方案详情
文
本文由CEM公司首席科学家 Michael J Collins Jr 撰写,主要介绍了目前微波在有机化学的应用,以及微波技术的发展进程。同时也讨论了微波技术在未来的发展趋势,这其中包括:化学家们对微波能量的理解,当前主流的使用方法,现有的硬件以及微波技术在材料合成、生命科学、放大以及流动化学中的应用等等。
微波在合成化学中的起源
什么是微波
微波合成的接受度
微波合成的发展方向
微波合成的潜在应用领域
微波合成是一种安全且高效快速的有机合成方法。
微波能量可迅速加热反应物,使化学反应更快捷进行的同时也减少副反应的产生。
微波技术在实验室中已被普遍接受。
微波合成的继续增长必须克服微波操作困难的错觉。
随着微波合成进入越来越多的本科实验课程中,很多化学家在很早时候就接触到了微波仪器。
微波能量势必在材料合成和生物化学中得到更多的应用,此技术是在放大和和流动化学中取得更好的应用。
方案详情

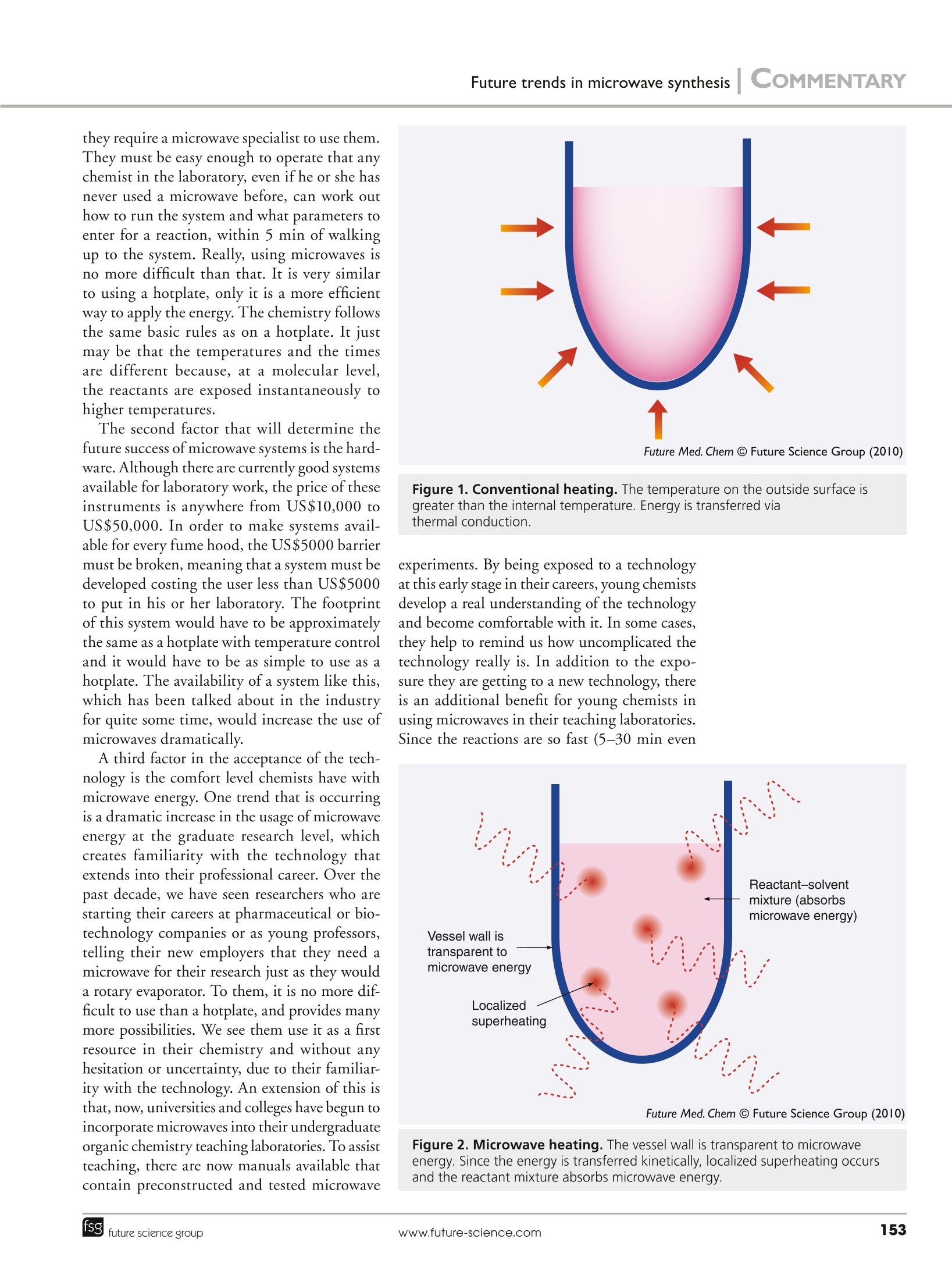
COMMENTARY COMMENTARYI Collins For reprint orders, please contact reprints@future-science.com Future trends in microwave synthesis This article discusses the current status of microwave organic chemistry and briefly explains how the technologyevolved to this point.Several trends in the technology and where it is likely to go in the future are also discussed.These trends include the way in which chemists think about microwave energy, the current method of use and thehardware presently available. Some of the future trends explored are microwave use in relation to materialssynthesis, bioscience applications, scale up and flow chemistry. The beginning of microwaves insynthetic chemistry Microwave energy began to be applied to organicchemistry reactions in the mid-1980s [1,2].The initial work was performed in kitchenmicrowaves or in multiple-mode systemswith 50-100-ml pressure vessels, which weredesigned for acid-digestion applications. Theproblems with performing chemistry in akitchen microwave are reproducibility andsafety; there is no temperature control or stir-ring, there are limitations to the power that canbe applied and the placement of the reactionvessel in the cavity is critically important. Themultimode systems, although capable of tem-perature measurement, stirring and running atelevated pressures, struggle with sample size.Typically, you need to run a total sample sizeof 20 ml or more in these systems. It was notuntil around 2000 that single-mode reactors,which were designed for smaller samples, beganto emerge in the market. A single-mode cavityis a smaller cavity design that allows a standingwave to be propagated in the cavity, resultingin a single mode of high microwave intensity.These cavities must be designed specifically tostay in tune with the load in the microwavecavity. One of the tricks of microwave cavitydesign is that, as the sample in the microwavecavity changes, so does the field. So, the cavityhas to be allowed to adjust to different samplesin order to ensure that the sample is in theregion of high microwave intensity. This alsoaddresses the issue of reproducibility. It ensuresthat if the same reaction is run in a single-modecavity multiple times, the reaction yield willremain fairly consistent. An additional benefitof the smaller, single-mode cavities is that thefootprint of the entire system is much smaller and weighs less than a multimode system, whichis more amenable to a synthetic chemistry labo-ratory, even allowing the microwave to reside inthe fume hood. Initially, the main advantage of microwaveswas thought to be in combination with pres-sure vessels, where microwave energy was used toquickly drive a reaction mixture to elevated tem-peratures and hold it at these temperatures; how-ever, it appears that microwaves actually offeran advantage beyond that, in the application ofthe energy itself. Applying energy to the systemdirectly and instantaneously provides the mol-ecules in the reaction mixture with the energyneeded to react and form chemical bonds, thus,the bulk temperature is a side product involvingthe entire reaction mixture rising to an elevatedenergy level. More microwave users have begunto think of the technology as an efficient wayto apply energy directly