental compositions (at%) Surface area (mg) Pore volume (cm/g) Li Mn Ni Co Zr Mn Ni Co Zr F Pristine LLO 2.398 0.9224e-2 1.198 0.538 0.131 0.133 0.000 64.76 17.42 17.82 0.00 0.00 MDZ@LLO 6.290 1.7430e-2 1.196 0.538 0.128 0.131 0.007 65.43 16.58 16.49 0.62 0.88 the rate capability, cyclability, and first coulombic efficiency of the LLOin the MOF-derived ZrO2 coated LLO (MDZ@LLO) was significantlyimproved. 2. Experimental Pristine Li1.2Mno.54Coo.13Nio.1302 (LLO) microspheres were synthe-sized by a facile microwave hydrothermal method. Stoichiometricamounts of acetates of Mn, Ni, and Co (Mn:Ni:Co=4:1:1, total0.35 mol) were dissolved in 120 ml solvent comprising 1:1 (v/v) mix-ture of ethanol and water. Excess of 50 mol% NH4HCO3 solution (11.9 gNHHCO3 dissolved in 50 ml distilled water) was then quickly addedinto the mixed metal-acetate solution with constant stirring. The mixedsolution was put into a microwave hydrothermal system (XH-8000)maintained at 200℃ for 1 h under 3 MPa. After filtration and drying,calcination with 5 wt% excess LiOH, first at 600℃ for 6h and then at850°℃ for 12 h in air produced the pristine LLO. MDZ@LLO was synthesized using an MOF-assisted treatment. The coating precursor, Zr-TFBDC (UIO-66-F4) was synthesized by the re-action between Zr(NO3)4 and TFBDC to form a coordination complex.First, 0.037 g TFBDC was dissolved in 50 ml ethanol, and 1.0 g powderof pristine LLO was added into the ethanol solution with continuousstirring for 30 min (pH=1.5). Then, Zr(NO3)4 solution (prepared bydissolved 0.064g Zr(NO3)4 in 25 ml ethanol) were added dropwise intothe solution mixture, and the Zr-TFBDC-modified LLO microsphereswere obtained by heating at 70℃ until the solvent fully evaporated.Finally, the Zr-TFBDC-modified LLO was calcined at 600℃ for 6 h in airto get MDZ@LLO. The crystal structure, morphology, and surface characteristics ofsamples were investigated by X-ray diffraction (XRD, PANalyticalPW3040/60), field emission scanning electron microscopy (FESEM,JSM-7001F), transmission electron microscopy (TEM, JSM-7001FJEOL) aided with energy dispersive X-ray spectrometer (EDS, INCA),Brunauer-Emmett-Teller (BET) surface area analyzer (ASAP 2020)and X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi). The electrochemical properties of pristine LLO and MDZ@LLO were Fig. 3. SEM images of carbonate precursors (a, d), pristine LLO (b, e), and MDZ@LLO (c, f). TEM (g) and HRTEM (h) images of MDZ@LLO. measured using 2032 coin-type cells with a battery test system(Neware, CT-3008W). An electrochemistry work station (SolartronSI1287) was used to perform Cyclic voltammetric (CV) curves and theelectrochemical impedance spectroscopy (EIS). The EIS was measuredin the frequency range from 1 Hz to 10 Hz with the amplitude of theAC signal of 10 mV. CV tests were measured from 2.0 to 4.8 V at ascanning rate of 0.1 mVs-1. For fabrication of the electrodes, thecathode powder was mixed with conductive carbon (TIMCAL) andpolyvinylidene fluoride (ARKEMA) (80:10:10 by weight) in N-methyl-pyrrolidinone. The mass loading of the active electrode material in eachslice was 1.5-2.5 mg/cm. The electrolyte solution was 1 M LiPF dis-solved in ethylene carbonate-dimethyl carbonate (1:1 in volume). 1 Cwas equivalent to 200 mA g-and the cell charge/discharge was testedin the range of 2.0-4.8 V at different current densities. 