方案详情
文
利用带有圆二色检测器的jasco手性拆分液相色谱对(R)-和(S)-沙利度胺(反应停)体外羟基化代谢产物进行分析,得到所有代谢产物的绝对构型.
方案详情

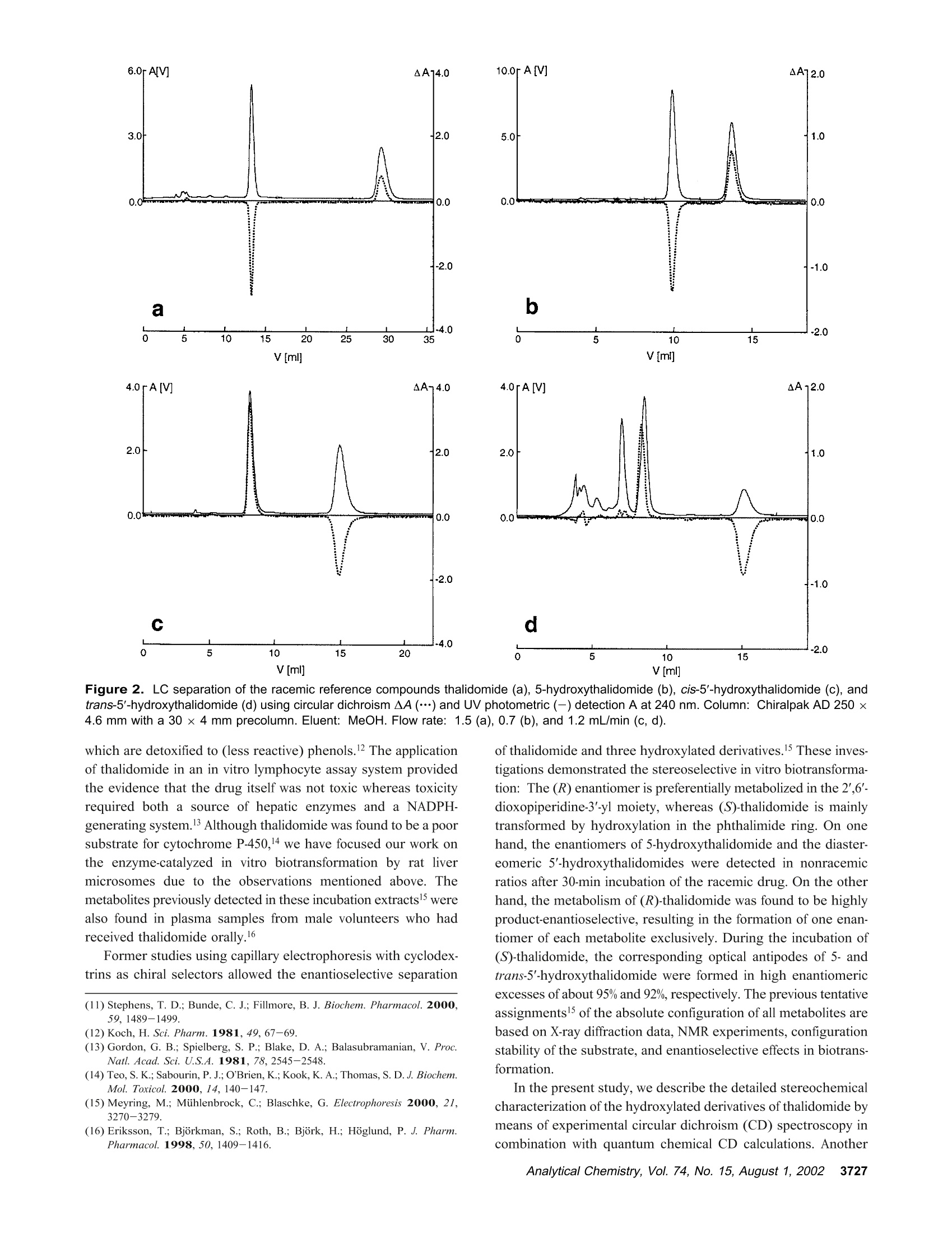
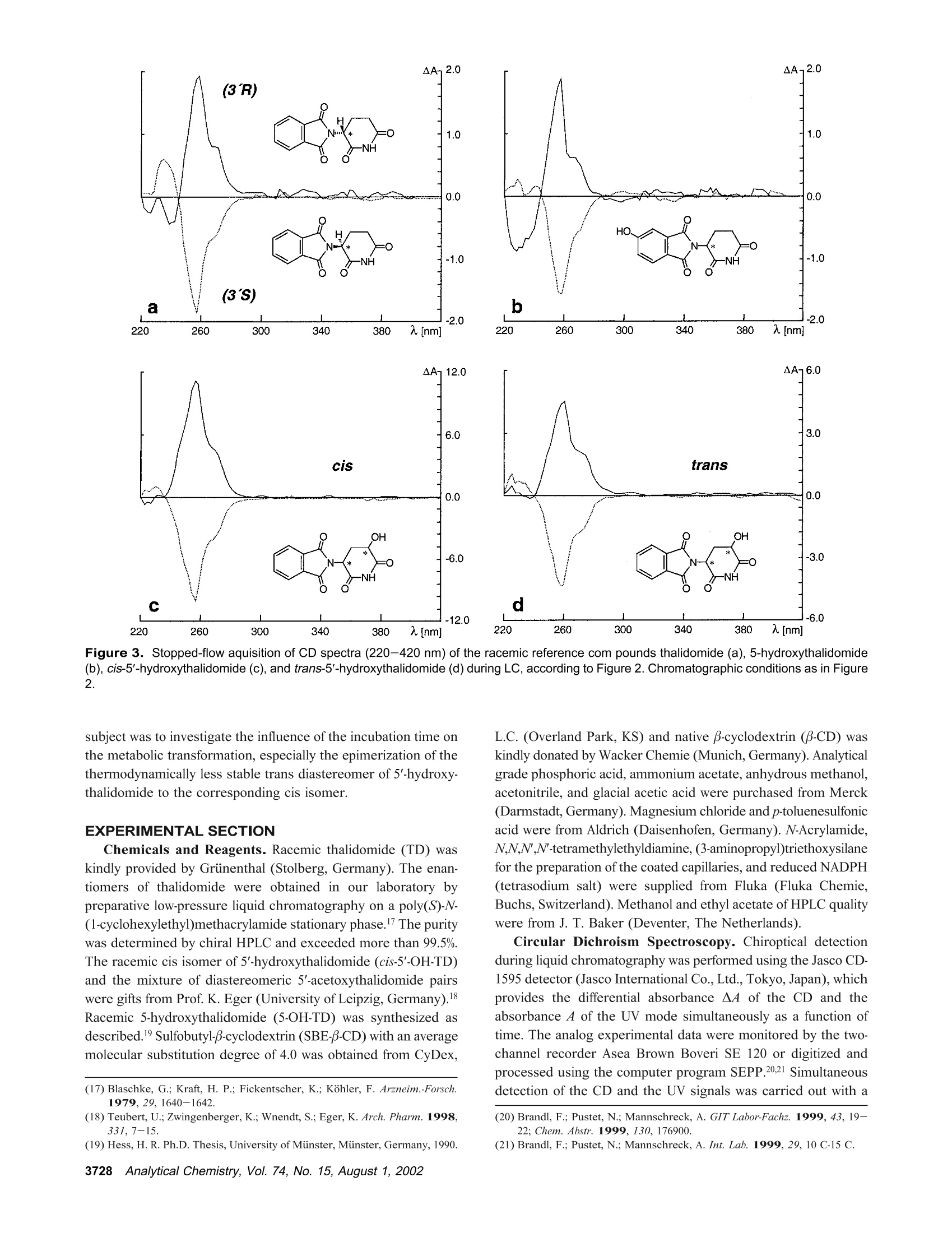
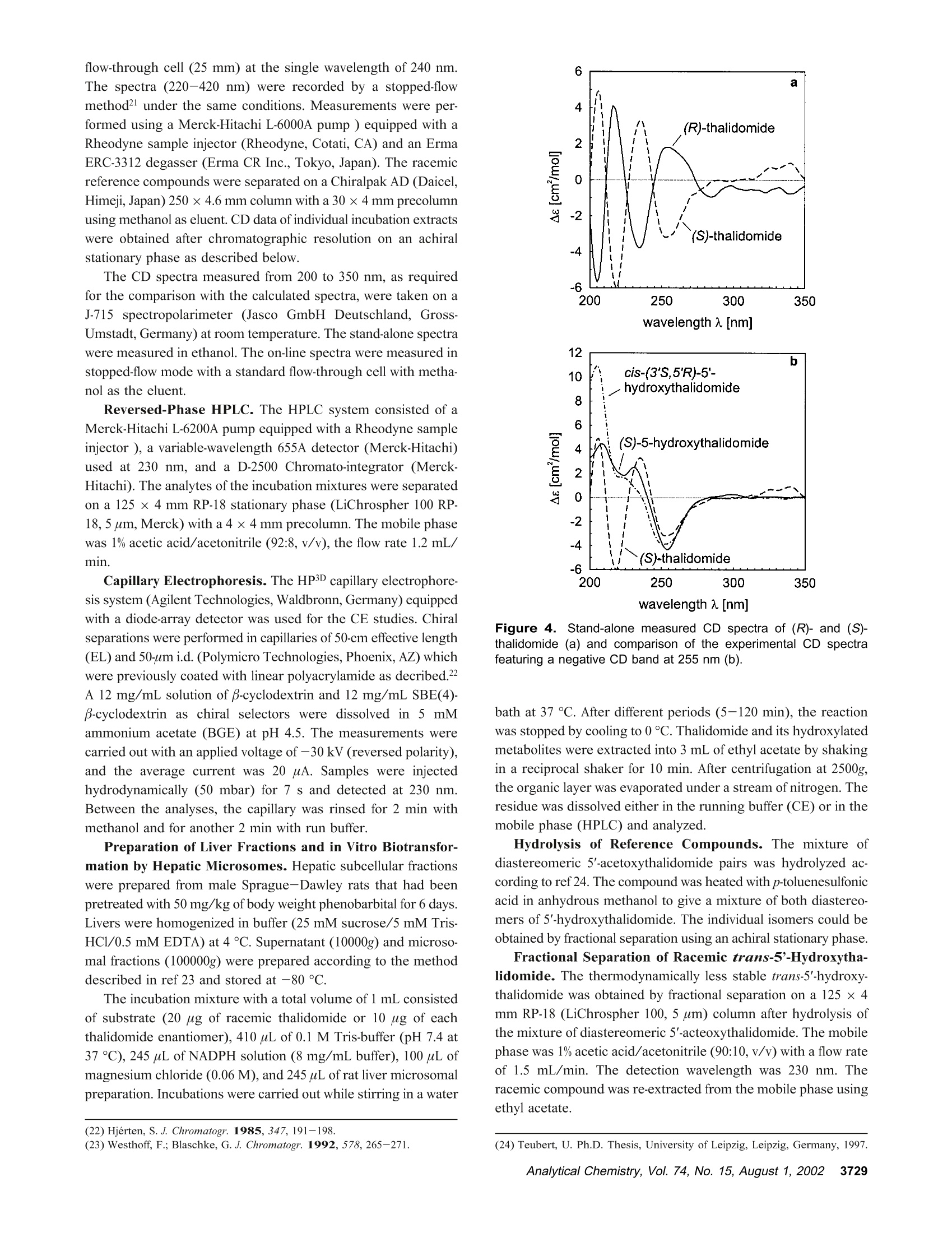
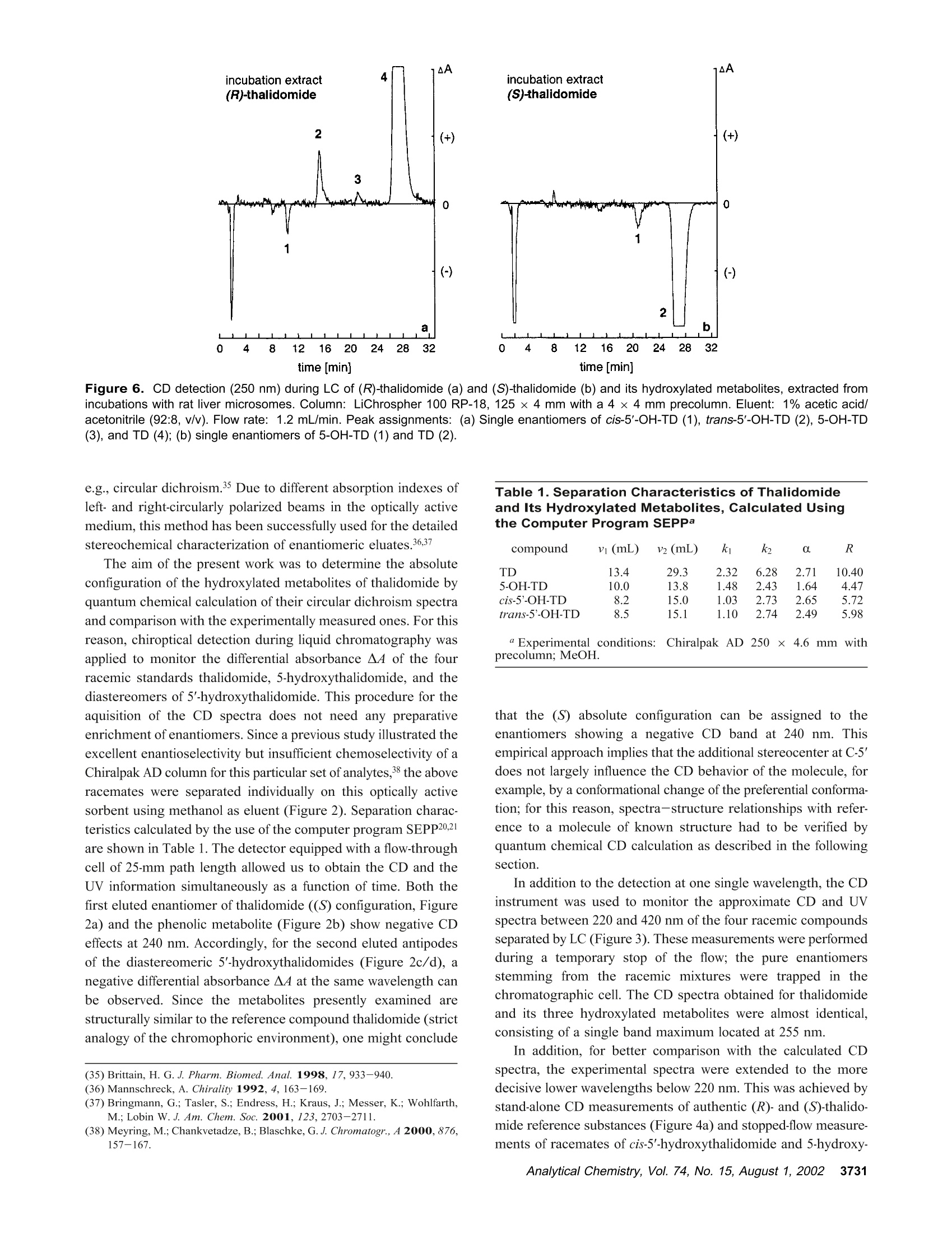
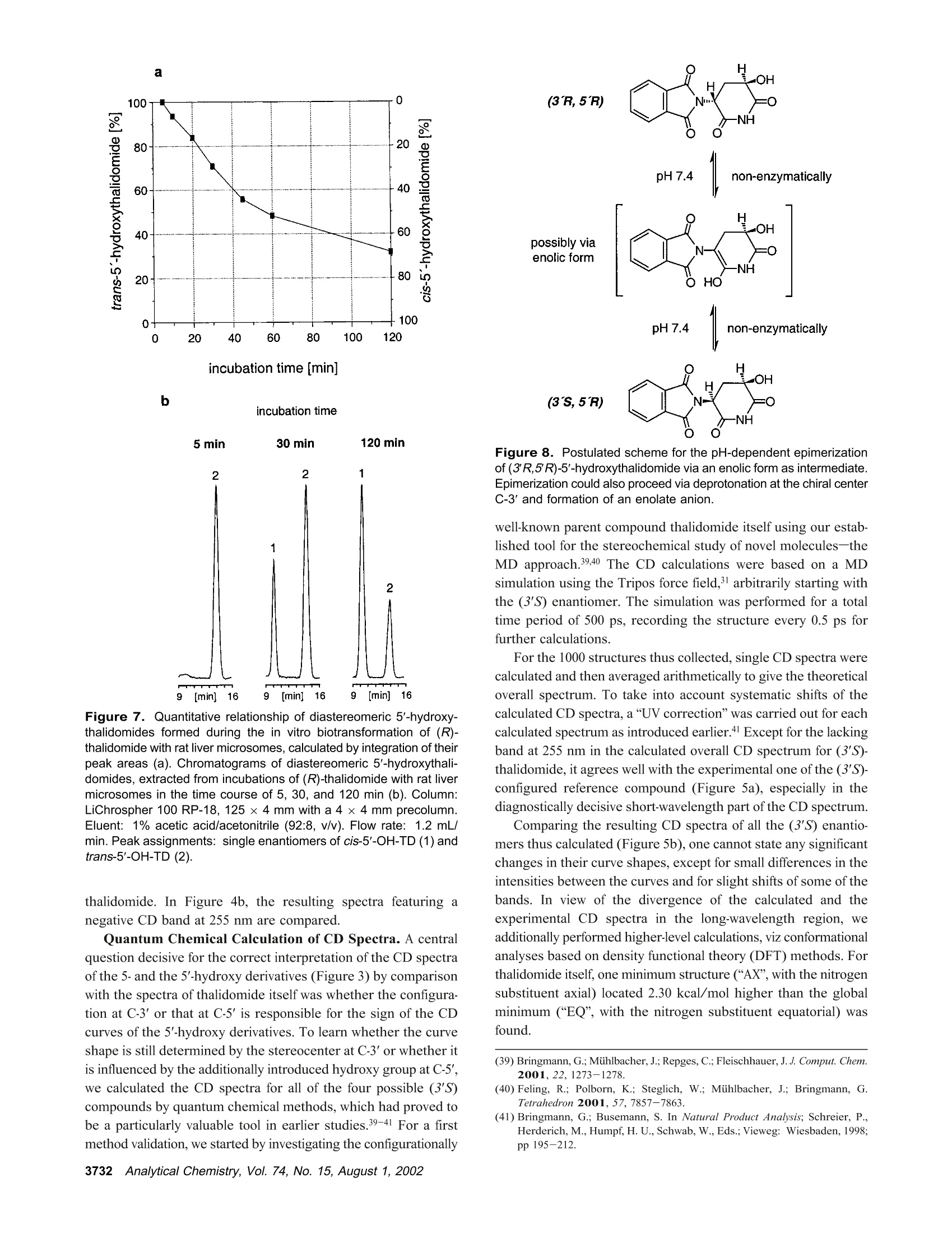
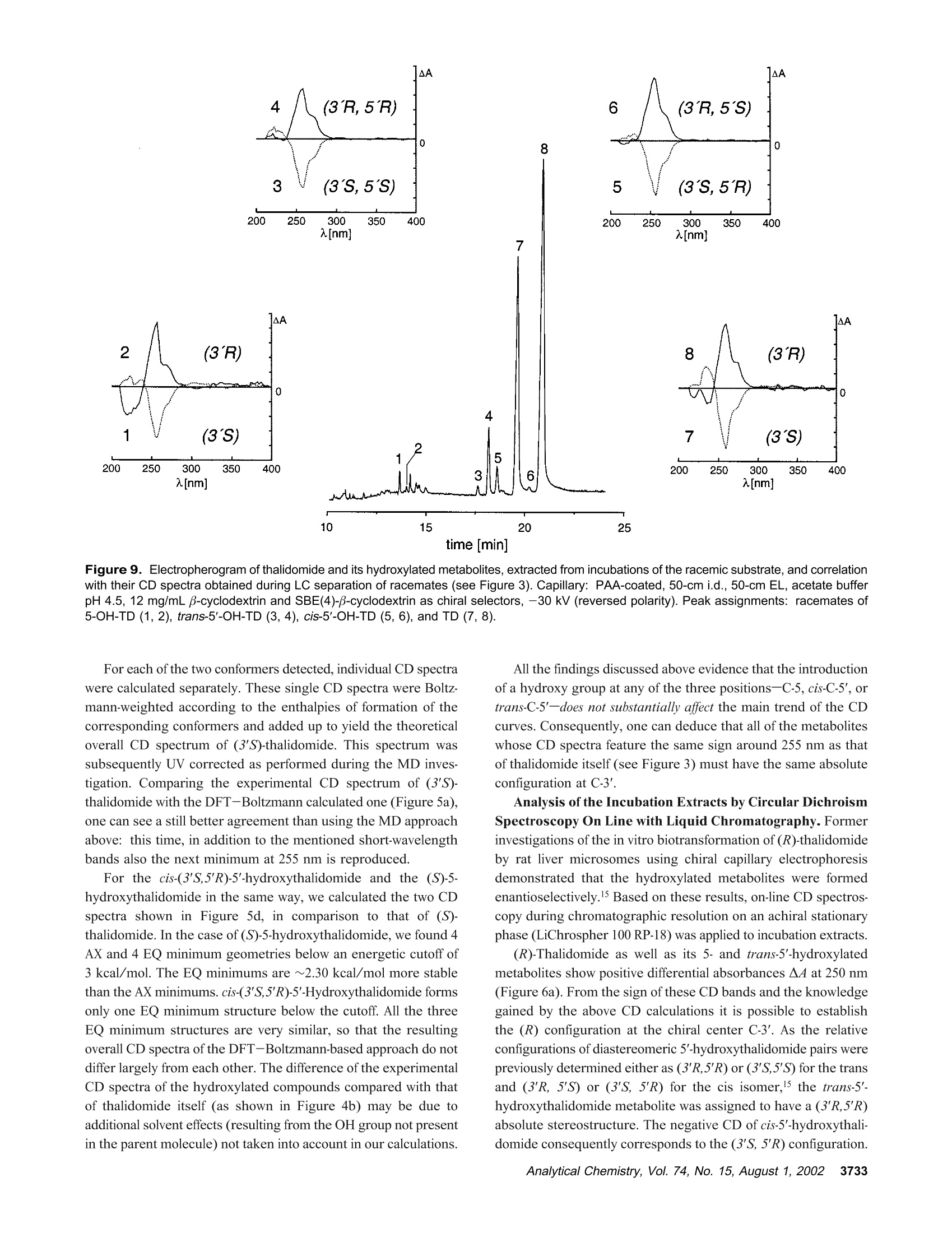
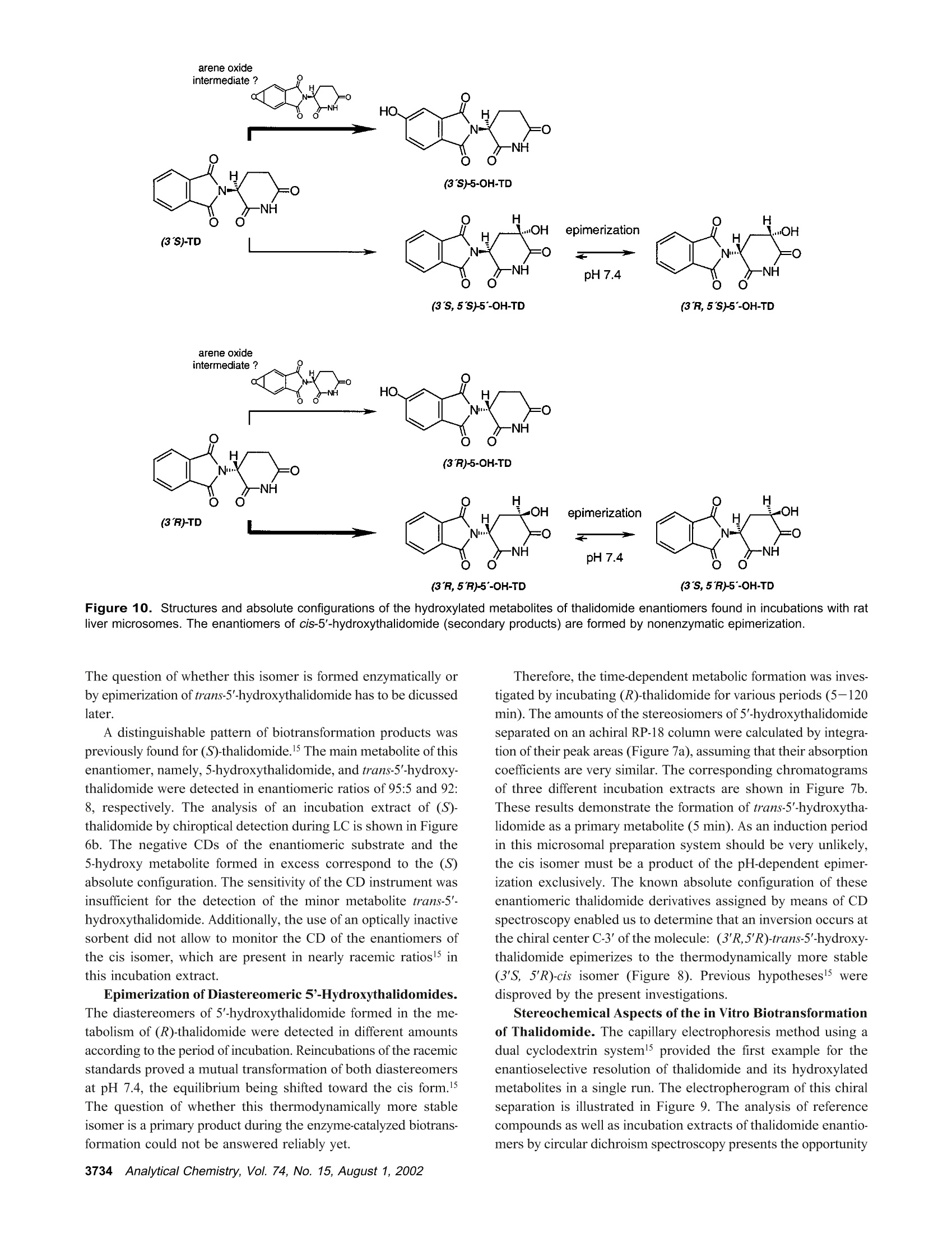
Anal. Chem. 2002, 74,3726-3735 AA-2.0AA-2.0 In Vitro Biotransformation of (R)-and(S)-Thalidomide: Application of Circular DichroismSpectroscopy to the StereochemicalCharacterization of the Hydroxylated Metabolites Michael Meyring,t Jorg Muhlbacher,* Kim Messer,* Nikola Kastner-Pustet,s Gerhard Bringmann,*,+Albrecht Mannschreck,*,s and Gottfried Blaschke*,s Institute of Pharmaceutical Chemistry, University of Munster, Hittorfstrasse 58-62, 48149 Muinster, Germany, Institute ofOrganic Chemistry, University of Wuirzburg, Am Hubland, 97074 Wuirzburg, Germany, and Institute of Organic Chemistry,University of Regensburg, Universitatsstrasse 9, 93040 Regensburg, Germany Circular dichroism (CD) spectroscopy was successfullyused for the stereochemical characterization of the hy-droxylated metabolites formed during the in vitro biotrans-formation of (R)-and(S)-thalidomide. Incubation extractsof the individual enantiomers were analyzed by HPLC onan achiral stationary phase combined with CD detection.The CD data of the almost enantiopure eluates of themetabolites were compared with the CD spectra quantumchemically calculated for the respective structures.Theresults allowed us a reliable determination of the absolutestereostructure for all of the metabolites.The chiral centerof thalidomide is unaffected by the stereoselective biotrans-formation process.(3'R,5'R)-trans-5'-hydroxythalidomideis the main metabolite of (R)-thalidomide, which epimer-izes spontaneously to give the more stable (3'S,5'R)-cisisomer.On the contrary,(S)-thalidomide is preferentiallymetabolized by hydroxylation in the phthalimide moiety,resulting in the formation of (S)-5-hydroxythalidomide. Thalidomide, identified to be a human teratogen in the early1960s,12 was recently approved for sale in the United States forthe treatment of erythema nodosum leprosum, a complication ofHansen’s disease. The originally sedative-hypnotic drug wasfound to suppress the release of the cytokine tumor necrosis factor(TNF)-a and to inhibit angiogenesis.4.5 Due to its antiinflammatoryand immunomodulatory properties thalidomide has been usedsuccessfully in graft-versus-host disease, rheumatoid arthritis, andseveral dermatologic disorders, e.g., Behcet's syndrome and lupus ( * Corresponding authors. G .B.: (e-mail) b laschg@uni-muenster.de; (tel) +4 9 2 51 8 333311;(fax) + 49 251 : 8 332144. G .B.: (e-mail) b ringman@chemie.uni-wuerzburg.de; (tel ) + 49 931 8 885323;( f ax) + 4 9 931 88 8 4755.A.M.: (e-mail) a lbrecht.mannschreck@chemie.uni-regensburg.de;(t e l) + 4 9 941 9434503; (fax)+49 941 9434617. ) ( T Univer s ity of Miinster. ) ( U n iversity of Wiirzburg. ) ( s University of Regensburg. ) ( (1 ) Lenz, W. Dtsch. Med . Wochensch r . 1961,86 , 2555-2556. ) ( (2)McBride, W . G. L ancet 1961,2,1358. ) ( (3)Marwick, C . J. Am. Med. Assoc . 1997,278,1135-1137. ) ( (4) Koch, H. Prog . Med . Chem . 1985, 2 2,166-242. ) ( (5)D'A m ato, R. J. ; L o ughnan, M. S .; Fly n n, E.; Fol k man, J. Proc . Natl . Acad.Sci. U . S.A . 1994, 91 , 4082-4085. ) Figure 1. Structure of thalidomide enantiomers. erythematosus.6.Notably, the enantiomers of thalidomide (Figure1) show a significant enantioselectivity in pharmacodynamics. Theimmunomodulatory effects may chiefly be exerted by (S)-thali-domide. whereas sedation was related to the blood concentrationof the (R) enantiomer. About four decades after Lenz and McBride independentlyassociated the use of thalidomide in early pregnancy with theoccurrence of phocomelia, the exact mechanism of teratogenesisremains unknown. Several different hypotheses were discussed,suggesting, for example, the bioactivation of thalidomide byembryonic prostaglandin H synthase, which causes oxidativedamage to DNA. The latest results proposed that the drug affectsthe pathway of insulin-like growth factor I and fibroblast growthfactor 2 stimulation of a-v and 3 integrin subunit genes duringdevelopment. Another tenable explanation of the teratogenic effects involvesthe metabolic formation of reactive arene oxide intermediates, ( (6) Stirling,D . Annu . Rep . Med . Chem. 1995, 30, 319-327. ) ( (7) Ochonsky, J . S.; Revuz, J . Eur. J . Dermato l . 