
方案详情
文
加速食品氧化过程---新型的食品氧化分析设备
意大利VELP公司和米兰的大学科研机构联合进行了为期半年的实验:使用新型的食品氧化分析设备OXITEST,来加速食品的氧化过程;在提高样品的温度、大量的空气或氧气情况,分析了食品的保存时间、食品的氧化速度的变化情况;使用OXITEST食品油脂氧化测定仪对葵花籽油和橄榄油进行了不同条件的实验,建立了一套关于植物油的分析方法。文章的内容对从事食品油脂氧化测定和分析的科研工作者有十分重要的参考价值。
方案详情

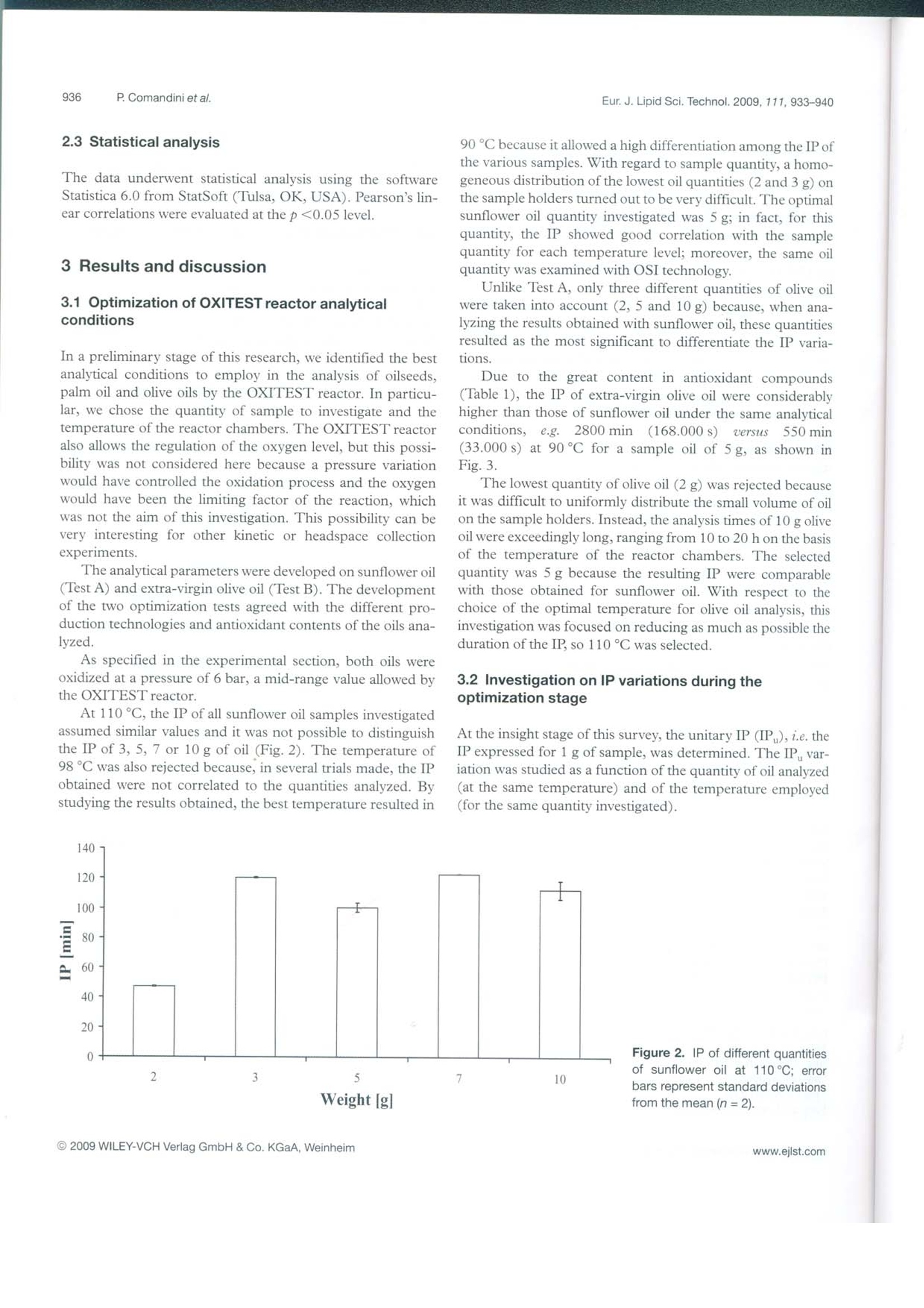
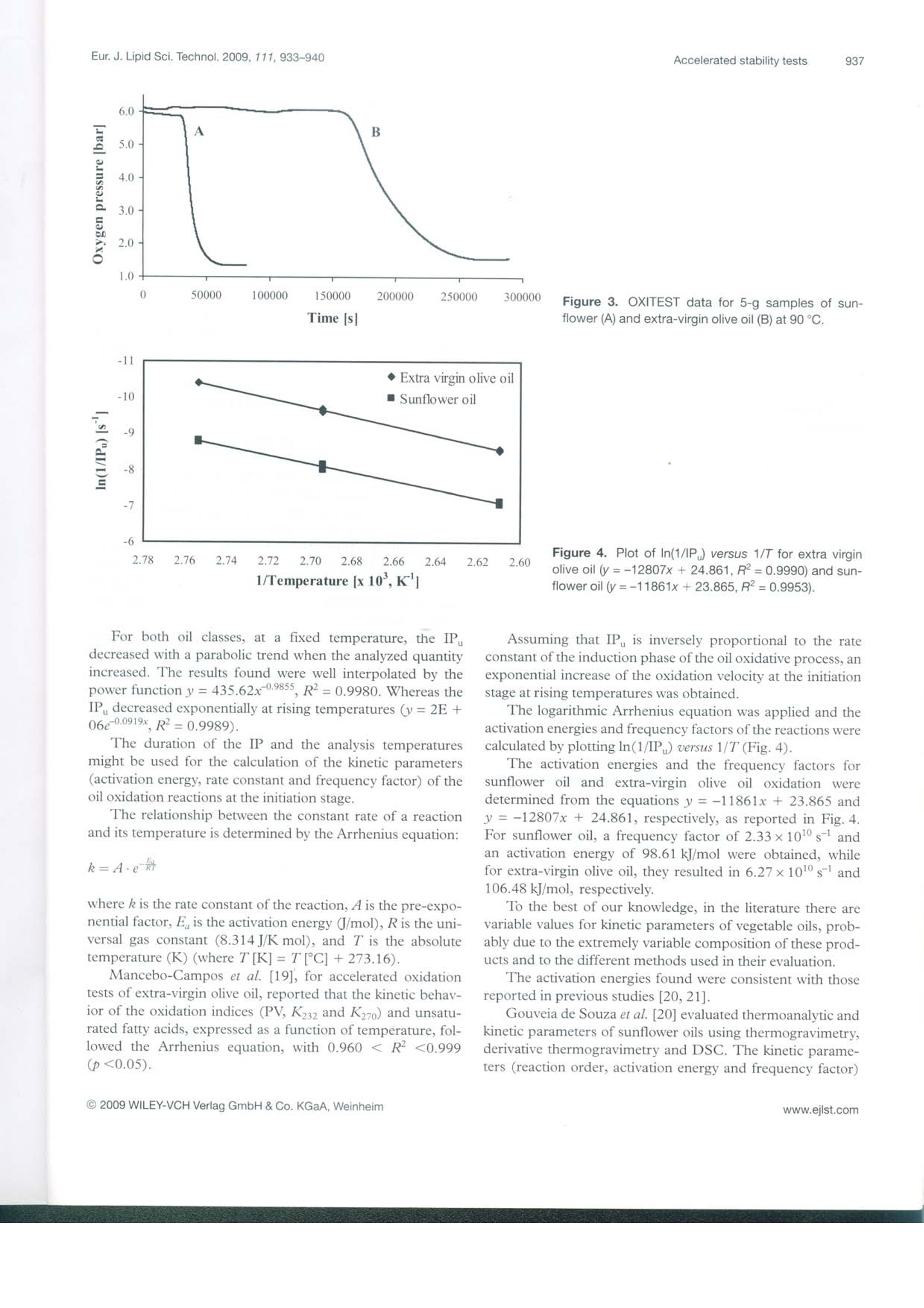
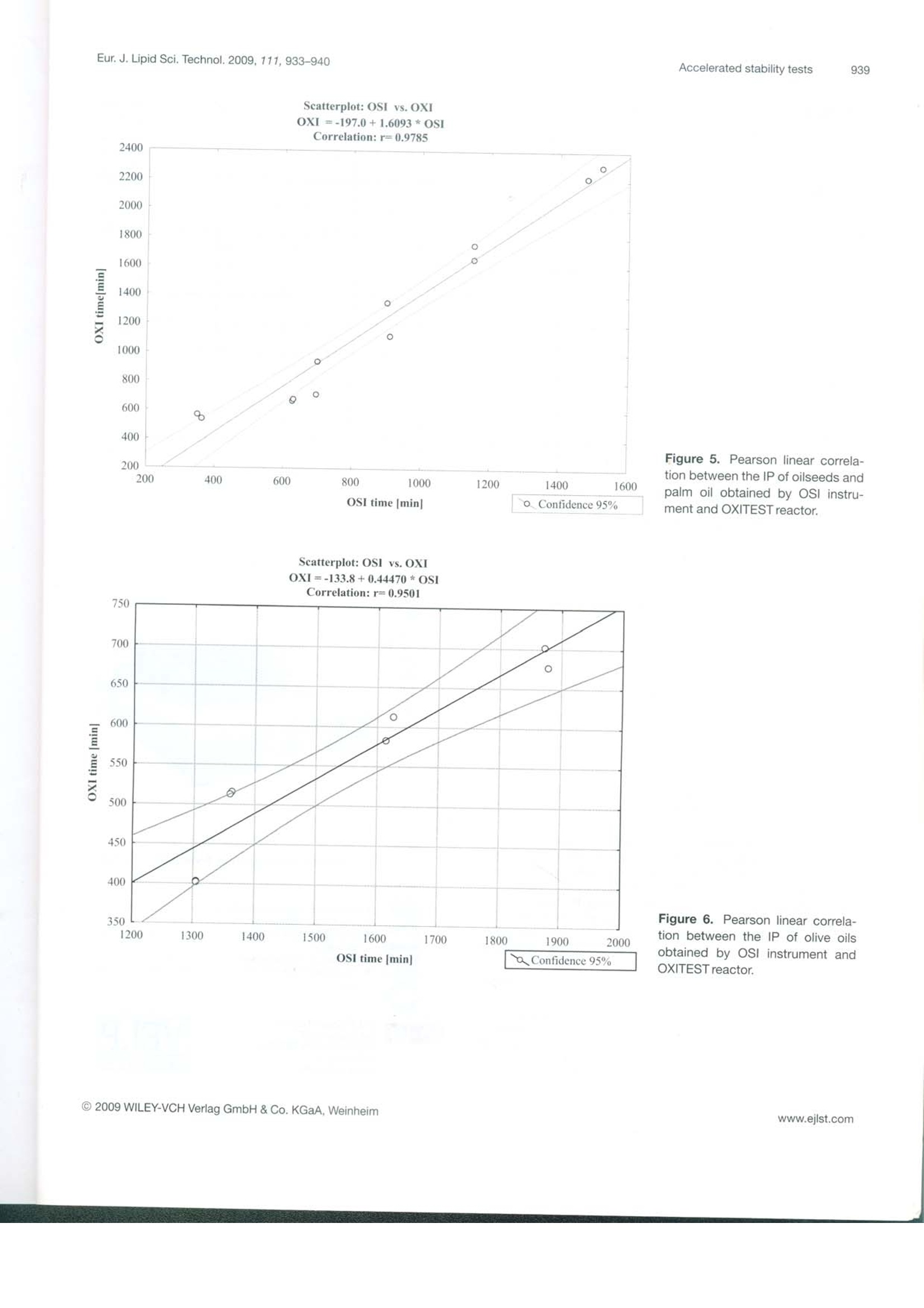
D 14215 ISSN 1438-7697EJLST 855-950 (2009)Vol.111·No.99/2009 Eur. J. Lipid Sci. Technol. 2009, 111, 933-940933 European journal of Lipid Science and Technology Lipidomics·Nutrition·Health·EngineeringAnalytics·Chemistry Special Issue: Euro Fed Lipid Highlights Euro fed Lipid www.eilst.com Research Paper Accelerated oxidation: Comparative study of a new reactorwith oxidation stability instrument Patrizia Comandini, Vito Verardo1, Paola Maiocchi?and Maria Fiorenza Caboni Dipartimento di Scienze degli Alimenti, Universita di Bologna, Cesena, Italy VELP Scientifica srl, Usmate, Italy Accelerated oxidation tests, such as the determination of the induction period, increase the lipid oxidationrate by exposing a food to elevated temperatures, in the presence of excess quantities of air or oxygen. Inaddition to the well-founded oxidative stability index (OSI) method, an innovative and promising tech-nique is the oxidation test (OXITEST) reactor. A new analytical method was developed with OXITESTto oxidize vegetable oils. At a preliminary stage of the investigation, the induction periods of sunflower andextra-virgin olive oil obtained by the OXITEST reactor were plotted against temperature, on the basis ofthe Arrhenius law; the activation energy and the frequency factor of lipid oxidation were calculated andresulted in 98.61 kJ/mol and 2.33×10s, respectively, for sunflower oil and 106.48 kJ/mol and6.27×10s, respectively for extra-virgin olive oil. The new oxidative technique was employed todetermine the induction periods of vegetable oils; the results obtained were well correlated with thoseachieved with OSI technology, with a Pearson correlation coefficient r=0.9785 (p<0.05) for oilseeds andpalm oil and r= 0.9501 (p<0.05) for extra-virgin olive oils. Keywords: Food /Oil/OSI /Oxidation /OXITEST Received: January 21, 2009; accepted: March 19, 2009 1 Introduction The oxidative stability of foods could be defined as theirresistance to oxidation [1], one of the most important reac-tions associated with the lipid portion, occurring by autoxida-tion, thermooxidation or photosensitized oxidation [2]. The oxidative state is a significant indicator of perfor-mance and shelf life of foods [1]; it can be evaluated by usingseveral analytical techniques due to the complexity of thechemical reactions involved in oxidation and the broad set ofcompounds produced [3]. Lipid oxidation is influenced by different factors such asthe energy input (light or heat), the composition of fatty acidsand their degree of unsaturation, the type and concentrationof oxygen (atmospheric triplet or singlet oxygen), and the ( Corresp on d e n c e : V ito Verardo and Maria F i orenza Caboni, P iazza Goi-danich 60, 4 7023 Cesena (FC ) , It a ly. ) ( E -mai l : v ito.verardo@unibo.it and maria.caboni@unibo.it ) Fax: +39 0547 382348 ( A bb r e viatio n s : IP , i nduction period; IP u , un i tary in d ucti o n per i o d; O S I , oxidative s tability index; O X ITES T, o x idation tes t . ) presence of minor compounds such as metals, pigments,phospholipids, free fatty acids, mono- and diacylglycerols,thermally oxidized compounds and antioxidants. Manyefforts have been made to improve the oxidative stability offoods by systematic studies on the effects of these factors.However, they interactively influence the oxidation processand it is not easy to differentiate the individual consequencesof each factor. Such implications make it impossible to assess the extentof oxidation by