
方案详情
文
对二氧化碳超临界萃取中的夹带剂,茶叶颗粒大小,提取温度和时间对去除咖啡因和儿茶酸的绿茶用超临界二氧化碳法的影响进行了研究。结果表明,乙醇为最佳助溶剂。水和50 %乙醇水溶液为夹带剂不适合,因为有很大比例儿茶素被去除。基于正交设计试验,脱咖啡因的绿茶,筛选最优条件10克0.2 - 0.6毫米粒径的绿茶,在80℃温度,用15毫升乙醇夹带剂,压力为300Bar,流速为1.5 L /min。经过两小时萃取 70.2 %咖啡因和6.2 %儿茶酸被去除。
方案详情

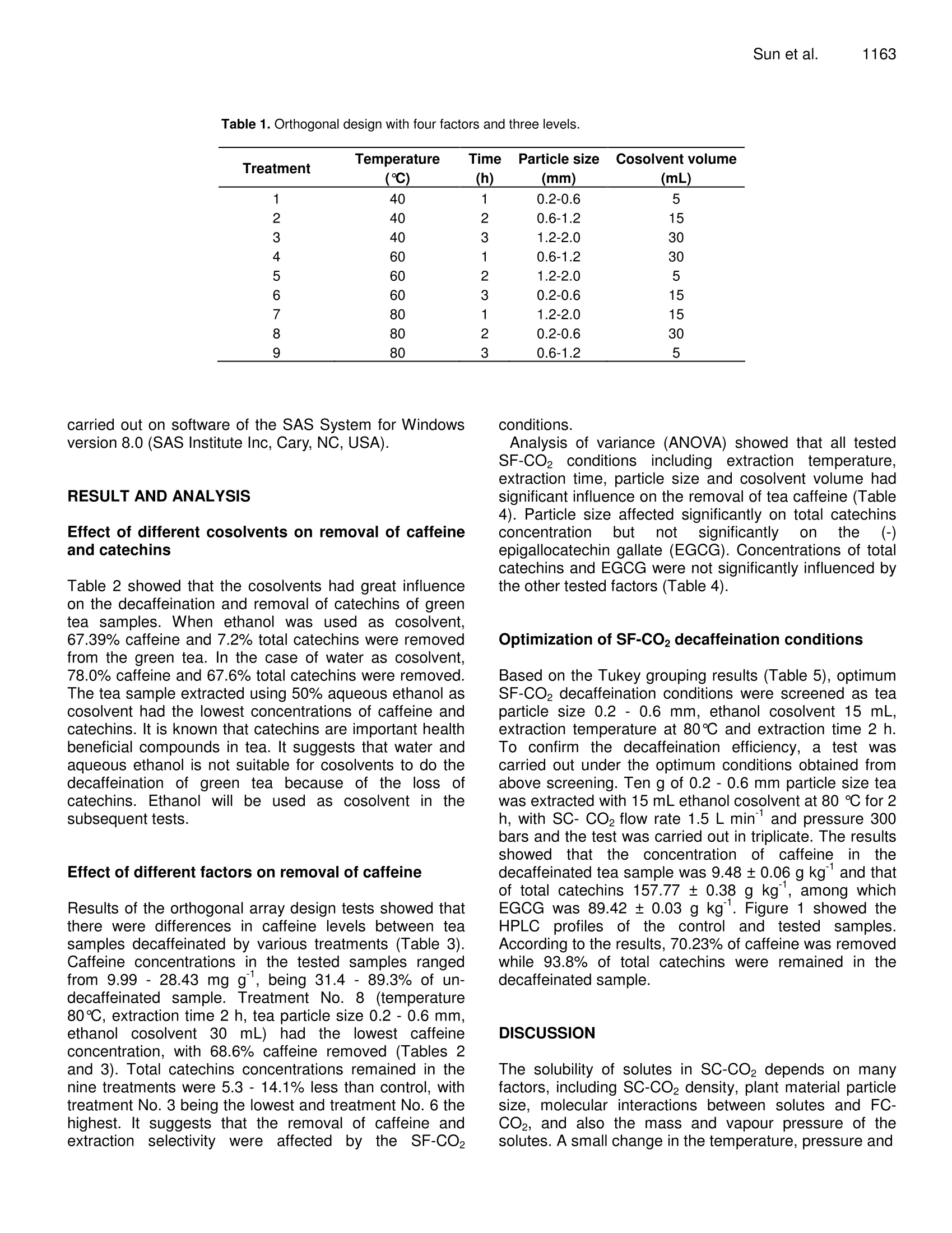
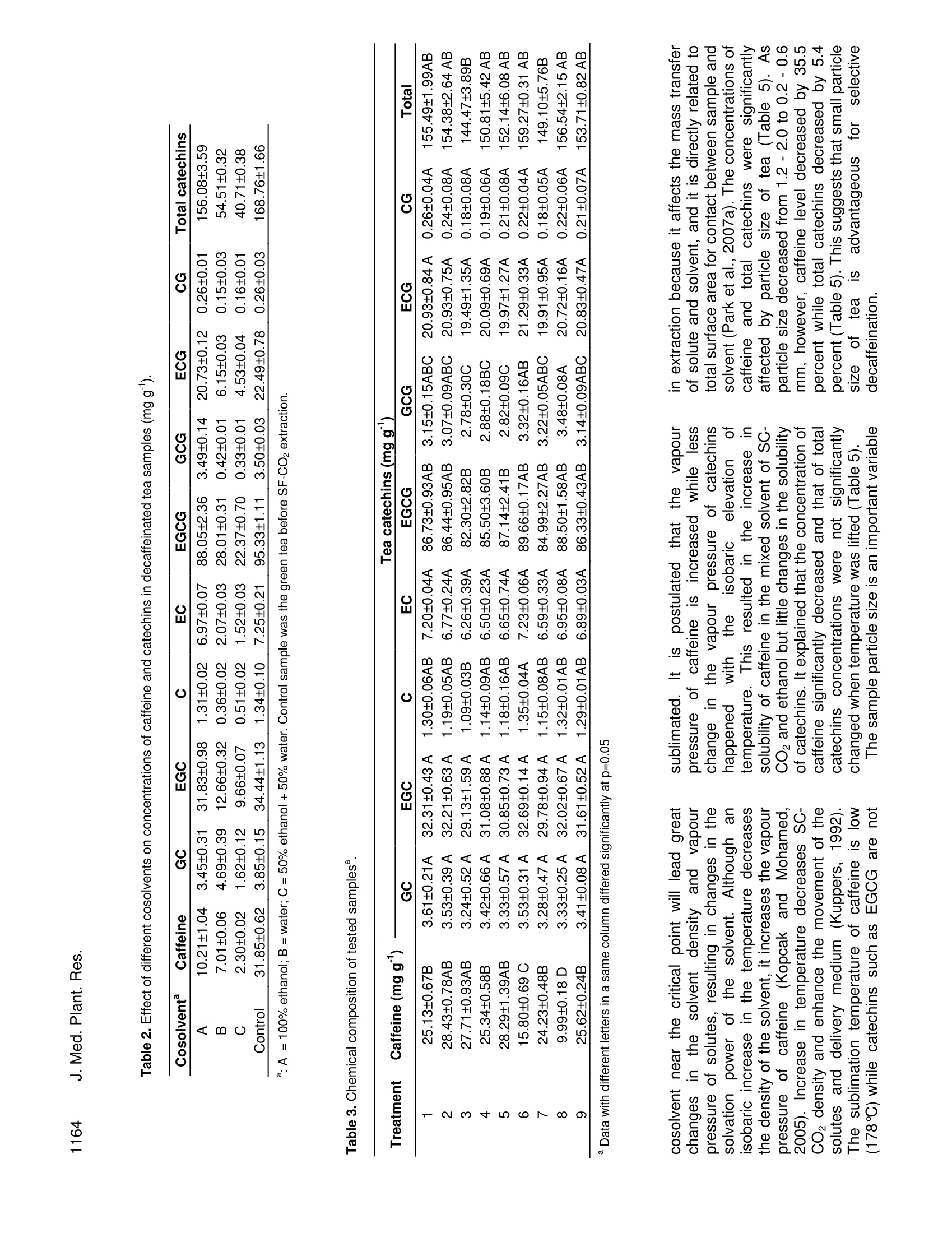
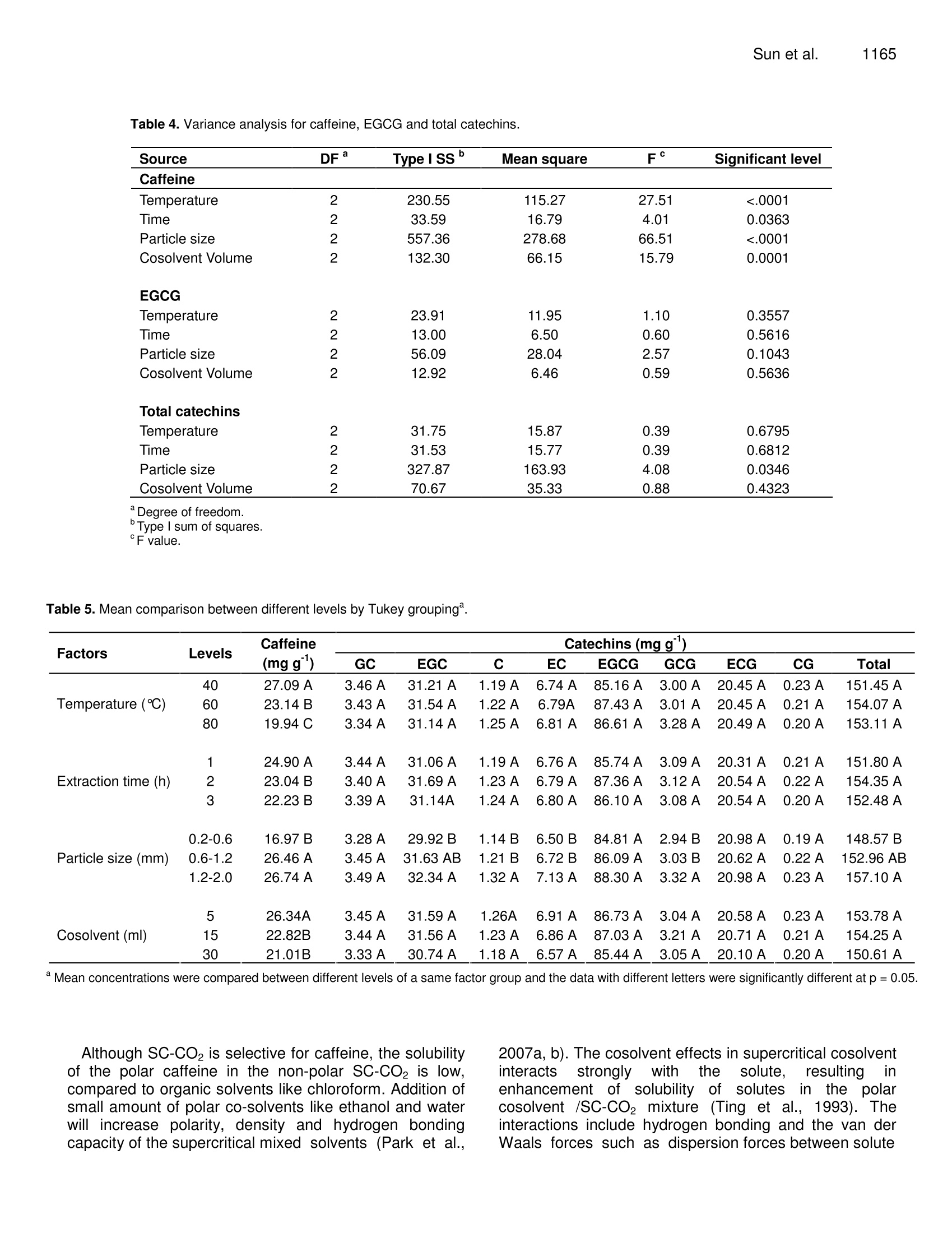
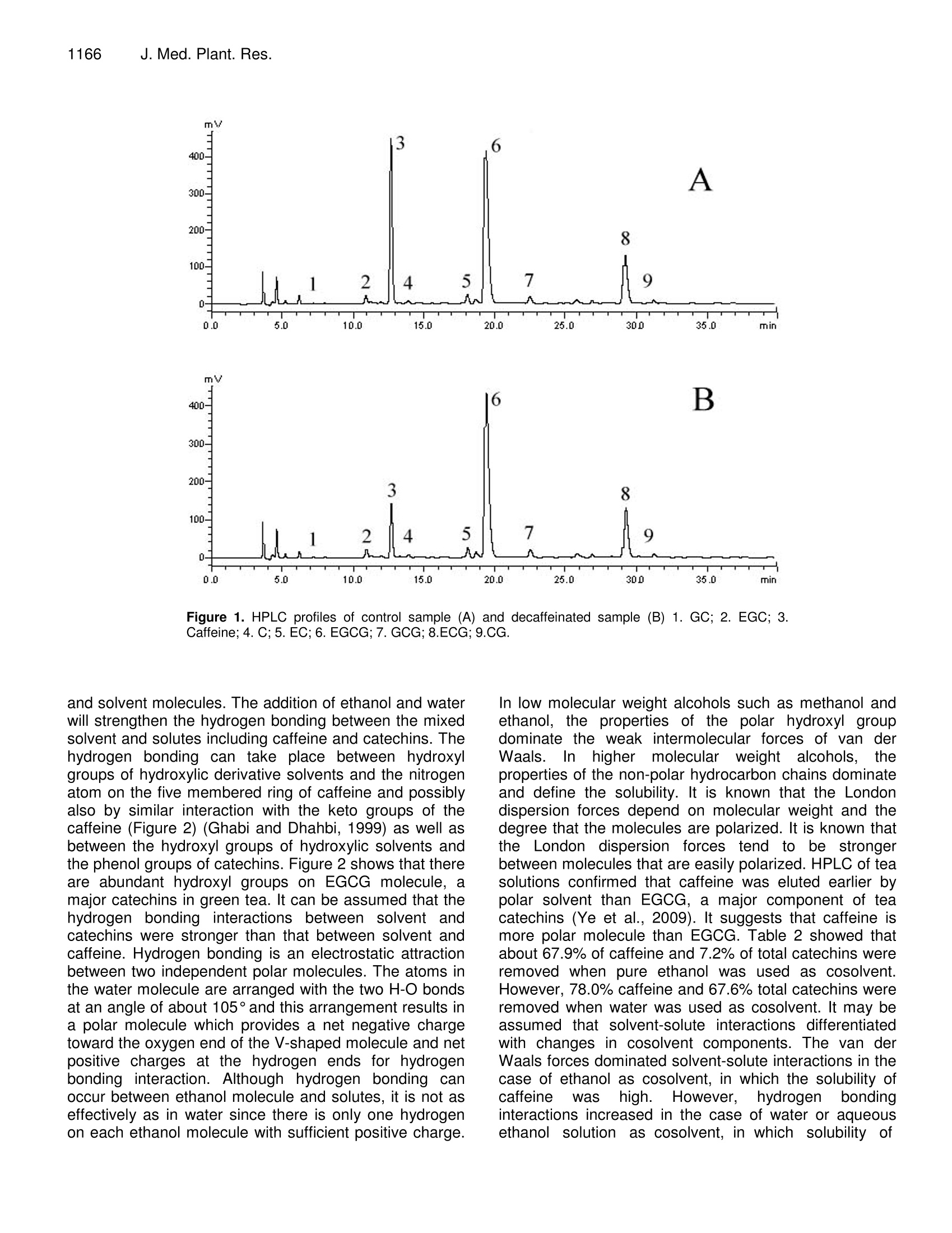
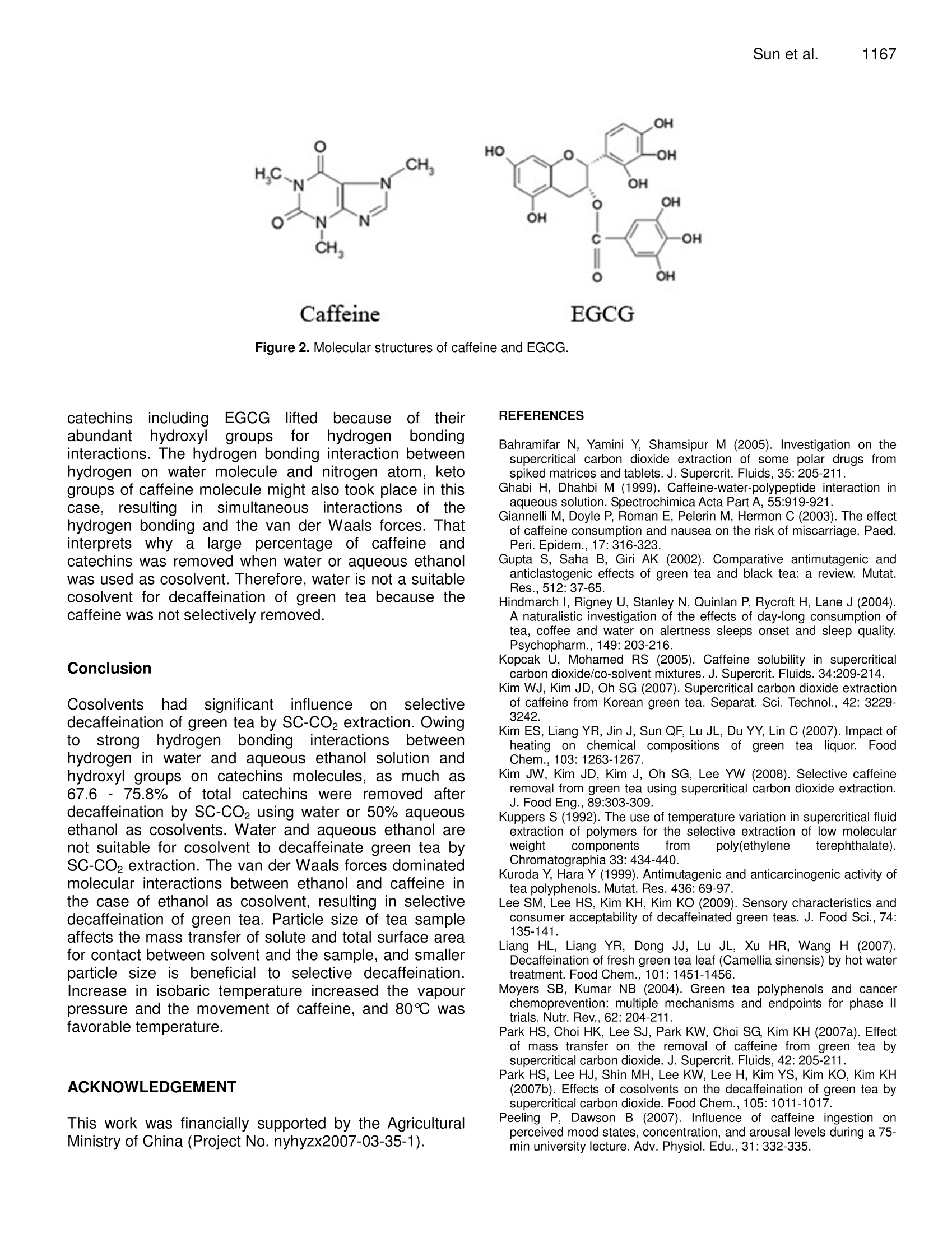
Journal of Medicinal Plants Research Vol. 4(12), pp. 1161-1168, 18 June, 2010Available online at http://www.academicjournals.org/JMPRISSN 1996-0875 C2010 Academic Journals 1162J. Med. Plant. Res. Full Length Research Paper Decaffeination of green tea by supercritical carbondioxide Qing-Lei Sun, Shu Hua', Jian-Hui Ye', Jian-Liang Lu², Xin-Qiang Zheng and Yue-RongLiang * 'Zhejiang University Tea Research Institute, Hangzhou 310029, PR China. The State Agriculture Ministry, Laboratory of Horticulture, Plant Growth Development and Quality Improvement,Hangzhou 310029, PR China. Accepted 6 April, 2010 Effects of cosolvent, tea particle size, extraction temperature and time on the removal of caffeine andcatechins from green tea using supercritical carbon dioxide method were investigated. It showed thatethanol was optimal cosolvent. Water and 50% aqueous ethanol were not suitable for cosolventsbecause a large percentage of catechins were removed. Based on the orthogonal array design tests,optimal protocol for green tea decaffeination was screened as 10 g of 0.2-0.6 mm particle size greentea was extracted using 15 ml ethanol cosolvent at 80℃ for 2 h when flow rate and pressure of SC- CO2were at 1.5 L min° and 300 bars, during which 70.2% caffeine and 6.2% catechins were removed,respectively. Key words: Decaffeinated tea, supercritical fluid extraction, cosolvent, particle size, temperature, caffeine,catechins. Green tea catechins have received great attention bothfrom scientific community and general public recently dueto their health benefits and functionality suchasantioxidation, anticancer, and anti-inflammation (Gupta etal., 2002; Kuroda and Hara, 1999; Moyers and Kumar,2004). Although caffeine can be used medicinally as acardiac, cerebral and respiratory stimulant as well as adiuretic (Peeling and Dawson, 2007), it has someadverse effects on humans, including sleep deprivation(Hindmarch et al., 2004), abortions and miscarriages(Giannelli et al, 2003). The caffeine in green tea has beena limitation for those who want to avoid it. The limitationbrought increase in need for decaffeinated products inthe green tea market (Kim et al., 2007; Lee et al., 2009).Thus, it is important to selectively remove caffeine fromthe green tea while to remain catechins in the green teafor healthy tea production (Kim et al.,2008). Researchers attempted to selectively remove caffeinefrom tea or tea extracts by many methods includingsolvent extraction, column chromatography, hot water ( *Corresponding author.E-mail: yrliang@zju.edu.cn. Fax: 86 571 8697 1704. ) treatment and supercritical CO2 (SC-CO2) extraction.Many organic solvents such as chloroform and methylenechloride are not accepted owing to their toxicities thoughthey are effective to remove caffeine from tea leaf.Column chromatography can be used to decaffeinate teaextracts but is not fit for decaffeination of tea leaf(Sakanaka, 2003; Ye et al., 2009). Hot water treatment isan inexpensive and safe method to prepare decaffeinatedgreen tea, but it can only