1994, 4,9- 1 5 . ) ( (8)Wnendt, S . ; Finkam, M . ; Winter, W.; Ossig, J.; Raabe, G.; Zwingenberger, K . Chirality 1996, 8,390-396. ) ( ( 9) Eriksson, T.;Bjorkman, S.;Roth, B .; Hoglund, P .J. Pharm. Pharmacol.2 0 00, 52,807-817. ) ( (10) Parman, T .; Wiley: M. J.; Wells , P. G. Na t . Med . 1999,5,582-585. ) V [ml] V[ml] Figure 2. LC separation of the racemic reference compounds thalidomide (a), 5-hydroxythalidomide (b), cis-5'-hydroxythalidomide (c), andtrans-5'-hydroxythalidomide (d) using circular dichroism AA (…) and UV photometric (-) detection A at 240 nm. Column: Chiralpak AD 250×4.6 mm with a 30 x4 mm precolumn. Eluent: MeOH. Flow rate: 1.5 (a), 0.7 (b), and 1.2 mL/min (c,d). which are detoxified to (less reactive) phenols.2 The applicationof thalidomide in an in vitro lymphocyte assay system providedthe evidence that the drug itself was not toxic whereas toxicityrequired both a source of hepatic enzymes and a NADPH-generating system.3Although thalidomide was found to be a poorsubstrate for cytochrome P-450,14 we have focused our work onthe enzyme-catalyzed in vitro biotransformation by rat livermicrosomes due to the observations mentioned above. Themetabolites previously detected in these incubation extractsl5 werealso found in plasma samples from male volunteers who hadreceived thalidomide orally.6 Former studies using capillary electrophoresis with cyclodex-trins as chiral selectors allowed the enantioselective separation ( (11) S tephens, T. D .; B unde, C . J .; F illmore, B . J . Biochem. P harmacol. 2 0 00, 59, 1 489- 1 499. ) (12) Koch, H. Sci. Pharm. 1981, 49, 67-69. ( (1 3 ) Gordon, G . B . ; S p ielberg, S . P . ; B l ake, D. A. ; Balasubramanian, V. P r oc . N atl. A cad. Sci. U .S.A . 1981, 7 8,2545-2548. ) ( (14) T eo, S. K .; Sabourin, P . J.;O' B rien, K .; Kook,K. A.; T h omas, S. D. J. B i ochem.Mol. Toxicol. 2000,14, 1 40-147. ) ( ( 1 5) Meyring, M .; M i ihlenbrock, C.; B laschke, G. Electrophoresis 2000, 21,3270-3279. ) ( (16) Eriksson, T .; Bjorkman, S . ; R oth, B . ; Bjork, H . ; H o glund, P. J. P h arm. Pharmacol.1998,50,1409-1416. ) of thalidomide and three hydroxylated derivatives.15 These inves-tigations demonstrated the stereoselective in vitro biotransforma-tion: The (R) enantiomer is preferentially metabolized in the2',6'-dioxopiperidine-3'-yl moiety, whereas (S)-thalidomide is mainlytransformed by hydroxylation in the phthalimide ring. On onehand, the enantiomers of 5-hydroxythalidomide and the diaster-eomeric 5'-hydroxythalidomides were detected in nonracemicratios after 30-min incubation of the racemic drug. On the otherhand, the metabolism of (R)-thalidomide was found to be highlyproduct-enantioselective, resulting in the formation of one enan-tiomer of each metabolite exclusively. During the incubation of(S)-thalidomide, the corresponding optical antipodes of 5- andtrans-5'-hydroxythalidomide were formed in high enantiomericexcesses of about 95% and 92%, respectively. The previous tentativeassignments15 of the absolute configuration of all metabolites arebased on X-ray diffraction data, NMR experiments, configurationstability of the substrate, and enantioselective effects in biotrans-formation. In the present study, we describe the detailed stereochemicalcharacterization of the hydroxylated derivatives of thalidomide bymeans of experimental circular dichroism (CD) spectroscopy incombination with quantum chemical CD calculations. Another Figure 3. Stopped-flow aquisition of CD spectra (220-420 nm) of the racemic reference com pounds thalidomide (a), 5-hydroxythalidomide(b), cis-5'-hydroxythalidomide (c), and trans-5'-hydroxythalidomide (d) during LC, according to Figure 2. Chromatographic conditions as in Figure2. subject was to investigate the influence of the