a single investigation, and often many analysesare necessary to evaluate the oxidative state of food. Thewidely used techniques include analytical methods, instru-mental investigations and indirect tests. The first studied thequantity and the composition of primary (hydroperoxides andconjugated dienes) and secondary oxidation products (alde-hydes, ketones, alcohols and hydrocarbons), the others evalu-ate the loss of unsaturated fatty acids, vitamins or antioxidantsnormally present in food [4]. For the food industry, it is very important to determine asquickly as possible the shelf life of fresh and processed foods.This kind of information allows a correct managing of fooddistribution activities and is also useful in evaluating the oxi-dative effects on food preservability of formulations. Lipid oxidation is a process that occurs fairly slowly atroom temperature, and the time required to reach the rancid-ity threshold and the consumer rejection of a food ranges fromdays to months, on the basis of the product considered. Rou-tine analysis with this duration is unacceptable, but has to beperformed in a relatively short period of time [5]. To speed up the studies of food deterioration kinetics,since 1930 a large number of tests for accelerated shelf lifeevaluation (named accelerated shelf life tests (ASLT)) havebeen developed [6]. These investigations allow the assessmentof the stability of fats and oils in a considerably shorter timethan the real degradation processes. In particular, acceleratedoxidation tests increase the lipid oxidation rate by exposing thefood to elevated temperatures, in the presence of excessquantities of air or oxygen [4]. Sometimes intense light,metallic catalysts or enzymes are applied to promote chemicalor physical changes [7]. The information provided by ASLT must be consideredonly as a guideline when determining the product stability,because such investigations cannot precisely reproduce theoxidative behavior of the food product under the real storageconditions. Frankel [8] highlighted that, when high tempera-tures (100℃ or above) are used to accelerate oil degrada-tion, the oxidation reactions differ from the characteristicones at lower temperatures. Therefore, it is advisable tocompare the relative stability of various oils at different tem-peratures. Up to now, several accelerated oxidation techniques havebeen developed to obtain the induction period (IP) or oxida-tive stability index (OSI): Originally, oxidative food stabilitywas evaluated by using the Schaal oven test and the activeoxygen method (AOM) [7]; nowadays, automatically workinginstruments are used, such as the Oxidative Stability Instru-ment (Omnion, Decatur, IL, USA)[9, 10] and the Rancimatinstrument (Metrohm AG, Herisau, Switzerland) [11]. Manyauthors who evaluated the oxidative state of oils by differentialscanning calorimetry (DSC) [12] and pressure DSC (PDSC)[13] highlighted an elevated correlation between thermalanalysis and traditional oxidative tests. Recently, also electronspin resonance (ESR) has been applied to assess the oxidativestability of oils [14]; unlike OSI and thermal analysis, ESRevaluates the earlier stage of the oxidative process, by mon-itoring the tendency to radical formation under mild acceler-ated oxidation conditions. Velasco et al.