into a system instead ofsimply a way to heat things rapidly. This showsbenefits for chemistries performed at reflux, aswell as chemistries carried out in pressure vessels.The advantage of being able to run an open ves-sel is that it applies to a broader range of chem-istries for the medicinal chemist and is less of achange from what they are doing conventionally.It also allows the evolution of gaseous productsand operation at larger scales. ■ Microwaves explained An important topic to address here is howmicrowave energy works, because that has abearing on the future direction of the tech-nology. Microwave energy is spectrum in thesame way as visible light, infrared irradiationand UV irradiation. Microwaves occupy theregion of the spectrum from approximately3iar00-300,000 MHz, just lower in quantum MichaeljCollins jr CEM Corporation, PO Box 200,Matthews, NC,USATel.: +| 704 821 7015Fax: +| 704 821 8710E-mail: michael.collins_jr@cem.com energy than infrared irradiation. This meansthat microwave energy does not have the quan-tum energy necessary to make or break chemi-cal bonds. There is not even sufficient quan-tum energy in microwaves to rotate or vibratechemical bonds, the energy simply causes mol-ecules to attempt to orient along their dipolemoment with the electric field. Since the elec-tric field oscillates billions of times per second,the molecule is in constant motion, attemptingto align with the field. This motion causes fric-tion, which translates into heat or an increasein temperature around this molecule that isquickly dissipated in the bulk solution. In thecase of ions, any ions present in solution willmove through the solution based on theorienta-tion of the electric field and, again, since thisis in constant fluctuation, the ion is movingin constantly changing directions through thesolution, causing a local temperature rise dueto friction. Another interesting phenomenon isthat microwave energy induces electron move-ment in metals, which causes rapid heating.Traditionally, metals are thought to be incom-patible with microwave energy; however, this isnot true of all cases. This electron movementin metals is what causes arcing in some caseswhen the electrons jump a gap from one metalsurface to another. There are ways to preventthe arcing of metals in a microwave cavity, suchas ensuring that the metals are in solution - theliquid provides an insulator between the metalsurfaces making it less likely that electrons willjump the gap. Using these unique properties ofmetals can actually be to a chemist’s advantagein microwave chemistry. For metal-catalyzedreactions, a metal catalyst can be used in a sol-uble form or as an insoluble metal to drasticallyenhance reaction rates and product yields. Thisis due to the fact that the surface of the catalyst,where the reaction occurs, is superheated, thus,essentially, you have supercharged your cata-lyst. This is one of the main reasons why pal-ladium-catalyzed coupling reactions have beenexplored so extensively in the microwave. Thesemechanisms help illustrate that the advantagesseen when using microwave synthesizers reallyboil down to the fact that microwaves allowenergy to be transferred rapidly and specifi-cally and that the application of the energycan be turned on and off instantaneously(FIGUREs I & 2). The reasons for the speculationthat microwave energy is doing something otherthan what is achievable conventionally are thatthe reactions seem to follow unusual kinetics or the results of the chemistry are different. Thisis likely because the measured bulk tempera-ture of the reaction mixture is not indicativeof the instantaneous molecular temperaturesthat are driving the reaction. If