incubation time onthe metabolic transformation, especially the epimerization of thethermodynamically less stable trans diastereomer of 5'-hydroxy-thalidomide to the corresponding cis isomer. EXPERIMENTAL SECTION Chemicals and Reagents. Racemic thalidomide (TD) waskindly provided by Griinenthal (Stolberg, Germany). The enan-tiomers of thalidomide were obtained in our laboratory bypreparative low-pressure liquid chromatography on a poly(S)-N-(1-cyclohexylethyl)methacrylamide stationary phase.17 The puritywas determined by chiral HPLC and exceeded more than 99.5%.The racemic cis isomer of 5'-hydroxythalidomide (cis-5'-OH-TD)and the mixture of diastereomeric 5'-acetoxythalidomide pairswere gifts from Prof. K. Eger (University of Leipzig, Germany).Racemic 5-hydroxythalidomide (5-OH-TD) was synthesized asdescribed.19 Sulfobutyl-B-cyclodextrin (SBE-B-CD) with an averagemolecular substitution degree of 4.0 was obtained from CyDex, ( ( 17 ) Blaschke, G .; K raft, H. P .; Fickentscher, K .; Kohler, F . Arzneim.-Forsch. 1979,29,1640-1642. ) ( (18) Te ubert, U.; Zwingenberger, K.; Wnendt, S .; E ger, K. Arch . Pharm. 1998, 331,7-15. ) ( (19) H ess, H. R . Ph.D. T h esis, University of Miinster, Mi i nster, Germany, 1990. ) ( 3728 Analytical Chemistry, V ol. 7 4, No. 1 5, August 1 , 2002 ) L.C. (Overland Park, KS) and native B-cyclodextrin (B-CD) waskindly donated by Wacker Chemie (Munich, Germany). Analyticalgrade phosphoric acid, ammonium acetate, anhydrous methanol,acetonitrile, and glacial acetic acid were purchased from Merck(Darmstadt, Germany). Magnesium chloride and p-toluenesulfonicacid were from Aldrich (Daisenhofen, Germany). N-Acrylamide,N,N,N',N'-tetramethylethyldiamine, (3-aminopropyl)triethoxysilanefor the preparation of the coated capillaries, and reduced NADPH(tetrasodium salt) were supplied from Fluka (Fluka Chemie,Buchs, Switzerland). Methanol and ethyl acetate of HPLC qualitywere from J. T. Baker (Deventer, The Netherlands). Circular Dichroism Spectroscopy. Chiroptical detectionduring liquid chromatography was performed using the Jasco CD-1595 detector (Jasco International Co., Ltd., Tokyo,Japan), whichprovides the differential absorbance AA of the CD and theabsorbance A of the UV mode simultaneously as a function oftime. The analog experimental data were monitored by the two-channel recorder Asea Brown Boveri SE 120 or digitized andprocessed using the computer program SEPP.20,21 Simultaneousdetection of the CD and the UV signals was carried out with a ( (20) Brandl, F. ; Pustet, N.; Mannschreck , A. GI T Labor-Fachz . 1999, 43, 19-22; Chem. Abstr . 1999,130,176900. ) ( (21) Brandl, F.; Pustet, N. ; Mannschreck, A . Int. Lab. 1999, 29, 10 C-15 C. ) flow-through cell (25 mm) at the single wavelength of 240 nm.The spectra (220-420 nm) were recorded by a stopped-flowmethod under the same conditions. Measurements were per-formed using a Merck-Hitachi L-6000A pump) equipped with aRheodyne sample injector (Rheodyne, Cotati, CA) and an ErmaERC-3312 degasser (Erma CR Inc., Tokyo, Japan). The racemicreference compounds were separated on a Chiralpak AD (Daicel,Himeji, Japan) 250 × 4.6 mm column with a 30x4 mm precolumnusing methanol as eluent. CD data of individual incubation extractswere obtained after chromatographic resolution on an achiralstationary phase as described below. The CD spectra measured from 200 to 350 nm, as requiredfor the comparison with the calculated spectra, were taken on aJ-715 spectropolarimeter (Jasco GmbH Deutschland,Gross-Umstadt, Germany) at room temperature. The stand-alone spectrawere measured in ethanol. The on-line spectra were measured instopped-flow mode with a standard flow-through cell with metha-nol as the eluent. Reversed-Phase HPLC. The HPLC system consisted of aMerck-Hitachi L-6200A pump equipped with a Rheodyne sampleinjector ),a variable-wavelength 655A detector (Merck-Hitachi)used at 230 nm, and a D-2500 Chromato-integrator (Merck-Hitachi). The analytes of the incubation mixtures were separatedon a 125×4 mm RP-18 stationary phase (LiChrospher 100 RP-18, 5 um, Merck) with a 4 ×4 mm precolumn. The mobile phasewas 1% acetic acid/acetonitrile (92:8,v/v), the flow rate 1.2 mL/min. Capillary Electrophoresis. The HP3D capillary electrophore-sis system (Agilent Technologies, Waldbronn, Germany) equippedwith a diode-array detector was used for the CE studies. Chiralseparations were performed in capillaries of 50-cm effective length(EL) and 50-um i.d. (Polymicro Technologies, Phoenix, AZ) whichwere previously coated with linear polyacrylamide as decribed.22A 12 mg/mL solution of B-cyclodextrin and 12 mg/mL SBE(4)-B-cyclodextrin as chiral selectors were dissolved in 5 mMammonium acetate (BGE) at pH 4.5. The measurements werecarried out with an applied voltage of-30 kV (reversed polarity),and the average current was 20 uA. Samples were injectedhydrodynamically (50 mbar) for 7 s and detected at 230 nm.Between the analyses, the capillary was rinsed for 2 min withmethanol and for another 2 min with run buffer. Preparation of Liver Fractions and in Vitro Biotransfor-mation by Hepatic Microsomes. Hepatic subcellular fractionswere prepared from male Sprague-Dawley rats that had beenpretreated with 50 mg/kg ofbody weight phenobarbital for 6 days.Livers were homogenized in buffer (25 mM sucrose/5 mM Tris-HCl/0.5 mM EDTA) at 4°C. Supernatant (10000g) and microso-mal fractions (100000g) were prepared according to the methoddescribed in ref 23 and stored at -80 °C. The incubation mixture with a total volume of 1 mL consistedof substrate (20 ug of racemic thalidomide or 10 ug of eachthalidomide enantiomer), 410 uL of 0.1 M Tris-buffer (pH 7.4 at37 ℃), 245 uL of NADPH solution (8 mg/mL buffer), 100 uL ofmagnesium chloride (0.06M), and 245 uL of rat liver microsomalpreparation. Incubations were carried out while stirring in a water ( (22) Hjerten, S. J. Chromatogr . 1985, 347, 191-198. ) ( (23) Westhoff, F.; Blaschke, G. J . Chromatogr . 1992,578,265-271. ) Figure 4. Stand-alone measured CD spectra of (R)-and (S)-thalidomide(a) and comparison of the experimental CD spectrafeaturing a negative CD band at 255 nm (b). bath at 37 C. After different periods (5-120 min), the reactionwas stopped by cooling to 0 °C. Thalidomide and its hydroxylatedmetabolites were extracted into 3 mL of ethyl acetate by shakingin a reciprocal shaker for 10 min. After centrifugation at 2500g,the organic layer was evaporated under a stream of nitrogen. Theresidue was dissolved either in the running buffer (CE) or in themobile phase (HPLC) and analyzed. Hydrolysis of Reference Compounds. The mixture ofdiastereomeric 5'-acetoxythalidomide pairs was hydrolyzed ac-cording to ref 24. The compound was heated with p-toluenesulfonicacid in anhydrous methanol to give a mixture of both diastereo-mers of 5'-hydroxythalidomide. The individual isomers could beobtained by fractional separation using an achiral stationary phase. Fractional Separation of Racemic trans-5'-Hydroxytha-lidomide. The thermodynamically less stable trans-5'-hydroxy-thalidomide was obtained by fractional separation on a 125 ×4mm RP-18 (LiChrospher 100, 5 um) column after hydrolysis ofthe mixture of diastereomeric 5'-acteoxythalidomide. The mobilephase was 1% acetic acid/acetonitrile (90:10, v/v) with a flow rateof 1.5 mL/min. The detection wavelength was 230 nm. Theracemic compound was re-extracted from the mobile phase usingethyl acetate. Figure 5. Comparisons of CD spectra: the calculated spectrum of (S)-thalidomide using the MD approach (---) with the experimental one(-) (a), the calculated spectra of all of the (3'S)-configured compounds using the MD approach (b), the experimental spectrum of (S)-thalidomide(-) with the one calculated using the DFT-Boltzmann approach (---) (c), and the spectra calculated for (S)-thalidomide (-), cis-(3'S,5'R)-5'-hydroxythalidomide (.