方案详情
文
The torch configuration (vertical torch, radial observation, sheath gas) allows easy analysis of the incineration solvents mixture. There is no requirement for dilution, cooled spray chamber or additional gases. The optimization was done on the plasma conditions and the sample uptake. The plasma and acquisition parameters are fixed in the analytical method and automatically controlled by computer for an optimum reproducibility from one operator to the other.
方案详情

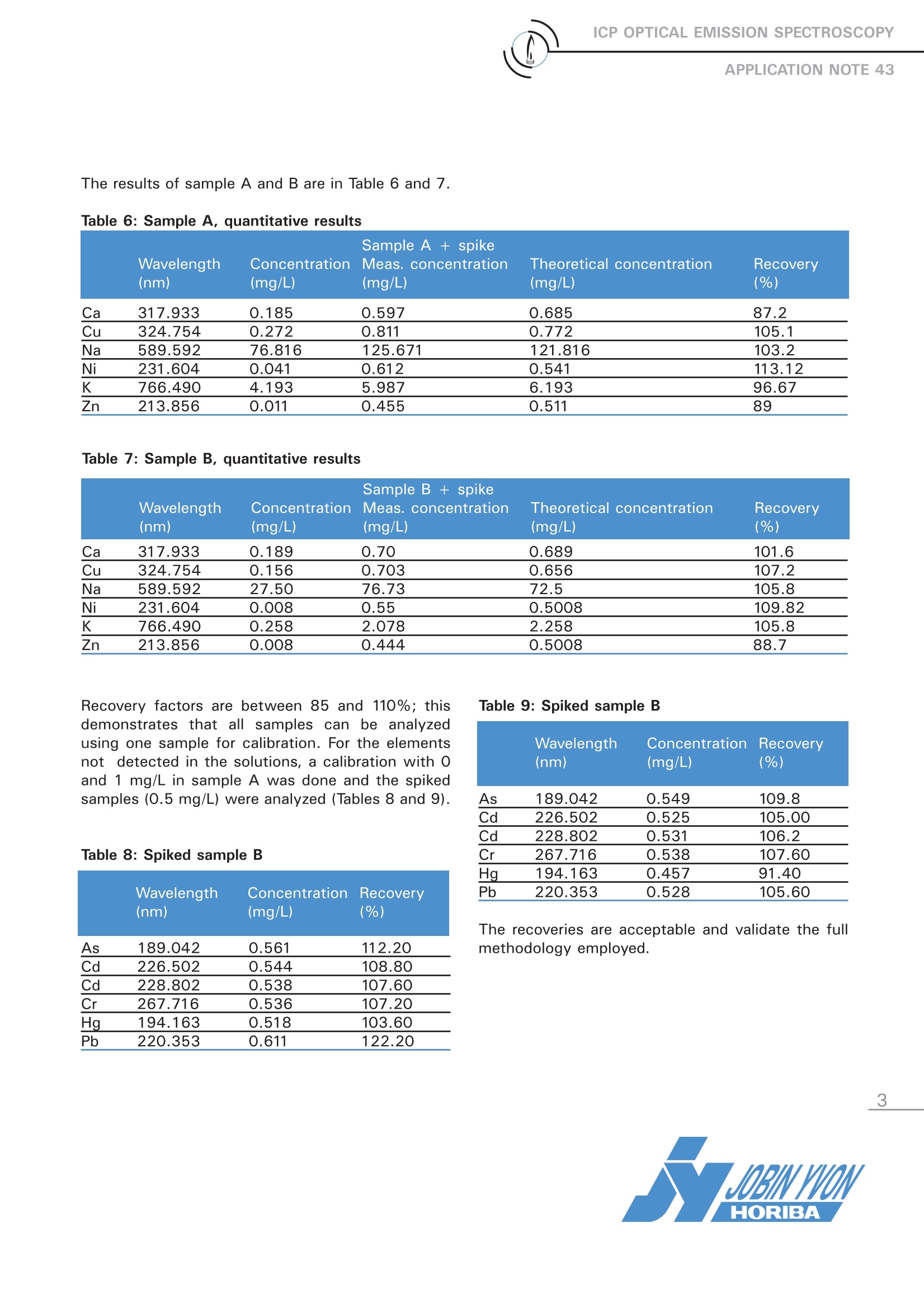
ICP OPTICAL EMISSION SPECTROSCOPY ICP OPTICAL EMISSION SPECTROSCOPYAPPLICATION NOTE 43 Analysis of Incineration Solvents Agnes Cosnier, Sebastien Velasquez, Jobin Yvon S.A.S., Horiba Group, Longjumeau, FranceKeywords: solvents, toxic metals, alcohol 1 Introduction Before solvent incineration, it is necessary toknow the level of toxic metals, such as Hg, Cd,Pb, etc. In this application note, we will demon-strate that ICP-OES can be an easy technique toperform solvent analysis, provided that you havea robust plasma. 2 Principle 2.1 Technique used The elemental analysis of solutions was under-taken by Inductively Coupled Plasma OpticalEmission Spectrometry (ICP-OES). The sample isnebulized then transferred to an argon plasma. Itis decomposed, atomized and ionized wherebythe atoms and ions are excited. We measure theintensity of the light emitted when the atoms orions return to lower levels of energy. Each ele-ment emits light at characteristic wavelengthsand these lines can be used for quantitativeanalysis after a calibration. 2.2 Wavelength choice The choice of the wavelength in a given matrixcan be made using the ·profilel function, or byusing Win-IMAGE, which is rapid semi-quantita-tive analysis mode using multiple wavelengths.The principle is the same in either case: recordthe scans of analytes at low concentration, andof the matrix. By superimposing the spectra, wesee possible interferences. 