you use theseinstantaneous temperatures as the temperaturesdriving the reaction, the kinetics of the reactioncan usually be shown to be the same for bothconventional and microwave methods. The rea-son for unusual results likely occurs becausethe bulk reaction mixture is not exposed to theelevated temperatures for extended amountsof time. Essentially, with microwave energyyou can achieve the temperatures needed tocause chemical conversion without the condi-tions that cause molecular degradation. Thiscan allow chemistries to take place that willnot react or will only occur minimally underconventional conditions. ■Where is microwave acceptance today? A reasonable estimate is that microwave synthe-sizers can be found in 15-20% of organic chem-istry laboratories and they are usually a sharedresource for everyone in that laboratory. Ofthechemists who actually use the microwave,thereis a dichotomy: those who use microwave as alast resort to see if they can make impossiblereactions happen and those who use it on a dailybasis for a large percentage of the reactions theyattempt. We have seen, and are seeing, a move-ment of chemists from the first category to thelatter, which is encouraging for the acceptanceof microwaves into the everyday workflow ofthe organic chemist. In addition to this trend, ahigher percentage of microwave users are usinglaboratory-grade instruments, which improvesthe repeatability of their results, as evidencedby the increasing number of microwave pub-lications each year. In addition, there is quitea number of books and review articles on thetopic of microwave chemistry [3-17]. Where are things going? The presence of microwaves in chemistrylaboratories is increasing, but there are stilla few roadblocks preventing the dramaticexpansion usage. The first of these is ease of use. Part of thisis the perception many chemists have of micro-waves as being difficult to use and requiringa specialist; part of this is that the systemsthemselves must become easier to operate.Microwave systems will never find themselvesin every fume hood or even every laboratory if they require a microwave specialist to use them.They must be easy enough to operate that anychemist in the laboratory, even if he or she hasnever used a microwave before, can work outhow to run the system and what parameters toenter for a reaction, within 5 min of walkingup to the system. Really, using microwaves isno more difficult than that. It is very similarto using a hotplate, only it is a more efficientway to apply the energy. The chemistry followsthe same basic rules as on a hotplate. It justmay be that the temperatures and the timesare different because, at a molecular level,the reactants are exposed instantaneously tohigher temperatures. The second factor that will determine thefuture success of microwave systems is the hard-ware. Although there are currently good systemsavailable for laboratory work, the price of theseinstruments is anywhere from US$10,000 toUS$50,000. In order to make systems avail-able for every fume hood, the US$5000 barriermust be broken, meaning that a system must bedeveloped costing the user less than US$5000to put in his or her laboratory. The footprintof this system would have to be approximatelythe same as a hotplate with temperature controland it would have to be as simple to use as ahotplate. The availability of a system like this,which has been talked about in the industryfor quite some time, would increase the use ofmicrowaves dramatically. A third factor in the acceptance of the tech-nology is the comfort level chemists have withmicrowave energy. One trend that is occurringis a dramatic increase in the usage of microwaveenergy at