be used to treat fresh tea leafand it could not selectively remove caffeine from dry tea(Liang et al., 2007; Ye et al., 2007). Supercritical CO2extraction is environmental but its selectivity depended onmany factors such as cosolvent, pressure, temperature,extraction time and moisture content of tea leaf (Park etal., 2007a, 2007b; Lee et al., 2009; Kim et al.,2008). Theextraction yield of caffeine increased with an increase intemperature at a constant pressure, and also increasedwith increasing pressure at a fixed temperature, and thatlarger particle size yielded the slower extraction rate,when SC-CO2 extraction was carried out by using 95%(v/v) ethanol as ia cosolvent. After supercritical CO2extraction, a substantial loss of EGCG, predominant teacatechins, as much as 37.8% of original content wasproved tobee uUnavoidable (Parkeet al., 2007a).Conditions for selective removal of tea caffeine by SC-CO2 need to be further optimized. Orthogonal array design (OAD) is a fractional factorialdesign and a series of trials are assigned by orthogonalarray. The advantages of OAD includee that it mayminimize test numbers and time to keep the experimentalcostataminimumlevel, and that[the optimumparameters obtained from the laboratory can be utilizedby larger scale of production (Yesilyurt,2004). It has beenadopted in different areas for the optimization of theanalyticalprocedure,includingextracting optimalprocedure of SC-CO2 extraction of some polar drugs fromspiked matrices and tablets (Bahramifar et al., 2005). Inthe present paper, an orthogonal design test with fourfactor and three levels each factor was carried out tooptimize the green tea decaffeination conditions of SC-CO2 extraction. MATERIALS AND METHODS Materials Green tea used was supplied by Qingcheng Tea Co. Ltd.,(Qingcheng County, Sichuan, China). The tea was driedin a dryer at 103℃C for 4 h to remove the moisturecompletely and then ground using a EUPA TSK-927Sgrinder (Cankun Co, Ltd, Shanghai, China) and siftedthrough sieves with different size meshes (Lantian Co.Ltd., Hangzhou, China) in order to obtain samples withthree different size particles (0.2 -0.6 mm, 0.6-1.2 mmand 1.2 - 2.0 mm in diameter). HPLC referencecompounds including (-)epigallocatechin gallate (EGCg),(-)epigallocatechin (EGC), (-)epicatechin gallate (ECg),(+)epicatechin (EC), (+)gallocatechin gallate (GCg), (-)gallocatechin (GC), (+)catechin gallate (Cg), (-)catechin(C) and caffeine were Sigma products (Sigma-Aldrich, St.Louis, USA). The other chemical reagents used were ofHPLC grade (Jinmei Biotech Corporation, Tianjin, China)except where stated otherwise. The water used in theexperiments was purified water prepared by a modelD7401 EASYPure Il UV Ultrapure system (BarnsteadInternational,lowa, USA). Extraction with different cosolvents A supercritical CO2 extractor Speed SFE-2 (AppliedSeparations Allentown Inc., PA, USA) was used in thisstudy. It was equipped with two pumps. The first pumpwas a master pump (Model SDC-6, Ningbo ScinentzBiotechnology Co. Ltd, China) for circulation of refrigerantin jacket, and the second was a cosolvent pump (ModelK-501, Knauer Co. Ltd, Berlin, Germany)') for thedelivering of cosolvent. Three kinds of cosolvents weretested, thatis, AR grade ethanol (Jinmei EBiotechCorporation, Tianjin, China), water and a mixture of 50%(v/v) AR grade ethanol and 50%(v/v) water. Fifteen milliliters cosolvent and teng tea sample (particle size 0.6- 1.2 mm) were used in each extraction. The