3 Instrument specification The work was done on a JY ULTIMA 2. The sam-ples were constitued of a mixture of 3 solvents(MeOH, EtOH, IsPrOH). The proportion of the 3solvents are different from one sample to anoth-er one. We prepared a mixture of one third eachMeOH, EtOH and IsPrOH to optimize the plasma, estimate the limits of detection and use as a rins-ing solution. The specifications of the instrumentare listed below in Tables 1 and 2. Table 1: Specification of spectrometer Parameters Specifications Mounting Czerny Turner Focal length 1m Nitrogen purge Yes Variable resolution Yes Grating number of grooves 2400 gr/mm Order 2nd order Table 2: Specification of RF Generator Parameters Specifications Type of generator Solid state Observation Radial Frequency 40.68 MHz Control of gas flowrate by computer Control of pump flow by computer Cooling air The operating conditions are listed in Table 3below. Table 3: Operating conditions Parameters Conditions RF Generator power 1400 W Plasma gas flowrate 18 L/min Auxiliary gas flowrate 1 L/min Sheath gas flowrate 0.65L/min Nebulizer flowrate 0.42 bars, 0.2 mL/min Pump tubing orange/white (solvflex) Pump speed 13 rpm Type of nebulizer Meinhard K3 type Type of spray chamber Cyclonic Argon humidifier No Injector tube diameter 3.0 mm 4 Semi-quantitative results To know the level of each element, a semi-quantita-tive method was used. The calibration was madewith two points for all elements: 0 and 5 mg/L. Theblank was made by a mixture of one third eachMeOH, EtOH and IsPrOH. This blank solution isused for the rinsing solution and for the calibrationsandards. Note the very good recovery for Na, which is anillustration of linearity and easy analysis of alkalineelements using one single method. Results of sample A are given in Table 4, as well assample A + spike. Semi-quantitative analyses are made with an errorof about 20%. The recovery factor is within thiserror so the matrix effect can be considered asminor, and the results a good approximation of thereal concentrations. Table 4: SampleA Sample A Sample A + spike Sample A + spike Wavelength Concentration Measured Theoretical Recovery (nm) (mg/L) concentration (mg/L) concentration (mg/L) % As 189.042 < LOQ 0.478 0.5 95.6 Ca 317.933 0.124 0.742 0.624 118.9 Cd 228.802
确定



还剩2页未读,是否继续阅读?
HORIBA(中国)为您提供《溶剂灰分中As元素分析检测方案(ICP-AES)》,该方案主要用于其他中重金属检测,参考标准--,《溶剂灰分中As元素分析检测方案(ICP-AES)》用到的仪器有HORIBA Ultima Expert高性能ICP光谱仪
该厂商其他方案
更多