the graduate research level, whichcreates familiarity with the technology thatextends into their professional career. Over thepast decade, we have seen researchers who arestarting their careers at pharmaceutical or bio-technology companies or as young professors,telling their new employers that they need amicrowave for their research just as they woulda rotary evaporator. To them, it is no more difficult to use than a hotplate, and provides manymore possibilities. We see them use it as a firstresource in their chemistry and without anyhesitation or uncertainty, due to their familiar-ity with the technology. An extension of this isthat, now, universities and colleges have begun toincorporate microwaves into their undergraduateorganic chemistry teaching laboratories. To assistteaching, there are now manuals available thatcontain preconstructed and tested microwave Future Med. Chem C Future Science Group (2010) Figure 1. Conventional heating. The temperature on the outside surface isgreater than the internal temperature. Energy is transferred viathermal conduction. experiments. By being exposed to a technologyat this early stage in their careers, young chemistsdevelop a real understanding of the technologyand become comfortable with it. In some cases,they help to remind us how uncomplicated thetechnology really is. In addition to the expo-sure they are getting to a new technology, thereis an additional benefit for young chemists inusing microwaves in their teaching laboratories.Since the reactions are so fast (5-30 min even Future Med. Chem C Future Science Group (2010) Figure 2. Microwave heating. The vessel wall is transparent to microwaveenergy. Since the energy is transferred kinetically, localized superheating occursand the reactant mixture absorbs microwave energy. for difficult reactions), they can perform reac-tions that were previously too difficult and there--fore required too much time to run in a 2-3-hlaboratory period. Who would have thought ofrunning Diels-Alder reactions, multistmep reac-tions, S Ars or Suzuki couplings inm OrganicLaboratory 101? But are these not the ereactionswe run in the real world? Areas of future exploration There are a few things that are likely to occurwith microwave technology in the next fewyears. First is the advancement of microwavesinto new areas such as materials science and bio-chemistry. Second is the future role of micro-wave in flow and scale-up chemistries. Third,and finally, is the evolution of the ways in whichmicrowaves are used. Microwaves offer some unique advantagesto materials synthesis. Owing to the ability ofmicrowaves to interact directly with the sam-ple and to turn on and off quickly, the particlegrowth of these materials is much more con-trolled and uniform, which results in an improve-ment in the desired property of these materials.There is only a small number of researchers usingmicrowave synthesizers in this field currently,but they are seeing remarkable results. This willmost likely lead to an increased adoption rate ofthe technology over the next few years. Another trend that is occurring is the use ofmicrowaves in biochemistry. This helps illustratethe real advantage of the technology. Most bio-molecules are fairly temperature-sensitive andwt9ill lose their biological function if exposed toelevated temperatures for extended periods oftime; however, there are a lot of biochemicalinteractions that are slow or difficult to occur. Fortemperature-stable small molecules, the solutionwould typically be to apply heat to promote thereaction, but this is less feasible in the ‘bio'world.Microwaves, however, fit this need perfectly. Theyare able to push biochemical interactions to