pressure,temperature and flow rate of SC-CO2 were maintainedconstant at 300 bars, 60℃ and 1.5 L min, respectively. Orthogonal array design test Extraction tests with different cosolvents showed thatwater and 50% (v/v) aqueous ethanol were not suitablefor cosolventsiebecausea large percentage of teacatechins, the antioxidants in tea, were removed duringthe decaffeination. AR grade ethanol was screened ascosolvent in the subsequent tests. To obtain optimum SC-CO2 decaffeination conditions, orthogonal array design(OAD) design with four factors and three levels eachfactor [Lg (3)] was carried out on software of the SAsSystem for Windows version 8.0 (SAS Institute Inc, Cary,NC, USA). The four tested factors were tea particle size,extraction temperature, extraction time and cosolventvolume. The details of the experimental design wereshown in Table 1. Ten g tea sample was used in eachextraction as above. The tests were carried out atisobaric 300 bars and SC-CO flow rate 1.5 L min. HPLC analysis of caffeine and catechins Teasamples before or after supercritical CO2decaffeination were dried at 103℃ for 4 h and groundinto particles less than 0.2 mm in diameter. One gram ofthe ground sample was extracted with 100 mL of 50%(V/V) aqueous ethanol at room temperature for 45 min,and then the extract was centrifuged at 5000 xg and 4℃for 10 min. Theisupernatantt was used for highperformanceliquid chromatography(HPLC, ModelLC20A, Shimadzu Co, Kyoto, Japan) to determinecaffeine and catechins (Kim and Liang et al, 2007). TheHPLC conditions were as follows: injection volume,10 pL;5 pm TC-C1: column (4.6x250 mm, Agilent TechnologiesInc, CA, USA), oven temperature at 28℃, mobile phaseA being acetonitrile /acetic acid /water (6/1/193,v/v/v) andmobile phase B acetonitrile /acetic) acid /water(60/1/139,v/v/v), linear gradient elution with mobile phaseB from 30 - 85% (v) during the early 35 min and holdingat 85% mobile phase B for further 5 min at flow rate 1mLmin, detecting at 280 nm. Caffeine and catechins in thetested sample were determined by comparing retentiontimes and peak heights with those of the correspondingauthentic references. Data statistics All the tests were carried out in triplicate and the meanvalues of the triplicate tests were presented. Analysis ofvariance (ANOVA) and Tukey grouping of the data were Treatment Temperature Time Particle size Cosolvent volume (℃) (h) (mm) (mL) 1 40 1 0.2-0.6 5 2 40 2 0.6-1.2 15 3 40 3 1.2-2.0 30 4 60 1 0.6-1.2 30 5 60 2 1.2-2.0 6 60 3 0.2-0.6 7 80 1 1.2-2.0 8 80 2 0.2-0.6 9 80 3 0.6-1.2 5 carried out on software of the SAS System for Windowsversion 8.0 (SAS Institute Inc, Cary, NC, USA). RESULT AND ANALYSIS Effect of different cosolvents on removal of caffeineand catechins Table 2 showed that the cosolvents had great influenceon the decaffeination and removal of catechins of greentea samples. When ethanol was used as cosolvent,67.39% caffeine and 7.2% total catechins were removedfrom the green tea. In the case of water as cosolvent,78.0% caffeine and 67.6% total catechins were removed.The tea sample extracted using 50% aqueous ethanol ascosolvent had the lowest concentrations of caffeine andcatechins. It is known that catechins are important healthbeneficial compounds in tea. It suggests that water andaqueous ethanol is not suitable for cosolvents to do thedecaffeination of green tea because of the loss ofcatechins. Ethanol will be used as cosolvent in thesubsequent tests. Effect of different factors