[14] found high linearcorrelations between ESR and Rancimat and between ESRand DSC,suggesting the utilization of ESR as a valid alter-native in predicting the oxidative stability of oils. Here, we present the potential of the oxidation test(OXITEST) reactor (Velp Scientifica, Usmate, Milan, Italy),an innovative instrument able to evaluate the oxidative state offoods and leading to the kinetics of oxidation processes. The OXITEST reactor (Fig.1) subjects the sample to ahigh-oxidative-stress environment (high temperature andoxygen overpressure) in order to evaluate, in a short period oftime, the resistance to fat oxidation. This instrument monitors Figure 1. Schematic diagram of the OXITEST reactor. the drop in oxygen pressure inside oxidation chambers,according to the ability of the food to oxidize. The OXITESTreactor allows temperature regulation from room temperatureto 110 °C, while the oxygen pressure may vary up to 8 bar (0-8 bar). These values allow the assessment of the full intrinsicresistance of any type of food (solid or liquid samples anddough) to becoming rancid. The internal parts in contact withthe food samples (oxidation chambers, sample holders andcovers) are in titanium, in order to avoid chemical corrosion.The speed of the oxygen absorption is a useful parameter alsoto foresee food shelf life. The aim of this report was to compare a well-establishedanalytical technique, such as the OSI, with the OXITESTreactor; this new instrument is able to evaluate the IP of thesample analyzed, as reported in this preliminary research, andit is also useful in the evaluation of the oxidative kinetics of thesamples studied. This investigation consisted of two main steps: firstly, theoptimization of the analytical conditions in the OXITESTreactor and, secondly,the comparison of the IP obtained withthis new instrument with those achieved with conventionalOSI technology. The evaluation of the OXITEST perfor-mances was our objective realized by the analysis of vegetableoils at different oxidation states. The OXITEST reactor may be used to directly analyzesolid foods, without a previous lipid extraction [15], but in thisinsight study vegetable oils were tested because there are con-solidated analytical techniques to evaluate the oxidative stateof this kind of foodstuff. 2 Experimental 2.1 Evaluation of the IP by the OXITEST reactor duringthe optimization step 2.1.1 Oil samples used in the optimization step The samples examined during the first stage of the investiga-tion for optimization of the analytical conditions were sun-flower oil, as representative of oilseeds, and palm oil, as well asan unrefined vegetable oil rich in polyphenols, such as extra- virgin olive oil. The sample oils were bought on the localmarket in Cesena (Italy) and were protected against light untilanalysis. 2.1.2 Analytical conditions employed during theoptimization step An OXITESTreactor (Velp Scientifica, Usmate, Milan, Italy)fitted with two separate oxidation chambers was used. During the optimization of the analytical conditions, theoxidations were performed at 90, 98 and 110℃, with anoxygen pressure of 6 bar, as indicated by Velp Scientifica [15].Sample quantities were 2, 3, 5, 7 and 10 g for sunflower oiland 2, 5 and 10 g for the extra-virgin olive oil. Each oil samplewas monitored twice and the IP, expressed in minutes, wereobtained by using the two-tangent method. 2.2 Comparison of IP obtained by the OXITESTreactor and by OSI technology 2.2.1 Oil samples used in the comparison step During comparison of the OXITEST reactor and OSI meth-od performances, oilseeds (sunflower and peanut oils), palmoil and olive oils (extra-virgin olive and olive oils) were used.All samples were purchased on a local market in Cesena(Italy). As reported in Table 1, different brands of oils areidentified by progressive numbers. The sunflower oils used inthis investigation were different both in unsaturation and inoxidation level: Precisely, the samples named Sunflower oil 2and 3 (Table 1) were submitted to preliminary heating by microwaves (for 12 and 15 min, respectively), in order toevaluate the instrumental response when lipid oxidation hasbeen initiated. The oil samples were stored in a dark place atroom temperature until analysis. 