occurwithout the high bulk temperatures that wouldcause loss of activity or degradation. This helpsto show that the great advantage of microwaveenergy is efficient energy transfer rather thana method to rapidly heat a solution to elevatedtemperatures. Think of a microwave as a scalpelcompared with a sledgehammer. This property iswhat has caused a tremendous amount of accep-tance in areas including peptides and proteomics.The challenge in this field is on the instrumentproviders to deliver the right hardware to themarket for these different applications. Flow chemistry is now emerging as an areaof interest and with it is the desire to coupleflow with microwave energy. Although it is aninteresting field and there are many examples ofvery good chemistries being performed in a flowmicrowave, it remains to be seen whether flowwill be adopted more broadly in the medicinalchemistry world. Organic chemists have beenmixing things in pots for over 100 years, andmoving from batch to flow, although techni-cally feasible, would require a significant con-certed movement.The eventual role of flowin a field where the chemistries are varied andconstantly changing is unclear, so it remains tobe seen where microwaves will fit in this area. One of the main questions asked about micro-waves is related to scale up; when will scale-upsystems be available for microwaves? Currently,there are microwave systems to address scale upto the kilogram level, but off-the-shelf’sys-tems are not available for larger scales. Thereare several industrial microwave companies thatspecialize in large-scale microwave systems, butmost of these systems are developed for appli-cations other than bulk chemical processing,such as curing, drying and food processing,for example. The problem with developing ascale-up microwave system for bulk chemicalprocessing is that you start to question theadvantage of microwave. In some of the cur-rent applications where scale-up microwaves areapplied, there are clear advantages to using amicrowave; however, for bulk heating of a largevat of chemicals to drive a reaction, microwaveenergy may not be the way to go. Even continu-ous flow on that scale has its issues, such as thehandling of solids, residence time in the micro-wave field versus throughput and dealing withelevated pressures. The application of micro-wave energy on the research scale demonstratesclear advantages to conventional heating, butit is unclear whether microwaves will ever findtheir way into the manufacturing facilities. Asmore chemical reactions are run in a micro-wave as a primary method, this will push themicrowave scale-up discussion to the forefrontand solutions will have to be found. It may bethat microwave energy is only the solution incertain cases and, in these cases, the solutionmay be a custom design versus an off-the-shelfsystem that will find broad acceptance acrossthe board in manufacturing plants. A decade from now, a microwave will not beuncommon or unique in a chemistry laboratory.It will be much like having a hotplate or a rotary evaporator in your laboratory. The unitswill be small, almost unnoticeable, but theywill be used every day for a majority of thechemistry performed in order to allow rapidreactions and push the boundaries of chemis-tries that will afford good results. The technol-ogy will open up new synthetic pathways andallow the use of more environmentally friendlysolvents. It will yield cleaner products thatwill not require as much purification. In addi-tion, the scientists using this equipment willunderstand how to apply this technology totheir workflow, but they will not be microwave specialists. Microwaves will simply be anothertool in their