-·), and (S)-5-hydroxythalidomide (---) using the DFT-Boltzmann approach (d). COMUPTATIONAL METHODS Conformational Analysis. The conformational analyses ofthalidomide, cis-5'-hydroxythalidomide, and 5-hydroxythalidomidewere performed on Linux iPII and iPIII workstations by meansof the DFT approach in the TZP25 basis set employing the B3LYPfunctional.26,27 All calculations were performed using the programpackage Turbomole28 starting from preoptimized geometriesgenerated by the AM129parametrization as implemented in theprogram package VAMP.3 Molecular Dynamics (MD). The MD simulations wereperformed on Silicon Graphics Octane (R10000) workstationsusing the Tripos3l force field as implemented in the molecularmodeling package Sybyl.3l The molecules were weakly coupled ( (25) S chafer, A.; Horn, H . ; Ahlrichs, R. J . Chem. Phys. 1992, 97,2571-25 77 . ) ( (26) Becke, A. D . J . Che m . Phy s . 1993,98 , 1372 - 1377. ) ( (27) Lee, C.; Yang, W.; P arr, R. G. Phys. Rev. B 1 988, 3 7 ,78 5 -78 9. ) ( (28) D ewar, M . J. S . ; Zoebisch, E . G .; He a ly,E. ; Steward, J. J. P. J . A m. C h em.Soc . 1985, 1 07,3902- 39 09. ) ( (29) Rauhut, G .; Chandrasekhar, J.; A l ex, A . ; Beck, B . ; S a uer,W.; Clark, T.;Program package VA M P 6.5,Oxford Molecular Ltd., The Medevar C entre, Oxford Science Park, Sandford-on-Thames, Oxford OX4 4GA, England. ) ( (30) A hlrichs, R.; e t al. Program p a ckage Turbomole 5.3, Inst i tut fii r Ph y sikalische Chemie und Elektrochemie,Lehrstuhl fiir Theoretische Chemie, Universitat Karlsruhe. ) ( (31 ) SY B YL 6.5; Tripos Associates: 1 699 H anley Rd., S uite 303, St. Louis , MO63144. ) to a virtual thermal bath at T=700 K,32 with a temperaturerelaxation time t =0.1 ps. CD Calculations. The wave functions for the calculation ofthe rotational strengths for the electronic transitions from theground state to excited states were obtained by CNDO/S-CI33,34calculations, in which the CI expansion33,34 takes into account theground state and all n and orbitals. These calculations werecarried out on Linux iPII and iPIII workstations using theBDZDO/MCDSPD33 program package. For a better comparisonof the theoretical CD spectra with the experimental ones, Gaussianband shape functions were generated over the calculated rotationalstrength values. RESULTS AND DISCUSSION Circular Dichroism of Authentic Standards. Moleculescontaining one or more centers of dissymmetry can be effectivelystudied using the various techniques of chiroptical spectroscopy, ( (32) B e rendsen, H . J. C.; Postma, J . P . M . ; v a n Gunsteren, W. F . ; DiNola, A.; Haak, J. R. J . Che m . Phys.1984,8I,3684-3690. ) ( (33) Downing, J . W. Program package BDZDO/MCDSPD, De p artment of C hemistry and Biochemistry, Uni v ersity of Colorado: Bo u lder, CO.; mo d ifiedby Fleischhauer, J.; Schleker, W.; Kramer; B .; ported to Linux by Gulden,K.-P. ) ( ( 34)Del B e ne, J . ; Jaffe, H. H. J. Chem Phys. 1968,48,1 8 07-1813. ) time [min] Figure 6. CD detection (250 nm) during LC of (R)-thalidomide (a) and (S)-thalidomide (b) and its hydroxylated metabolites, extracted fromincubations with rat liver microsomes. Column: LiChrospher 100 RP-18, 125×4 mm with a 4 × 4 mm precolumn. Eluent: 1% acetic acid/acetonitrile (92:8, v/v). Flow rate: 1.2 mL/min. Peak assignments: (a) Single enantiomers of cis-5'-OH-TD(1), trans-5'-OH-TD (2), 5-OH-TD(3), and TD (4); (b) single enantiomers of 5-OH-TD (1) and TD (2). e.g., circular dichroism.35 Due to different absorption indexes ofleft- and right-circularly polarized beams in the optically activemedium, this method has been successfully used for the detailedstereochemical characterization of enantiomeric eluates.36,37 The aim of the present work was to determine the absoluteconfiguration of the hydroxylated metabolites of thalidomide byquantum chemical calculation of their circular dichroism spectraand comparison with the experimentally measured ones. For thisreason, chiroptical detection during liquid chromatography wasapplied to monitor the differential absorbance A4 of the fourracemic standards thalidomide, 5-hydroxythalidomide, and thediastereomers of 5'-hydroxythalidomide. This procedure for theaquisition of the CD spectra does not need any preparativeenrichment ofenantiomers. Since a previous study illustrated theexcellent enantioselectivity but insufficient chemoselectivity of aChiralpak AD column for this particular set of analytes,38 the aboveracemates were separated individually on this optically activesorbent using methanol as eluent (Figure 2). Separation charac-teristics calculated by the use of the computer