2.2.2 Chemical characterization of the oil samples Total fatty acids and peroxide values (PV) were determinedaccording to Chiavaro et al. [16]. The oxidized fatty acids(OFA) were evaluated by HPLC method according to Rovel-lini and Cortesi [17]. The method proposed by Carrasco-Pancorbo [18] was employed to determine the total phenoliccontent. 2.2.3 Evaluation of IP by the OXITEST reactor The best heating conditions, as determined during the opti-mization stage, resulted in 90℃ for sunflower, peanut andpalm oil analysis, and 110℃ for olive oil investigations; theoxygen pressure was set at 6 bar. Each oil sample (5 g) wasmonitored twice and the IP, expressed in minutes, were cal-culated by using the two-tangent method. 2.2.4 Evaluation of IP by OSI method An eight-channel OSI instrument (Omnion, Decatur, IL,USA) was used. The instrument was set at 110℃ and an airflow rate of 120 mL/min was used, following the analyticalprotocol described by Poerio et al.[10]. Each sample (5g) wasanalyzed in duplicate (n=2) and the IP,determined with thetwo-tangent method, were expressed in minutes. Table 1. Chemical characterization of oilseeds, palm and olive oils. Sample Phenolic compound 16:0 16:1 18:0 18:1 18:2 18:3 20:0 20:1 22:0 24:0 PVs OFA Palm oil n.d. 58.0" 0.1 5.5 27.2 5.4 0.3 0.3 0.0 Peanut oil n.d. 8.1 1.5 49.9 35.4 1.1* 0.9 2.1* 1.0° 0.5d 0.1 Sunflower oil 1 n.d. 7.6° 0.1 4.7a.b 22.5 75.5" 0.3 0.4 0.2 1.0 0.4 0.74 0.1 Sunflower oil 2 n.d. 3.5 3.2 79.7 12.5~ 0.7 0.1 7.9 2.1 Sunflower oil 3 n.d. 3.7 3.6 78.8* 12.3 0.5 0.1 13.4 Sunflower oil 4 n.d. 3.7 3.5 79.1 12.1 0.8 0.3 0.5d 0.0° Olive oil 107.1 10.9 0.8 3.7 71.5" 8.5d 1.3" 11.6° 0.15 EVO oil1 169.8 10.6° 0.8" 2.1 77.1* 8.3 0.8 6.4 0.0 EVO oil 2 209.5° 11.8° 0.7 1.4 73.5" 7.6 1.1" 4.2° EVO oil3 284.7 12.1 0.7 2.3 75.6" 8.1° 1.0" 3.0° 0.o n.d., Not detectable; EVO, extra-virgin olive. Mean values in the same column with different letters show statistically significant differences (p≤0.05). 'mg of phenolic compound/kg of oil. meq O,/kg fat. "mg OFA/100 g fat. Fatty acids are expressed as g/100 g of fat. 2.3 Statistical analysis The data underwent statistical analysis using the softwareStatistica 6.0 from StatSoft (Tulsa, OK, USA). Pearson’s lin-ear correlations were evaluated at the p <0.05 level. 3 Results and discussion 3.1 Optimization of OXITEST reactor analyticalconditions In a preliminary stage of this research, we identified the bestanalytical conditions to employ in the analysis of oilseeds,palm oil and olive oils by the OXITEST reactor. In particu-lar, we chose the quantity of sample to investigate and thetemperature of the reactor chambers. The OXITEST reactoralso allows the regulation of the oxygen level, but this possi-bility was not considered here because a pressure variationwould have controlled the oxidation process and the oxygenwould have been the limiting factor of the reaction, whichwas not the aim of this investigation. This possibility can bevery interesting for other kinetic or headspace collectionexperiments. The analytical parameters were developed on sunflower oil(Test A) and extra-virgin olive oil (Test B). The developmentof the two optimization tests agreed with the different pro-duction technologies and antioxidant contents of the oils ana-lyzed. As specified in the experimental section, both oils wereoxidized at a pressure of 6 bar, a mid-range value allowed bythe OXITEST reactor. At 110℃, the IP of all sunflower oil samples investigatedassumed similar values and it was not possible to distinguishthe IP of 3, 5, 7 or 10 g of oil (Fig. 2). The temperature of98℃ was also rejected because,in several trials made, the IPobtained were not correlated to the quantities analyzed. Bystudying the results obtained, the best temperature resulted in 90℃ because it allowed a high differentiation among the IP ofthe various samples. With regard to sample quantity, a homo-geneous distribution of the lowest oil quantities (2 and 3 g) onthe