standard toolkit, much like thinlayer chromatography. Financial & competing interests disclosure The author has no relevant affiliations or financial involve-ment with any organization or entity with a financial interestin or financial conflict with the subject matter or materialsdiscussed in the manuscript. This includes employment, con-sultancies, honoraria, stock ownership or options, experttestimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of.this manuscript. Executive summary Microwave energy is a safe and efficient method of rapidly performing organic synthesis. Microwave energy enables chemists to instantaneously heat their reactants, allowing chemistries to proceed much more rapidly andwith fewer side reactions than conventional methods. Acceptance of microwave technology for the laboratory is growing. The perception of microwave energy as difficult to work with must be overcome in order for the technology to grow, but with the useof microwave synthesis in undergraduate teaching laboratories, many chemists are becoming familiar with the equipment early on. The use of microwave energy in materials synthesis and biochemistry will grow, but it remains to be seen if it is the right technology forscale-up or flow chemistry. Bibliography ( 1 Gedye R, S mith F, Westaway K et al. The use of microwave-ovens for r a pid organic-synthesis. Tetrabedron Lett. 27(3), 279-282 (1986). ) 2 Giguere RJ, Bray TL, Duncan SM,Majetich G. Application of commercialmicrowave-ovens to organic-synthesis.Tetrabedron Lett.27(41), 4945-4948 (1986). Hayes BL. Microwave Synthesis: Chemistry atthe Speed ofLight. CEM Publishing,Matthews, NC, USA (2002). 4 Loupy A (Ed.). Microwaves in OrganicSynthesis. Wiley-VCH, Weinheim, Germany(2002). Kappe CO, Stadler A. Microwaves in Organicand Medicinal Chemistry. Wiley-VCH,Weinheim, Germany (2005). 6 B Lidstrom P, Tierney JP (Eds). Microwave-Assisted Organic Synthesis. Lackwell, Oxford,UK (2005). 7 Loupy A (Ed.). Microwaves in OrganicSynthesis. Wiley-VCH, Weinheim, Germany(2006). ( 8 Kappe CO, D a llinger D, Murphee S S . Practical Microwave Synthesis for OrganicChemists: Strategies, I nstruments, andProtocols. Wiley-VCH, Weinheim, Germany (2009). ) 9 Larhed M, Moberg C, Hallberg A.Microwave-accelerated homogeneouscatalysis in organic chemistry.Acc. Chem.Res. 35(9),717-727(2002). 10 Lew A, Krutzik PO, Hart ME,Chamberlin AR. Increasing rates of reaction:microwave-assisted organic synthesis forcombinatorial chemistry. J. Comb. Chem.4(2),95-105(2002). 11 Wathey B, Tierney J, Lidstrom P, WestmanJ. The impact of microwave-assisted organicchemistry on drug discovery. Drug Discov.Today 7(6), 373-380 (2002). 12 Kappe CO. Controlled microwave heating inmodern organic synthesis. Angew. Chem. Int.Ed. Engl. 43(46),6250-6284(2004). 13 Man AK, Shahidan R. Microwave-assistedchemical reactions. J.Macromol. Sci.A44(4-6), 651-657(2007). 14 Kappe CO. Microwave dielectric heating insynthetic organic chemistry. Chem. Soc. Rev.37(6),1127-1139,2008. 15 Caddick S, Fitzmaurice R. Microwaveenhanced synthesis. Tetrahedron 65(17),3325-3355 (2009) 16 Kappe CO,Dallinger D. Controlledmicrowave heating in modern organicsynthesis: highlights from the 2004-2008literature. Mol. Diversity 13(2),71-193 (2009). 17 Santagada V, Frecentese F, Perissutti E,Fiorino,F, Severino, B, Caliendo G.Microwave assisted synthesis: a newtechnology in drug discovery. Mini-Rev. Med.Chem. 9(3),340-358(2009). FMC.C Future Science LtdFuture Med. Chem. (, SSN uture science groupfssFuture Med. Chem.(
确定




还剩3页未读,是否继续阅读?
培安有限公司为您提供《微波合成法中发展趋势检测方案(微波合成仪)》,该方案主要用于其他中发展趋势检测,参考标准--,《微波合成法中发展趋势检测方案(微波合成仪)》用到的仪器有CEM Discover 2.0 300mL腔体环形聚焦单模微波合成仪
相关方案
更多
该厂商其他方案
更多