on removal of caffeine Results of the orthogonal array design tests showed thatthere were differences in caffeine levels between teasamples decaffeinated by various treatments (Table 3).Caffeine concentrations in the tested samples rangedfrom 9.99 - 28.43 mgg, being 31.4-89.3% of un-decaffeinated sample. Treatment No. 8 (temperature80℃, extraction time 2 h, tea particle size 0.2 -0.6 mm,ethanol cosolvent 30 mL) had tthe lowest caffeineconcentration, with 68.6% caffeine removed (Tables 2and 3). Total catechins concentrations remained in thenine treatments were 5.3 - 14.1% less than control, withtreatment No. 3 being the lowest and treatment No. 6 thehighest. It suggests that the removal of caffeine andextractioniselectivitylyvwereeaffected bby tthe SF-CO。 conditions. Analysis of variance (ANOVA) showed that all testedSF-CO2 conditions including extraction temperature,extraction time, particle size and cosolvent volume hadsignificant influence on the removal of tea caffeine (Table4). Particle size affected significantly on total catechinsconcentrationbut notitsignificantlyontthe (-)epigallocatechin gallate (EGCG).Concentrations of totalcatechins and EGCG were not significantly influenced bythe other tested factors (Table 4). Optimization of SF-CO2 decaffeination conditions Based on the Tukey grouping results (Table 5), optimumSF-CO2 decaffeination conditions were screened as teaparticle size 0.2 - 0.6 mm, ethanol cosolvent 15 mL,extraction temperature at 80℃ and extraction time 2 h.To confirm the decaffeination efficiency, a test wascarried out under the optimum conditions obtained fromabove screening. Ten g of 0.2 -0.6 mm particle size teawas extracted with 15 mL ethanol cosolvent at 80 ℃ for 2h, with SC-CO flow rate 1.5 L min°and pressure 300bars and the test was carried out in triplicate. The resultsshowed thattthee concentration of caffeine inntthedecaffeinated tea sample was 9.48 ±0.06 g kg and thatof total catechins 157.77 ±0.38 g kg , among whichEGCG was 89.42 ±0.03 g kg’. Figure 1 showed theHPLC profiles of the control and tested samples.According to the results, 70.23%of caffeine was removedwhile 93.8% of total catechins were remained in thedecaffeinated sample. DISCUSSION The solubility of solutes in SC-CO depends on manyfactors, including SC-CO2 density, plant material particlesize, molecular interactions between solutes and FC-CO2, and also the mass and vapour pressure of thesolutes. A small change in the temperature, pressure and Table 2. Effect of different cosolvents on concentrations of caffeine and catechins in decaffeinated tea samples (mg g). Cosolvent° Caffeine GC EGC C EC EGCG GCG ECG CG Total catechins A 10.21±1.04 3.45±0.31 31.83±0.98 1.31±0.02 6.97±0.07 88.05±2.36 3.49±0.14 20.73±0.12 0.26±0.01 156.08±3.59 B 7.01±0.06 4.69±0.39 12.66±0.32 0.36±0.02 2.07±0.03 28.01±0.31 0.42±0.01 6.15±0.03 0.15±0.03 54.51±0.32 C 2.30±0.02 1.62±0.12 9.66±0.07 0.51±0.02 1.52±0.03 22.37±0.70 0.33±0.01 4.53±0.04 0.16±0.01 40.71±0.38 Control 31.85±0.62 3.85±0.15 34.44±1.13 1.34±0.10 7.25±0.21 95.33±1.11 3.50±0.03 22.49±0.78 0.26±0.03 168.76±1.66 :A =100% ethanol; B =water;C=50% ethanol+50% water. Control sample was the green tea before SF-CO2 extraction. Table 3. Chemical composition of tested samples. Tea catechins (mg g) Caffeine (mg g )GC EGC C EC EGCG GCG ECG CG Total 25.13±0.67B 3.61±0.21A 32.31±0.43 A 1.30±0.06AB 7.20±0.04A 86.73±0.93AB 3.15±0.15ABC 20.93±0.84 A 0.26±0.04A 155.49±1.99AB 2 28.43±0.78AB 3.53±0.39 A 