program SEPP20,2Iare shown in Table 1. The detector equipped with a flow-throughcell of 25-mm path length allowed us to obtain the CD and theUV information simultaneously as a function of time. Both thefirst eluted enantiomer of thalidomide ((S) configuration, Figure2a) and the phenolic metabolite (Figure 2b) show negative CDeffects at 240 nm. Accordingly, for the second eluted antipodesof the diastereomeric 5'-hydroxythalidomides (Figure 2c/d), anegative differential absorbance A4 at the same wavelength canbe observed. Since the metabolites presently examined arestructurally similar to the reference compound thalidomide (strictanalogy of the chromophoric environment), one might conclude ( (35) Brittain, H. G . J. Phar m . Biomed. Ana l .1998, 1 7,933-940. ) ( (36) Mannschreck,A. Chirality 1992,4,1 6 3-169. ) ( (37) B ringmann, G.; T a sler, S . ; E ndress, H . ; K r aus, J; M e sser, K.; Wo h lfarth, M .; Lobin W. J. Am. Chem. Soc. 2001,123,2 7 03-2711. ) ( (38) Meyring, M .; Chankvetadze, B.; Blaschke, G.J. Chromatogr., A 2 000, 8 7 6,157-167. ) Table 1. Separation Characteristics of Thalidomide and Its Hydroxylated Metabolites, Calculated Using the Computer Program SEPPa compound vi(mL) v2(mL) k k2 R TD 13.4 29.3 2.32 6.28 2.71 10.40 5-OH-TD 10.0 13.8 1.48 2.43 1.64 4.47 cis-5'-OH-TD 8.2 15.0 1.03 2.73 2.65 5.72 trans-5'-OH-TD 8.5 15.1 1.10 2.74 2.49 5.98 a Experimental conditions:Chiralpak AD 250 x 4.6 mm withprecolumn; MeOH. that the (S) absolute configuration can be assigned to theenantiomers showing a negative CD band at 240 nm. Thisempirical approach implies that the additional stereocenter at C-5’does not largely influence the CD behavior of the molecule, forexample, by a conformational change of the preferential conforma-tion; for this reason, spectra-structure relationships with refer-ence to a molecule of known structure had to be verified byquantum chemical CD calculation as described in the followingsection. In addition to the detection at one single wavelength, the CDinstrument was used to monitor the approximate CD and UVspectra between 220 and 420 nm of the four racemic compoundsseparated by LC (Figure 3). These measurements were performedduring a temporary stop of the flow; the pure enantiomersstemming from the racemic mixtures were trapped in thechromatographic cell. The CD spectra obtained for thalidomideand its three hydroxylated metabolites were almost identical,consisting of a single band maximum located at 255 nm. In addition, for better comparison with the calculated CDspectra, the experimental spectra were extended to the moredecisive lower wavelengths below 220 nm. This was achieved bystand-alone CD measurements of authentic (R)- and (S)-thalido-mide reference substances (Figure 4a) and stopped-flow measure-ments of racemates of cis-5'-hydroxythalidomide and 5-hydroxy- incubation time [min] Figure 7. Quantitative relationship of diastereomeric 5'-hydroxy-thalidomides formed during the in vitro biotransformation of (R)-thalidomide with rat liver microsomes, calculated by integration of theirpeak areas (a). Chromatograms of diastereomeric 5'-hydroxythali-domides, extracted from incubations of (R)-thalidomide with rat livermicrosomes in the time course of 5, 30, and 120 min (b). Column:LiChrospher 100 RP-18, 125 x 4 mm with a 4 ×4 mm precolumn.Eluent: 1% acetic acid/acetonitrile (92:8, v/v). Flow rate: 1.2 mL/min. Peak assignments: single enantiomers of cis-5'-OH-TD(1) andtrans-5'-OH-TD (2). thalidomide. In Figure 4b, the resulting spectra featuring anegative CD band at 255 nm are compared. Quantum Chemical Calculation of CD Spectra. A centralquestion decisive for the correct interpretation of the CD spectraof the 5-and the 5'-hydroxy derivatives (Figure 3) by comparisonwith the spectra of thalidomide itself was whether the configura-tion at C-3' or that at C-5’is responsible for the sign of the CDcurves of the 5'-hydroxy derivatives. To learn whether the curveshape is still determined by the stereocenter at C-3'or whether itis influenced by the additionally introduced hydroxy group at C-5',we calculated the CD spectra for all of the four possible (3'S)compounds by quantum chemical methods, which had proved tobe a particularly valuable tool in earlier studies.39-41 For a firstmethod validation, we started by investigating the configurationally Figure 8. Postulated scheme for the pH-dependent epimerizationof (3'R,5'R)-5'-hydroxythalidomide via an enolic form as intermediate.Epimerization could also proceed via deprotonation at the chiral centerC-3' and formation of an enolate anion. well-known parent compound thalidomide itself using our estab-lished tool for the stereochemical study of novel molecules-theMD approach.39,40 The CD calculations were based on a MDsimulation using the Tripos force field,3 arbitrarily starting withthe (3'S) enantiomer. The simulation was performed for a totaltime period of 500 ps, recording the structure every 0.5 ps forfurther calculations. For the 1000 structures thus collected, single CD spectra werecalculated and then averaged arithmetically to give the theoreticaloverall spectrum. To take into account systematic shifts ofthecalculated CD spectra, a“UV correction”was carried out for eachcalculated spectrum as introduced earlier.4 Except for the lackingband at 255 nm in the calculated overall CD spectrum for (3'S)-thalidomide, it agrees well with the experimental one of the(3'S)-configured reference compound (Figure 5a), especially in thediagnostically decisive short-wavelength part of the CD spectrum. Comparing the resulting CD spectra of all the (3’S) enantio-mers thus calculated (Figure 5b), one cannot state any significantchanges in their curve shapes, except for small differences in theintensities between the curves and for slight shifts of some of thebands. In view of the divergence of the calculated and theexperimental CD spectra in the long-wavelength region, weadditionally performed higher-level calculations, viz conformationalanalyses based on density functional theory (DFT) methods. Forthalidomide itself, one minimum structure (“AX", with the nitrogensubstituent axial) located 2.30 kcal/mol higher than the globalminimum (“EQ”, with the nitrogen substituent equatorial) wasfound. ( (39) Bringmann, G.;Muihlbacher,J. ; Repges, C.; Fleischhauer,J.J. C omput. Chem. 2001,22,1273-1 2 78. ) (40) Feling, R.; Polborn, K.; Steglich, W.; Muihlbacher, J.; Bringmann, G.Tetrahedron 2001,57,7857-7863. (41) Bringmann, G.; Busemann, S. In Natural Product Analysis; Schreier, P.,Herderich, M., Humpf, H. U., Schwab, W., Eds.; Vieweg: Wiesbaden, 1998;pp 195-212. Figure 9. Electropherogram of thalidomide and its hydroxylated metabolites, extracted from incubations of the racemic substrate, and correlationwith their CD spectra obtained during LC separation of racemates (see Figure 3). Capillary: PAA-coated,50-cm i.d., 50-cm EL, acetate bufferpH 4.5, 12 mg/mL B-cyclodextrin and SBE(4)-B-cyclodextrin as chiral selectors, -30 kV (reversed polarity). Peak assignments: racemates of5-OH-TD (1, 2), trans-5'-OH-TD (3, 4), cis-5'-OH-TD (5, 6), and TD (7, 8). Foreach of the two conformers detected, individual CD spectrawere calculated separately. These single CD spectra were Boltz-mann-weighted according to the enthalpies of formation of thecorresponding conformers and added up to yield the theoreticaloverall CD spectrum of (3'S)-thalidomide. This spectrum wassubsequently UV corrected as performed during the MD inves-tigation. Comparing the experimental CD spectrum of (3'S)-thalidomide with the DFT-Boltzmann calculated one (Figure 5a),one can see a still better agreement than using the MD approachabove: this time, in addition to the mentioned short-wavelengthbands also the next minimum at 255 nm is reproduced. For the cis-(3'S,5'R)-5'-hydroxythalidomide and the (S)-5-hydroxythalidomide in the same way, we calculated the two CDspectra shown in Figure 5d, in comparison to that of (S)-thalidomide. In the case of (S)-5-hydroxythalidomide, we found 4AX and 4 EQ minimum geometries below an energetic cutoff of3 kcal/mol. The EQ minimums are ~2.30 kcal/mol more stablethan the AX minimums. cis-(3'S,5'R)-5'-Hydroxythalidomide formsonly one EQ minimum structure below the cutoff. All the threeEQ minimum structures are very similar, so