3. Results and discussion The diffraction peaks in the XRD patterns (Fig. 1a) of pristine LLOand MDZ@LLO indicate the successful synthesis of Li-rich layeredoxide. Pristine LLO and MDZ@LLO have similar XRD patterns, whereinthe main peaks can be indexed to a layered hexagonal a-NaFeO2structure. A series of weak peaks between 20° and 23°can be ascribed tothe super-lattice ordering, which confirm the existence of Li-rich phase[12,13]. Compared with XRD pattern of pristine LLO, a new peak at30.2°was detected in MDZ@LLO, which is the characteristic (011) peakof tetragonal ZrO2 (JCPDS card No. 50-1089). The (020) peak origi-nating from the tetragonal ZrO2 at 50.71°was also detected in MDZ@LLO. The XPS spectra of MDZ@LLO (Fig. 1b) show the presence of Zr3d5/2 (182.0 eV) and Zr 3d3/2(184.4eV), which are in good agreementwith its tetravalency in ZrO2 [10]. The XPS spectra of MDZ@LLO alsoshow the presence of F 1s (685.1 eV) (Fig. 1c). Both the XRD patternand XPS spectra indicated the presence of Zr and F in MDZ@LLO. Tofurther analyze the element disturbance of MDZ@LLO, EDS elementaldistribution mapping of MDZ@LLO was performed (Fig. 2). Zr, F, Mn,Ni and Co elements are homogeneously distributed in the MDZ@LLOmicrospheres. ICP results also showed the relative ratio between atomscomposed of LLO (Table 1). The chemical compositions of Pristine LLOand MDZ@LLO were closed to their theoretical ratio, confirming thesuccessful synthesis of LLO through our proposed method. The SEM micrographs show a homogeneous smooth LLO micro-sphere-like carbonate precursors (Fig. 3a, d) with a particle size of about1-2 um. The morphology of pristine LLO and MDZ@LLO were main-tained even after calcination at 850℃. Moreover, the pristine LLO con-sisted of closely packed irregular primary nanoparticles approximately100-300 nm in diameter (Fig. 3b,e), while some new pores were ob-served between the primary nanoparticles on MDZ@LLO upon the MOF-assisted treatment (Fig. 3c, f). In the step of synthesizing MDZ@LLO, thepristine LLO was immersed into the TFBDC solution with continuousstirring for 30 min. In this process, the strongly acidic character of TFBDCfacilitates a significantly porous structure on the LLO microspheres bythe boundary-etching. The BET results showed that the surface area ofMDZ@LLO increased to 6.290m’g, almost three times higher thanthat of Pristine LLO (2.398m²g ), and the pore volume also increasedfrom 0.9224×10-2 to 1.7430×10-2cm/g (Table 1). These newpores may enable an efficient transportation of the electrolyte and ensurerapid Li*intercalation/deintercalation [14]. These results demonstratedthat the MOF-assisted treatment can increase the surface area of thehierarchical secondary LLO particle, and still maintain the microspheresstructure of LLO. The morphology of MDZ@LLO was further investigated by TEM andHRTEM.Fig. 3g and h clearly show a uniform MDZ coating at the edgeof the LLO surface. The surface of MDZ@LLO showed a weak ZrO2crystalline structure, approximately 10 nm thick, after calcination at600°℃ for 6h (Fig. 3h). This also revealed the formation of MOF-de-rived ZrO2 layer on the surface of LLO microspheres. In order to confirmthis result, Energy dispersive X-ray line scanning from coating layer to Fig. 4. Energy dispersive X-ray line scanning from coating layer to bulkstructure of MDZ@LLO particles. (a) TEM image of MDZ@LLO for line scan-ning, (b-c) Zr and F characteristic X-rays intensity observed. bulk structure of MDZ@LLO particles was carried out (Fig.4). The EDXline scanning showed that the concentration of zirconium on thecoating layered was significantly higher than that of the bulk structure,confirming the existence of the ZrO2 coating layer, and at the sametime, the fluorine element was also detected, confirming the doping of Fin LLO particle. Cyclic voltammetric (CV) performances were carried out to under-stand the effect of MOF-derived