32.21±0.63 A 1.19±0.05AB 6.77±0.24A 86.44±0.95AB 3.07±0.09ABC 20.93±0.75A 0.24±0.08A 154.38±2.64 AB 3 27.71±0.93AB 3.24±0.52A 29.13±1.59A 1.09±0.03B 6.26±0.39A 82.30±2.82B 2.78±0.30C 19.49±1.35A 0.18±0.08A 144.47±3.89B 4 25.34±0.58B 3.42±0.66 A 31.08±0.88A 1.14±0.09AB 6.50±0.23A 85.50±3.60B 2.88±0.18BC 20.09±0.69A 0.19±0.06A 150.81±5.42 AB 5 28.29±1.39AB 3.33±0.57 A 30.85±0.73A 1.18±0.16AB 6.65±0.74A 87.14±2.41B 2.82±0.09C 19.97±1.27A 0.21±0.08A 152.14±6.08 AB 6 15.80±0.69 C 3.53±0.31 A 32.69±0.14 A 1.35±0.04A 7.23±0.06A 89.66±0.17AB 3.32±0.16AB 21.29±0.33A 0.22±0.04A 159.27±0.31 AB 7 24.23±0.48B 3.28±0.47 A 29.78±0.94 A 1.15±0.08AB 6.59±0.33A 84.99±2.27AB 3.22±0.05ABC 19.91±0.95A 0.18±0.05A 149.10±5.76B 8 9.99±0.18 D 3.33±0.25A 32.02±0.67A 1.32±0.01AB 6.95±0.08A 88.50±1.58AB 3.48±0.08A 20.72±0.16A 0.22±0.06A 156.54±2.15AB 9 25.62±0.24B 3.41±0.08A 31.61±0.52 A 1.29±0.01AB 6.89±0.03A 86.33±0.43AB 3.14±0.09ABC 20.83±0.47A 0.21±0.07A 153.71±0.82 AB Data with different letters in a same column differed significantly at p=0.05 cosolvent near the critical point will lead greatchangesin the solvent density and vapourpressure of solutes, resulting in changes in thesolvation power of the solvent. Although anisobaric increase in the temperature decreasesthe density of the solvent, it increases the vapourpressure of caffeine (Kopcak andMohamed.2005). Increase in temperature decreases SC-CO density and enhance the movement of thesolutes and delivery medium (Kuppers, 1992).The sublimation temperature of caffeine is low(178℃) while catechins such as EGCG are not sublimated. It ispostulatedthatt the vapourincreased while lesschange in the vapour of catechinshappened with the isobaric elevation oftemperature. This resulted in the increase insolubility of caffeine in the mixed solvent of SC-CO2 and ethanol but little changes in the solubilityof catechins. It explained that the concentration ofcaffeine significantly decreased and that of totalcatechins concentrations werenot significantlychanged when temperature was lifted (Table 5). The sample particle size is an important variable in extraction because it affects the mass transferof solute and solvent, and it is directly related tototal surface area for contact between sample andsolvent (Park et al., 2007a). The concentrations ofcaffeine and total catechinss were significantlyaffected by particle size of tea (Table 5). Asparticle size decreased from 1.2 - 2.0 to 0.2-0.6mm, however, caffeine level decreased by 35.5percent while total catechins decreased by 5.4percent (Table 5). This suggests that small particlesizee ofttea Is advantageous forsselectivedecaffeination. Table 4. Variance analysis for caffeine, EGCG and total catechins. Source DF TypeISSs Mean square F° Significant level Caffeine Temperature 2 230.55 115.27 27.51 <.0001 Time 2 33.59 16.79 4.01 0.0363 Particle size 2 557.36 278.68 66.51 <.0001 Cosolvent Volume 2 132.30 66.15 15.79 0.0001 EGCG lemperature 2 23.91 11.95 1.10 0.3557 Time 2 13.00 6.50 0.60 0.5616 Particle size 2 56.09 28.04 2.57 0.1043 Cosolvent Volume 2 12.92 6.46 0.59 0.5636 Total catechins Temperature 2 31.75 15.87 0.39 0.6795 Time 2 31.53 15.77 0.39 0.6812 Particle size 327.87 163.93 4.08 0.0346 Cosolvent Volume 2 70.67 35.33 0.88 0.4323 “Degree of freedom. bTypeI sum of squares. ‘F value. Table 5. Mean comparison between different levels by Tukey grouping. Factors Levels Caffeine Catechins (mg g) (mgg) GC EGC C EC EGCG GCG ECG CG Total 40 27.09 A 3.46 A 31.21 