sample holders turned out to be very difficult. The optimalsunflower oil quantity investigated was 5 g; in fact, for thisquantity, the IP showed good correlation with the samplequantity for each temperature level; moreover, the same oilquantity was examined with OSI technology. Unlike Test A, only three different quantities of olive oilwere taken into account (2,5 and 10 g) because, when ana-lyzing the results obtained with sunflower oil, these quantitiesresulted as the most significant to differentiate the IP varia-tions. Due to the great content in antioxidant compounds(Table 1), the IP of extra-virgin olive oil were considerablyhigher than those of sunflower oil under the same analvticalconditions,e.g. 2800 min (168.000 s) uersils 550 min(33.000 s) at 90℃ for a sample oil of 5 g, as shown inFig. 3. The lowest quantity of olive oil (2 g) was rejected becauseit was difficult to uniformly distribute the small volume of oilon the sample holders. Instead, the analysis times of 10 g oliveoil were exceedingly long, ranging from 10 to 20 h on the basisof the temperature of the reactor chambers. The selectedquantity was 5 g because the resulting IP were comparablewith those obtained for sunflower oil. With respect to thechoice of the optimal temperature for olive oil analysis, thisinvestigation was focused on reducing as much as possible theduration of the IP, so 110℃ was selected. 3.2 Investigation on IP variations during theoptimization stage At the insight stage of this survey, the unitary IP (IP),i.e. theIP expressed for 1 g of sample, was determined. The IP,, var-iation was studied as a function of the quantity of oil analyzed(at the same temperature) and of the temperature employed(for the same quantity investigated). Figure 2. IP of different quantitiesof sunflower oil at 110℃; errorbars represent standard deviationsfrom the mean (n=2). Figure 3. OXITEST data for 5-g samples of sun-flower (A) and extra-virgin olive oil (B) at 90℃. Figure 4. Plot of In(1/IP) versus 1/T for extra virginolive oil (y=-12807x+24.861,R²=0.9990) and sun-flower oil (y=-11861x+23.865,R?=0.9953). For both oil classes, at a fixed temperature, the IPdecreased with a parabolic trend when the analyzed quantityincreased. The results found were well interpolated by thepower function y= 435.62x-0.9855,R²=0.9980. Whereas theIP decreased exponentially at rising temperatures (y=2E+06e-0.0919x、R²=0.9989). The duration of the IP and the analysis temperaturesmight be used for the calculation of the kinetic parameters(activation energy, rate constant and frequency factor) of theoil oxidation reactions at the initiation stage. The relationship between the constant rate of a reactionand its temperature is determined by the Arrhenius equation: where k is the rate constant of the reaction, A is the pre-expo-nential factor, Eis the activation energy (J/mol), R is the uni-versal gas constant (8.314 J/Kmol), and Tis the absolutetemperature (K)(where T[K]= T[C]+273.16). Mancebo-Campos et al.[19], for accelerated oxidationtests of extra-virgin olive oil, reported that the kinetic behav-ior of the oxidation indices (PV, K3 andK) and unsatu-rated fatty acids, expressed as a function of temperature, fol-lowed the Arrhenius equation, with 0.960 < R² <0.999(p<0.05). Assuming that IP is inversely proportional to the rateconstant of the induction phase of the oil oxidative process, anexponential increase of the oxidation velocity at the initiationstage at rising temperatures was obtained. The logarithmic Arrhenius equation was applied and theactivation energies and frequency factors of the reactions werecalculated by plotting ln(1/IP) versus 1/T (Fig.4). The activation energies and the frequency factors forsunflower oil and extra-virgin olive oil oxidation weredetermined from the equations y=-11861x + 23.865 andy=-12807x + 