ZrO2 coating on electrochemical per-formance of LLO cathode (Fig. S2). MDZ@LLO had similar CV curveswith Pristine LLO while the current density of MDZ@LLO was largerthan that of Pristine LLO, indicating better electrochemical perfor-mance of MDZ@LLO. The anodic and cathodic peaks potentials ofMDZ@LLO were consistent with that of LLO. These results implied thatthe surface modification did not destroy the bulk structure of LLO. MDZ@LLO exhibited enhanced first coulombic efficiency, cyclingstability and rate capability. When galvanostatically tested in the rangeof 2.0-4.8Vvs Li*/Li at 0.1 C, MDZ@LLO electrode showed higherinitial discharge capacity than the pristine LLO electrode (Fig. 5a); theinitial discharge capacity of MDZ@LLO reached 279.8mAhgwhilethat of pristine LLO was 228.4 mAh g. The higher discharge capacityof MDZ@LLO may be ascribed to abundant pores between the primarynanoparticles, which increases the specific surface area of MDZ@LLOmicrospheres, and hence Li* diffusion channels during cycling [10].Furthermore, the first coulombic efficiency of MDZ@LLO was 72%,which was better than that of pristine LLO(62%). The increase in thefirst coulombic efficiency can be attributed to the decrease in the cellimpedance for Litdiffusion upon F doping. This element doping canefficiently stabilize the crystal structure by suppressing the formation ofoxygen vacancies during the first cycle [15]. MDZ@LLO showed an excellent cycling performance (Fig. 5c, d andFig. S3). The discharge capacity of MDZ@LLO reached 171.3 mAhg (C)350- 100 300- b0 >Coulombic Efficiency 250 80 MDZ@LLO 83.8% .2200. -60 33 150- 叭 Capacity Retation>80% (after 200 cycle) 40 100- -20 50- -MDZ@LLO MDZ@LLO Coulombic Efficieney 1C 0 -0 0 20 40 60 80 100120140160180200 Cycle number Cycle number 300 (e) MDZ@LLO (f) 200 N' 100- After1 cycle After 200 cycle 0 0 100 200 300 400 Z'/ohm Z'ohm (capacity retention above 83.2%) after 200 cycles at 1C, whereas thedischarge capacity of pristine LLO was only 106.3 mAhg(capacityretention: 71.1%), which was much lower than that of MDZ@LLO.Additionally, MDZ@LLO presents much higher rate capability com-pared to pristine LLO (Fig. 5b). The discharge capacity of MDZ@LLOreached 110.0mAhgat 5 C while the same for pristine LLO was only58.5 mAhg. Even at a very high C-rate of 10 C (2000mAh g), thedischarge capacity of MDZ@LLO still maintained 50.2 mAhgForcomparison, tthe discharge capacity of Pristine LLO was only0.2 mAhgat 10 C. Such exquisite properties may be attributed to aunique combination of a suitable porous structure, F doping, and veryhomogeneous ZrO2 coating. Our work demonstrated that these threefunctional modifications were successfully introduced into LLO. TheLLO, only coated by ZrO2, normally showed relatively low rate capacityand low first coulombic efficiency [10]. The key to achieving such su-perior electrochemical performance lies in the use of Zr-TFBDC(UIO-66-F4), a new ideal precursor of ZrO2 coatings, which derive LLO posses-sing all three attributes (high rate capability, excellent cycling stability,and high coulombic efficiency of the first cycle). The ionic and elec-tronic conductivities of Zr-based MOF(UIO-66-F4) might affect funda-mental properties of cathode materials, therefore potentiostatic coulo-metry and EIS measurements were carried out to evaluate the electricconductivity of this material (Figs. S4 and S5). The values of ionicand electronic conductivities of Zr-based MOF(UIO-66-F4)Vwere 2.17×10-S/cm and 4.20 ×10-10 s/cm respectively. Although theZr-based MOF has low ionic and electronic conductivities, its specialstructure and structural changes in