A 1.19 A 6.74 A 85.16 A 3.00 A 20.45 A 0.23 A 151.45 A Temperature (C) 60 23.14 B 3.43 A 31.54 A 1.22 A 6.79A 87.43 A 3.01A 20.45 A 0.21 A 154.07 A 80 19.94 C 3.34 A 31.14 A 1.25 A 6.81 A 86.61 A 3.28 A 20.49 A 0.20 A 153.11 A 1 24.90 A 3.44 A 31.06 A 1.19 A 6.76 A 85.74 A 3.09 A 20.31A 0.21 A 151.80 A Extraction time (h) 2 23.04 B 3.40 A 31.69 A 1.23 A 6.79 A 87.36 A 3.12 A 20.54 A 0.22 A 154.35 A 3 22.23 B 3.39 A 31.14A 1.24 A 6.80 A 86.10 A 3.08 A 20.54 A 0.20 A 152.48 A 0.2-0.6 16.97 B 3.28 A 29.92 B 1.14 B 6.50 B 84.81 A 2.94 B 20.98 A 0.19 A 148.57 B Particle size (mm) 0.6-1.2 26.46A 3.45 A 31.63 AB 1.21 B 6.72B 86.09 A 3.03 B 20.62 A 0.22 A 152.96 AB 1.2-2.0 26.74 A 3.49 A 32.34 A 1.32 A 7.13 A 88.30 A 3.32 A 20.98 A 0.23 A 157.10 A 5 26.34A 3.45 A 31.59 A 1.26A 6.91 A 86.73 A 3.04 A 20.58 A 0.23 A 153.78 A Cosolvent (ml) 15 22.82B 3.44 A 31.56 A 1.23 A 6.86 A 87.03 A 3.21 A 20.71 A 0.21 A 154.25A 30 21.01B 3.33 A 30.74 A 1.18 A 6.57 A 85.44 A 3.05 A 20.10A 0.20 A 150.61 A Mean concentrations were compared between different levels of a same factor group and the data with different letters were significantly different at p=0.05. Although SC-CO2 is selective for caffeine, the solubilityof the polar caffeine in the non-polar SC-CO2 is low,compared to organic solvents like chloroform. Addition ofsmall amount of polar co-solvents like ethanol and waterwill increase polarity, density and hydrogen bondingcapacity of the supercritical mixed solvents (Park et al., 2007a, b). The cosolvent effects in supercritical cosolventinteracts stronglywith the solute. resultingl iirnenhancement of solubility/ of solutesiinnthe polarcosolvent /SC-CO2 mixture (Ting et al., 1993). Theinteractions include hydrogen bonding and the van derWaals forces such as dispersion forces between solute Figure 1. HPLC profiles of control sample (A) and decaffeinated sample (B) 1. GC; 2. EGC; 3.Caffeine;4. C;5. EC; 6. EGCG; 7. GCG; 8.ECG; 9.CG. and solvent molecules. The addition of ethanol and waterwill strengthen the hydrogen bonding between the mixedsolvent and solutes including caffeine and catechins. Thehydrogen bonding can take place betweenhydroxylgroups of hydroxylic derivative solvents and the nitrogenatom on the five membered ring of caffeine and possiblyalso by similar interaction with the keto groups50O of thecaffeine (Figure 2) (Ghabi and Dhahbi, 1999) as well asbetween the hydroxyl groups of hydroxylic solvents andthe phenol groups of catechins. Figure 2 shows that thereare abundant hydroxyl groups on EGCG molecule, amajor catechins in green tea. It can be assumed that thehydrogen bonding interactions between solvent andcatechins were stronger than that between solvent andcaffeine. Hydrogen bonding is an electrostatic attractionbetween two independent polar molecules. The atoms inthe water molecule are arranged with the two H-O bondsat an angle of about 105°and this arrangement results ina polar molecule which provides a net negative chargetoward the oxygen end of the V-shaped molecule and netpositive charges at the hydrogen ends for hydrogenbonding interaction. Although hydrogen bonding canoccur between ethanol molecule and solutes, it is not aseffectively as in water since there is only one hydrogenon each ethanol molecule with sufficient positive charge. In low molecular weight alcohols such as methanol andethanol, the properties of the polar hydroxyl groupdominate the weak intermolecular forces of van derWaals.Inhigher molecular