24.861, respectively, as reported in Fig. 4.For sunflower oil, a frequency factor of 2.33×10s andan activation energy of 98.61 kJ/mol were obtained, whilefor extra-virgin olive oil, they resulted in 6.27×10s- and106.48 kJ/mol, respectively. To the best of our knowledge, in the literature there arevariable values for kinetic parameters of vegetable oils, prob-ably due to the extremely variable composition of these prod-ucts and to the different methods used in their evaluation. The activation energies found were consistent with thosereported in previous studies [20, 21]. Gouveia de Souza et al. [20] evaluated thermoanalvtic andkinetic parameters of sunflower oils using thermogravimetry,derivative thermogravimetry and DSC. The kinetic parame-ters (reaction order, activation energy and frequency factor) were obtained by approximation and integral methods, usingkinetic equations proposed by Coats and Redfern (CR),Madhusudanan (MD), Horowitz and Metzger (HM) and VanKrevelen (VK); the activation energies determined with theHM and VK methods were the most similar to those we foundwith the OXITEST reactor. With respect to extra-virgin olive oil, more contrastingvalues for the activation energy were found in the literature.Jadhav et al. [21] related kinetic parameters of vegetables oilsto the content of transition metals: These elements increasethe rate of oil oxidation due to the reduction of the activationenergy of the initiation step in the autoxidation. For extra-vir-gin olive oils, which contain relatively high quantities of tran-sition metals (9.8ppb and 0.73 ppm are the concentrations ofcopper and iron, respectively, reported by [22]), the activationenergy ranges from 63 to 104 kJ/mol. 3.2 Comparison of the OXITEST reactor with the OSImethod In the following stage of the investigation, the analytical con-ditions optimized for sunflower oil (5 g of sample analyzed at90℃) and olive oils (5 g analyzed at 110C) were employedin the accelerated oxidation of commercial peanut, sunflower,palm and olive oil (extra-virgin olive oil and olive oil) samples.Table 1 shows the evaluated chemical parameters of the oilsamples. The IP obtained with the OXITEST reactor were com-pared to those achieved with OSI technology, as reported inTable 2. Analyzing the results obtained for both oil classes, wenoted that all the IP ofoilseeds and palm oil obtained with theOXITEST reactor were superior to those achieved with theOSI method, whereas in the case of olive oils, an oppositetrend was verified. The reasons for these different values hadto be related to the different analytical conditions used during OXITESTanalysis, in particular to the different temperaturesemployed (90℃ for peanut, sunflower and palm oils, and110℃ for olive oil OXITEST analysis). In fact, the purposeof this report was to verify the correlation degree of two dif-ferent ASLT techniques, regardless ofthe analytical condi-tions used for each sample class. As explained in Section 3.1, the choice to analyze byOXITESTolive oils at higher temperature was caused by theirmajor oxidative stability, which led to excessively long OXIT-EST investigation times at 90C. During the well-establishedOSI analysis, instead, the same temperatures (110℃) andquantities (5 g) were employed for both oils. The IP of oilseeds and palm oil obtained by the OXITESTturned out to be higher than the respective values achievedwith the OSI investigations, due to the lower reaction temper-ature used in the first technique (90C). Under the sameanalytical conditions, however, both oil classes presentedlonger IP with OSI than with OXITEST analysis (data notpublished). In order to verify the correlation degree between the twodifferent analytical techniques, the Pearson linear correlationindex was extrapolated. The obtained values, r=0.9785(p<0.05) for oilseeds and palm oil (Fig.5) and r=0.9501(p<0.05) for olive oils (Fig. 6), denote a statistical corre-spondence between the two analytical techniques. In addition to the good correlation with a well-foundedtechnology as the OSI method, the OXITEST reactor pre-sents several advantages: One of the most important advan-tages is the possibility to directly analyze solid, liquid anddoughy foods, without the need to perform a preliminary iso-lation of the fat portion [15]. Lipid extraction, necessary whenan OSI instrument or the Rancimat are used, might lead to amuch longer overall analysis time [23]; this characteristicmakes the OXITEST reactor a suitable technology also forrapid analysis of complex foods. Table 2. IP of oilseeds, palm and olive oils evaluated by OSI instrument and OXITEST reactor. Oil samples IP OSI [min] IP OXITEST [min] Repetition 1 Repetition 2 Repetition 1 Repetition 2 Palm oil 1479 1521 2220 2300 Peanut oil 627 630 667 679 Sunflower oil 1 351 363 567 539 Sunflower oil 2 696 699 713 940 Sunflower oil 3 909 900 1121 1349 Sunflower oil 4 1152 1152 1653 1752 Olive oil 1305 1305 403 402 EVO oil 1 1362 1359 516 513 EVO oil 2 1614 1626 583 613 EVO oil 3 1872 1878 702 677 EVO,Extra-virgin olive. Scatterplot: OSI vs.OXIOXI =-197.0+1.6093*OSICorrelation: r=0.9785 2400 2200 0 2000 1800 0 1600 一E1400XO 1200 1000 0 800 600 400 200 200 400 600 800 1000 1200 1400 1600 OSI time |min] o Confidence 95% Figure 5. Pearson linear correla-tion between the IP of oilseeds andpalm oil obtained by OSl instru-ment and OXITEST reactor. Scatterplot: OSl vs.OXI OXI=-133.8+0.44470* OSI Correlation: r= 0.9501 750 700 0 650 600EEvXO 0 550 500 450 400 350 Figure 6. Pearson linear correla-tion between the IP of olive oilsobtained by OSI instrument andOXITEST reactor. Conflict of interest statement The authors have declared no conflict of interest. References ( [1] M. D . G uille n , N . C a b o: Four ier t r ansform in f rared sp e c tr a d ata v ers us pero x ide and anisidine va l ues t o determine o xi da-tive stabili t y of edib le o il s. Foo d C he m. 2002, 7 7,5 0 3 -510. ) ( [2] J . A . E F ar i a , M . K . Mukai: U s e of a ga s chromatogr ap hicr e actor to s tudy l i p id p ho t ooxidation. j A m O il C he m So c .1983, 60 ,7 7 - 81 . ) ( [3 ] E D . G unst o n e : Re ac t ion o f o xy g en and un s aturated fa t ty a cids .j A m Oil C h e m Soc. 1 984, 61, 4 41- 44 7. ) ( [ 4 ] J . I . Gray : Measurement o f lipid o xida t io n : A r eview.j A m OilC h em S oc . 19 7 8 , 55,5 3 9-546. ) ( [ 5 ] S . G o mez- A lonso ,V . Ma n cebo- C a m pos, M. D . S a lvador, G .Frega p ane: Ox ida t i o n k i n etic s i n oli ve oi l tri a cylgl ycero lsunder ac celera t ed s h el f - l ife t e sting ( 25- 7 5℃) . Eu r y Lipid Sc i T e c hnol . 20 0 4 , 106, 3 69- 3 7 5 . ) ( [ 6] J.O. R agnarsson, T . P. Lab u za: A c celerated she l f life t esting f o r o xidative ranci d it y in f ood s - A re v i e w . F oo d C he m . 1 97 7 , 2, 2 91- 3 08. ) ( [ 7] P. J . Wan: Ac c e lera t ed s t ability t est s. In : Metho ds t o As s ess Q u ality an d S t ability of O i ls a nd Fa t -Co nt aining F o ods. E d s. K . Wa r ner,N. A . M. E sk in, AOAC P ress, C h ampaign,IL ( U S A ) 2 000, pp. 1 7 9- 1 89. ) ( [ 8] E . N. Fr ankel: In s earch o f better me t hods t o evaluate n a tural antioxidants and ox id ative sta bility in fo o d lipi d s. Tr ends Fo o dS c i T e ch nol. 1993 , 4 , 220- 2 25. ) ( [ 9 ] S . D . B a rtee, H . J . Kim, D . B . Min:Ef f e c ts of a n t ioxidant s onthe o xidative st abi l it y o f oil s c o nt aining ar a c h i d oni c , do c - osa pentaenoic and doco s ahexaenoic a cid s. Am Oil C h e m Soc . 