the preparation process of MDZ@LLO induced porous characteristic and F-doping, therefore improvingthe lithium ion conductivity of MDZ@LLO. EIS measurements were carried out to further study the electro-chemical properties of LLO. Fig. 5e and f showed the EIS spectra ofMDZ@LLO and LLO. All spectra were semicircular at high-to-mediumfrequencies, and the width of this semicircle corresponds to the chargetransfer resistance (Rct). The inclined line in the low-frequency range isconsidered as the Warburg impedance, which is attributed to Li* dif-fusion inside the electrode materials [14].The Rct of MDZ@LLO waslower than that of Pristine LLO, which can be ascribed to the lowerinterfacial reactive resistance of the MOF-modified LLO. After long timecycling, the increased resistance value of the MDZ@LLO electrode wasmuch less than that of pristine LLO, which was consistent with theimproved stability as demonstrated by the cycling tests. The above system is not yet studied thoroughly and has not beenoptimized using TFBDC as the organic ligand. However, it is still morepromising compared with that of the existing metal oxide coating.Significant improvements are expected with further investigations onthese new coating materials. This study provides a new area for ex-ploring a large number of MOF-derived coating for the fabrication ofpromising lithium cathode materials. 4. Conclusions We report a novel and effective surface modification of LLO, using aZr-based MOF precursor, to produce in-situ MOF-derived ZrO2 coatings.The strongly acidic character of TFBDC induces the H*-Li* exchangeon the surface of the Li-rich layered oxide particles, which facilitate theuniform coating of Zr-TFBDC and a significantly porous structure on theLLO microspheres. ZrO2 can easily form on the surface of LLO and F wassuccessfully doped into LLO particles by the facile one-step MOF-as-sisted modification. In this study, electrochemical performances such asthe rate capability, cyclability, and first coulombic efficiency of the LLOin the MOF-derived ZrO2 coated LLO (MDZ@LLO) was significantlyimproved. Acknowledgments Funding: This work was financially supported by the NationalNatural Science Foundation of China (project code 21776051); theScientific Research Foundation for Yangcheng Scholars (project code1201541563); Applied Science and Technology Planning Project ofGuangdong Province (project code 2017B090917002); GuangdongEducation Department YoungInnovatorsProject (project code2016KQNCX125); and 2016 Nansha District Research Project (projectcode 2016GG007). Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi.org/10.1016/j.elecom.2019.01.005. References ( (2013) 8527 - 8534 . ) [2]M.M. Thackeray, S.H. Kang, C.S. Johnson, J.T.Vaughey, R. Benedek, S.A. Hackney,Li2MnO3-stabilized LiMO2(M=Mn, Ni, Co) electrodes for lithium-ion batteries, J.Mater. Chem. 17 (2007)3112-3125. [3]M.M. Thackeray, C.S. Johnson, J.T. Vaughey, N. Li, S.A. Hackney, Advances inmanganese-oxide ‘composite'electrodes for lithium-ion batteries, J. Mater. Chem.15 (2005)2257-2267. [4]H. Yu, H. Kim, Y. Wang, P. He, D.Asakura, Y. Nakamura, H. Zhou, High-energy'composite' layered manganese-rich cathode materials via controlling Li2MnOsphase activation for lithium-ion batteries, Phys. Chem. Chem. Phys. 14 (2012)6584-6595. [5]J. Zheng, M. Gu, J. Xiao, P. Zuo, C. Wang, J.G. Zhang, Corrosion/fragmentation oflayered composite cathode and related capacity/voltage fading during cyclingprocess, Nano Lett. 13 (2013)3824. [6]S. Kim, W. Cho, X. Zhang, Y. Oshima, J.W. Choi, A stable lithium-rich surfacestbtructure for lithium-rich layered cathode materials, Nat. Commun. 