weighttalcohols,ttheproperties of the non-polar hydrocarbon chains dominateand define the solubility. It is known that the Londondispersion forces depend on molecular weight and thedegree that the molecules are polarized. It is known thatthe London dispersion forces tend to be strongerbetween molecules that are easily polarized. HPLC of teasolutions confirmed that caffeine was eluted earlier bypolar solvent than EGCG, a major component of teacatechins (Ye et al., 2009). It suggests that caffeine ismore polar molecule than EGCG. Table 2 showed thatabout 67.9% of caffeine and 7.2% of total catechins wereremoved when pure ethanol was used as cosolvent.However, 78.0% caffeine and 67.6% total catechins wereremoved when water was used as cosolvent. It may beassumed that solvent-solute interactions differentiatedwith changes in cosolvent components. The van derWaals forces dominated solvent-solute interactions in thecase of ethanol as cosolvent, in which the solubility ofcaffeine shigh. However, hydrogen bondinginteractions increased in the case of water or aqueousethanolsolutionas cosolvent, in whichhsolubility of Figure 2. Molecular structures of caffeine and EGCG. catechinsincludingEGCG; liftedbecauseof ttheirabundanthydroxyl groupsfor hydrogenbondinginteractions. The hydrogen bonding interaction betweenhydrogen on water molecule and nitrogen atom, ketogroups of caffeine molecule might also took place in thiscase, resulting in simultaneous interactions of thehydrogen bonding and the van der Waals forces. Thatinterpretswhyalarge percentage of caffeineeandcatechins was removed when water or aqueous ethanolwas used as cosolvent. Therefore, water is not a suitablecosolvent for decaffeination of green tea because thecaffeine was not selectively removed. Conclusion Cosolvents had significantinfluenceonnsselectivedecaffeination of green tea by SC-CO2 extraction. Owingto strong hydrogen bonding interactions betweenhydrogen in water and aqueous ethanol solution andhydroxyl groups on catechins molecules, as much as67.6-75.8% of total catechins were removed afterdecaffeination by SC-CO2 using water or 50% aqueousethanol as cosolvents. Water and aqueous ethanol arenot suitable for cosolvent to decaffeinate green tea bySC-CO2 extraction. The van der Waals forces dominatedmolecular interactions between ethanol and caffeine inthe case of ethanol as cosolvent, resulting in selectivedecaffeination of green tea. Particle size of tea sampleaffects the mass transfer of solute and total surface areafor contact between solvent and the sample, and smallerparticle size is beneficial to selective decaffeination.Increase in isobaric temperature increased the vapourpressure and the movement of caffeine, and 80℃ wasfavorable temperature. ACKNOWLEDGEMENT This work was financially supported by the AgriculturalMinistry of China (Project No. nyhyzx2007-03-35-1). REFERENCES Bahramifar N, Yamini Y, Shamsipur M (2005). Investigation on thesupercritical carbon dioxide extraction of some polar drugs fromspiked matrices and tablets. J. Supercrit.Fluids,35:205-211. Ghabi H, Dhahbi M (1999). Caffeine-water-polypeptide interaction inaqueous solution. Spectrochimica Acta Part A, 55:919-921. Giannelli M, Doyle P,Roman E, Pelerin M, Hermon C (2003). The effectof caffeine consumption and nausea on the risk of miscarriage. Paed.Peri. Epidem., 17:316-323. Gupta S, Saha B, Giri AK (2002). Comparative antimutagenic andanticlastogenic effects of green tea and black tea: a review. Mutat.Res.,512:37-65. Hindmarch I, Rigney U, Stanley N, Quinlan P, Rycroft H, Lane J (2004).A naturalistic investigation of the effects of day-long consumption oftea, coffee and water on alertness sleeps onset and sleep quality.Psychopharm., 149:203-216. Kopcak U, Mohamed RS (2005). Caffeine solubility in supercriticalcarbon dioxide/co-solvent mixtures. J. Supercrit. Fluids. 