2 0 07 , 84, 3 6 3 - 3 68 . ) ( [1 0 ] A. P oerio , A. B endini, L . Cerret a ni, M . B on o li-Car b ognin, G . Ler c k e r: Ef fect o f ol ive f r uit f r eezing on o xi d ativ e sta bi lity ofv i rgin o liv e oi l . E u r j L i p i d S ci Te chno l. 200 8, 110 , 36 8- 3 7 2 . ) [11] R. Farhoosh, R. Niazmand, M. Rezaei, M. Sarabi: Kineticparameter determination of vegetable oil oxidation underRancimat test conditions. Eurj Lipid Sci Technol.2008,110,587-592. [12] C. P. Tan, Y. B. Che Man,J. Selamat, M. S.A. Yusoff: Com-parative studies of oxidative stability of edible oils by differ-ential scanning calorimetry and oxidative stability indexmethods. Food Chem. 2002,76,385-389. [13] B. Kowalski, K. Ratusz, D. Kowalska, W. Bekas: Determina-tion of the oxidative stability of vegetable oils by differentialscanning calorimetry and Rancimat measurements. Eur yLipid Sci Technol. 2004,106165-169. [14]J. Velasco, M. L. Andersen, L. H. Skibsted: Evaluation of oxi-dative stability of vegetable oils by monitoring the tendency toradical formation. A comparison of electron spin resonancespectroscopy with the Rancimat method and differentialscanning calorimetry. Food Chem. 2004,85,623-632. ( [ 1 5] Vel p S c i e n t if ic a : OXIT E S T Re a c t or Ope ra t i n g Ma n u al . V E L P S cientifica s rl , Us mate (Ita ly ) 2 0 06 . ) ( [ 1 6 ] E. C h iavaro , E . Vittad i n i , M. T . R o dr i g ue z -E st r a da , L . C er - ret a ni , M. Bono l i , A . Ben d ini , G. L e rc k er: Mo n o v ariet a l ex tr a v i rgin olive o il s: Co rr e l a t i on b e twe en t hermal p ro p e r ti es an d chemical c o mpo si t i o n .j A g r ic Fo o d C h e m . 2 00 7 , 5 5, 1 0779 -1 0 786 . ) ( [1 7] P . R ov e l li n i , N . Corte si: O x i d ativ e sta t u s of ex t r a virgi n olive oi l s : H PLC ev al u at i on . I taly F oo d Sci . 2 0 0 4, 1 6 ,3 35- 3 4 4 . ) ( [1 8 ] A . C a rrasco - Pancor b o, L . Ce r retani, A. B en d ini, A. S eg u ra- C ar retero , G. Lercker, A. F e rn a ndez - Gutierrez: E v alu a ti o n o fthe influence of therma l ox idati on on t he p henolic c ompos i- t io n a nd o n the a nt ioxi d ant activ i ty of e x t ra-virg i n olive oi l s. y A g ric Fo o d C h e m. 20 07, 55 , 4 77 1- 4 7 8 0. ) ( [ 19 ] V. Mance b o- Ca m p os, G . Fr e gapa n e , M. D. S al v ado r : K i n etic s tudy f o r t he develo p ment of an accelerat e d oxidative s t ability t est t o estimate v i r gin ol i ve o i l p o t e ntial s h elf lif e. E u r Lip i d S c i T ec hn o l. 2 008,110 , 969 - 976 . ) ( [20] A. G ouveia d e Sou z a,J. C . O liveira Santos , M. M . C once icao , M. C. D a n tas S i lva, S . Prasa d : A thermoanalytic and kinetic s tudy of s unfl o wer o il . B ra zj C h e m E n g. 2004, 21, 265 -2 7 3 . ) ( [21] S. J. Jadha v, S . S . Nimbalka r , A . D. Kulkami , D . L. Madhavi: L ipid o xidation in bio l o g ical and f o o d s y s te ms. In : F o o d A nt i- ox idants . Eds. D. L . Madhavi, S. S . D eshpa nd e, D . K. Sa l u-nkhe, Marcel Dekker, New Yo r k, NY (US A) 1 996, p p. 5- 64. ) ( [ 2 2 ] Ministry o f A griculture , Fisheri es and F o od o f the U n itedKingdom: M et als in co l d- p ressed oil s . F oo d Sur u eil l a nce I n f ormation She e t . 199 7 ,138. ) ( [ 23] D . S andmeier, G . Zi e gle d er: Th e diff i culties i n the de t ermi- n atio n o f induc t ion time s o f fat s usin g th e Rancimat . Su ess w a ren . 1985、 2 9、 4 87 -4 89. ) 0c l Suitable for oxidation stability studies on foods, ols and fats■Easy to use.reliable. fast ■ Shelf-life analysis on whole food, without preliminary fatseparation ■ Analysis on the same sample in duplicate ■ The software controls the entire operation in a user friendlyway VELP Scientifica srlvia Stazione 1620040 Usmate (MI) Italyinse@velp.itwww.velp.com WWILEY-BLACKWELL iterScienceC WILEY-VCH Verlag GmbH & Co.KGaA, Weinheimwww.ejlst.com
确定






还剩7页未读,是否继续阅读?
嘉盛(香港)科技有限公司为您提供《食品中氧化过程检测方案(定氮仪)》,该方案主要用于其他食品中理化分析检测,参考标准--,《食品中氧化过程检测方案(定氮仪)》用到的仪器有瑞典OPSIS全自动6位脂肪测定仪(索氏抽提仪)SX-360、瑞典OPSIS 全自动凯氏定氮仪 KD-310
相关方案
更多
该厂商其他方案
更多