7 (2016) 8. [7] J. Zhao, X. Kuai, X. Dong, H. Wang, W. Zhao,L. Gao, Y. Wang, R. Huang, Phasetransitions and related electrochemical performances of Li-Rich layered cathodematerials for high-energy lithium ion batteries, J. Alloys Compd.732 (2018) 385-395. [8]SS. Li,X. Fu, J. Zhou, Y. Han, P. Qi, X. Gao, X. Feng, B. Wang, An effective approachto improve the electrochemical performance of LiNio.6C0o.2Mno.2O2 cathode by anMOF-derived coating, J. Mater. Chem. A 4 (2016) 5823-5827. ( [9] 1 M . Ho u , J . Li u , S. Guo, J. Yan g , C . Wang, Y . Xia, E nhanced e lectroc h emica l p er- f ormance o f L i - rich l ay er e d c athode material s by su rf a ce modif i catio n wi th P 2 0s, E lectroche m . Com m un. 4 9 (2014) 83-87. ) ( [10 ] Z . W ang, E. L i u , L . G u o, C. S h i , C . H e , J. L i , N . Z hao, C y cle p e r formance im- p roveme n t of Li- r i ch layered cathode m a terial L i [L i o.2 M no.54Nio.13Co0.13]O2 by Z rO2 coating, S ur f. Coat. Technol. 2 35 (2013) 5 7 0-576. ) [11]Q(. Xue, J. Li, G. Xu, H. Zhou, X. Wang, F. Kang, In situ polyaniline modified cathodematerial Li[Lio.2Mno.54Nio.13Coo.13]O2 with high rate capacity for lithium ion bat-teries, J.Mater. Chem. A 2 (2014) 18613-18623. [12]B. Song, M.O. Lai, Z. Liu, H. Liu, L. Lu, Graphene-based surface modification onlayered Li-rich cathode for high-performance Li-ion batteries, J. Mater. Chem. A 1(2013) 9954-9965. [13]J. Liu, A. Manthiram, Functional surface modifications of a high capacity layered Li[Lio.2Mno.54Nio.13Coo.13]02 cathode, J. Mater. Chem. 20 (2010) 3961-3967. [14] Y. Xie, S. Chen, W. Yang, H. Zou, Z. Lin, Improving the rate capability and decel-erating the voltage decay of Li-rich layered oxide cathodes by constructing a sur-face-modified microrod structure, J. Alloys Compd. 772 (2019)230-239. [15]L. Li, B.H. Song, Y.L. Chang, H. Xia, J.R. Yang, K.S. Lee, L. Lu, Retarded phasetransition by fluorine doping in Li-rich layered Li1.2Mn0.54Nio.13Co0.1302 cathodematerial, J. Power Sources 283 (2015) 162-170. 通过MOF辅助处理的表面改性,增强富锂层状氧化物Li1.2Mn0.54Co0.13Ni0.13O2的电化学性能 简介 富含锂的层状氧化物(LLO),无论是作为固体溶液还是作为层状Li2MnO3和Li(M)O2(M=Mn、Ni、Co)的纳米复合材料,作为锂离子电池正极材料的最有希望的候选材料之一,已经引起了广泛的关注。它们具有超过800Wh g-1的高能量密度,并且成本低、毒性小(由少量的Co和Ni组成)[1-4]。01020304 然而,为了获得高的比容量,富锂层状氧化物的电位必须被充电到4.5V的平台,这诱发了Li+作为Li2O的部分提取。此外,在充电/放电过程中,表面微观结构的持续分层到尖晶石的转变导致了低速率能力和LLO的容量衰减[5-7]。05速率能力低,第一个循环的库仑效率差,以及循环稳定性低,阻碍了LLO的商业应用。已经证实,表面涂层可以极大地改善LLO的循环的稳定性。例如,涂在LLO上的Al2O3、SiO2和TiO2薄层可以保护LLO的表面不受电解液的腐蚀,并在高电位下抑制LLO的副反应和减速氧的释放[8,9]。尽管这些涂层可以有效地提高LLO的循环性能,但它们在提高速率性能和库仑效率方面的贡献通常有限。 此外,在原位溶剂蒸发法中,整个颗粒上的金属氧化物的均匀涂层是困难的[10]。因此,探索新的表面涂层方法来进一步提高LLO的性能是非常可取的。金属有机框架(MOFs)是一类具有高表面积和丰富的Li+扩散通道的材料,是在电化学应用中具有重要性能的功能性混合材料的有前途的预示[8]。在此,我们报告了一种新的和有效的LLO表面改性,使用Zr基MOF(UIO-66-F4)前驱体,在原位生产MOF衍生的ZrO2(MDZ)涂层。TFBDC(2,3,5,6-四氟-1,4-苯二甲酸)是一种强有机酸,具有四个极强的电子撤回F取代基。它的强酸性特征诱导富含锂的层状氧化物颗粒表面的H+-Li+离子交换(质子化),这有利于在LLO微球上形成明显的多孔结构[11]。此外,TFBDC通常倾向于在碱金属氧化物附近聚集。LLO,从而形成Zr-TFBDC的均匀涂层。通过简单的热处理,ZrO2可以很容易地在LLO的表面产生;这种均匀的涂层可以保护LLO不与电解质直接接触,并且不妨碍Li+的传输。同时,通过简单的一步式MOF-as-sisted改性,成功地将F掺入LLO颗粒中。在这项研究中,MOF衍生的ZrO2包覆的LLO(MDZ@LLO)的电化学性能,如速率能力、循环能力和LLO的第一库仑效率得到了明显改善。 结论我们报告了一种新型的、有效的LLO表面改性方法,使用一种基于Zr的MOF前驱体来生产原位MOF衍生的二氧化锆涂层。Zr基MOF前体,生产原位MOF衍生的ZrO2涂层。TFBDC的强酸性特征诱导了H+-Li+交换。在富含锂的层状氧化物颗粒的表面进行H+-Li+交换,从而促进了Zr-TFBDC的均匀涂覆。这有利于Zr-TFBDC的均匀涂层,并在LLO微球上形成明显的多孔结构LLO微球。ZrO2很容易在LLO的表面形成,F被成功地掺入LLO颗粒中。通过简单的一步式MOF辅助改性,成功地将F掺入到LLO颗粒中。在这项研究中,电化学性能,如速率能力、循环能力和LLO的首次库仑效应。在MOF衍生的ZrO2包覆的LLO(MDZ@LLO)中的电化学性能得到了明显的改善和显著提高。
确定


还剩4页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《富含锂的层状氧化物中电化学性能检测方案(微波合成仪)》,该方案主要用于锂电池中电化学性能检测,参考标准--,《富含锂的层状氧化物中电化学性能检测方案(微波合成仪)》用到的仪器有多用途微波化学合成仪、微波水热合成仪 Hydrocube XH-800SE型
相关方案
更多