34:209-214. Kim WJ, Kim JD, Oh SG (2007). Supercritical carbon dioxide extractionof caffeine from Korean green tea. Separat. Sci. Technol., 42: 3229-3242. Kim ES, Liang YR, Jin J, Sun QF, Lu JL, Du YY, Lin C (2007). Impact ofheating on chemical compositionsofgreenteaI liquor. FoodChem., 103: 1263-1267. Kim JW, Kim JD, Kim J, Oh SG, Lee YW (2008). Selective caffeineremoval from green tea using supercritical carbon dioxide extraction.J.Food Eng., 89:303-309. Kuppers S (1992). The use of temperature variation in supercritical fluidextraction of polymers for the selective extraction of low molecularweight components from poly(ethylene terephthalate).Chromatographia 33: 434-440. Kuroda Y, Hara Y(1999). Antimutagenic and anticarcinogenic activity oftea polyphenols. Mutat. Res.436:69-97. Lee SM, Lee HS, Kim KH, Kim KO (2009). Sensory characteristics andconsumer acceptability of decaffeinated green teas. J. Food Sci., 74:135-141. Liang HL, Liang YR, Dong JJ, Lu JL, Xu HR, Wang H (2007).Decaffeination of fresh green tea leaf (Camellia sinensis) by hot watertreatment. Food Chem., 101:1451-1456. Moyers SB, Kumar NB (2004). Green tea polyphenols and cancerchemoprevention: multiple mechanisms and endpoints for phase Iltrials. Nutr.Rev., 62:204-211. Park HS, Choi HK, Lee SJ, Park KW, Choi SG, Kim KH (2007a). Effectof mass transfer on the removal of caffeine from green tea bysupercritical carbon dioxide. J. Supercrit. Fluids,42:205-211. Park HS, Lee HJ, Shin MH, Lee KW, Lee H, Kim YS, Kim KO, Kim KH(2007b). Effects of cosolvents on the decaffeination of green tea bysupercritical carbon dioxide. Food Chem., 105:1011-1017. ( Peeling P, Dawson B ( 2 007). I n fluence o f c affeine ingestion onperceived mood states, c o ncentration, and arousal le v els during a 75-min university lecture. Adv. Physiol. Edu., 31:332-335. ) ( Sakanaka S (2003). A n o vel c o nvenient p r ocess t o o b tain a r a wdecaffeinated t e a polyphenol fr a ction using a lignocellulose column. J.Agric. Food Chem., 5 1:3140-3143. ) ( AgngFoods T ing SST, Tomasko DL, M acnaughton SJ, F o ster NR (1993). Chemicalphysical interpretation o f c o solvent ef f ects in su pe rcritical fl u ids. I ndustr. Eng. Chem. Res., 32:1482-1487. ) ( Ye J H, J in J, Liang HL, Lu JL, Du YY, Zheng XQ, L i ang YR (2009).Using tea stalk li gnocellulose a s an a ds orbent fo r sep a ratingdecaffeinated t ea catechins. Biores. Technol., 100:622-628. ) ( Ye J H, L iang YR, J i n J, L iang HL, D u YY, Lu JL , Ye Q, Lin C (20 0 7).Preparation of partially decaffeinated in s tant gr e en tea. J. Agric. Food Chem.,55:3498-3502. ) ( Ye Q , C hen H , Z h ang LB , Ye JH, Lu JL, Liang YR (2009). Effects ofTemperature, I llumination , and Sodium Ascorbate o n Browning ofGreen Tea I n fusion. F ood Sci. B iotechnol.,18:932-938. ) ( Yesilyurt M (2004). Determination o f t he o ptimum c o nditions f o r t h e boric acid extractio n fro m colemanit e or e in HNO3 solutions . Chem. Eng. P roc.,43:1189-1194. )
确定



还剩6页未读,是否继续阅读?
香港环球分析测试仪器有限公司为您提供《绿茶中咖啡因检测方案(超临界萃取)》,该方案主要用于茶叶中食品添加剂检测,参考标准--,《绿茶中咖啡因检测方案(超临界萃取)》用到的仪器有
相关方案
更多