that the resultingoverall CD spectra of the DFT-Boltzmann-based approach do notdiffer largely from each other. The difference of the experimentalCD spectra of the hydroxylated compounds compared with thatof thalidomide itself (as shown in Figure 4b) may be due toadditional solvent effects (resulting from the OH group not presentin the parent molecule) not taken into account in our calculations. All the findings discussed above evidence that the introductionof a hydroxy group at any of the three positions-C-5, cis-C-5', ortrans-C-5'-does not substantially affect the main trend of the CDcurves. Consequently, one can deduce that all of the metaboliteswhose CD spectra feature the same sign around 255 nm as thatof thalidomide itself (see Figure 3) must have the same absoluteconfiguration at C-3'. Analysis of the Incubation Extracts by Circular DichroismSpectroscopy On Line with Liquid Chromatography. Formerinvestigations of the in vitro biotransformation of (R)-thalidomideby rat liver microsomes using chiral capillary electrophoresisdemonstrated that the hydroxylated metabolites were formedenantioselectively.15 Based on these results, on-line CD spectros-copy during chromatographic resolution on an achiral stationaryphase (LiChrospher 100 RP-18) was applied to incubation extracts. (R)-Thalidomide as well as its 5- and trans-5'-hydroxylatedmetabolites show positive differential absorbances ▲4 at 250 nm(Figure 6a). From the sign of these CD bands and the knowledgegained by the above CD calculations it is possible to establishthe (R) configuration at the chiral center C-3'. As the relativeconfigurations of diastereomeric 5'-hydroxythalidomide pairs werepreviously determined either as (3'R,5'R) or (3'S,5'S) for the transand (3'R, 5'S) or (3'S, 5'R) for the cis isomer,15 the trans-5'-hydroxythalidomide metabolite was assigned to have a (3'R,5'R)absolute stereostructure. The negative CD of cis-5'-hydroxythali-domide consequently corresponds to the (3'S, 5'R) configuration. Figure 10. Structures and absolute configurations of the hydroxylated metabolites of thalidomide enantiomers found in incubations with ratliver microsomes. The enantiomers of cis-5'-hydroxythalidomide (secondary products) are formed by nonenzymatic epimerization. The question of whether this isomer is formed enzymatically orby epimerization of trans-5'-hydroxythalidomide has to be dicussedlater. A distinguishable pattern of biotransformation products waspreviously found for (S)-thalidomide.15 The main metabolite of thisenantiomer, namely, 5-hydroxythalidomide, and trans-5'-hydroxy-thalidomide were detected in enantiomeric ratios of 95:5 and 92:8, respectively. The analysis of an incubation extract of (S)-thalidomide by chiroptical detection during LC is shown in Figure6b. The negative CDs of the enantiomeric substrate and the5-hydroxy metabolite formed in excess correspond to the (S)absolute configuration. The sensitivity of the CD instrument wasinsufficient for the detection of the minor metabolite trans-5'.hydroxythalidomide. Additionally, the use of an optically inactivesorbent did not allow to monitor the CD of the enantiomers ofthe cis isomer, which are present in nearly racemic ratios15 inthis incubation extract. Epimerization of Diastereomeric 5'-Hydroxythalidomides.The diastereomers of 5'-hydroxythalidomide formed in the me-tabolism of (R)-thalidomide were detected in different amountsaccording to the period of incubation. Reincubations of the racemicstandards proved a mutual transformation of both diastereomersat pH 7.4, the equilibrium being shifted toward the cis form.The question of whether this thermodynamically more stableisomer is a primary product during the enzyme-catalyzed biotrans-formation could not be answered reliably yet. Therefore, the time-dependent metabolic formation was inves-tigated by incubating (R)-thalidomide for various periods (5-120min). The amounts of the stereosiomers of 5'-hydroxythalidomideseparated on an achiral RP-18 column were calculated by integra-tion of their peak areas (Figure 7a), assuming that their absorptioncoefficients are very similar. The corresponding chromatogramsof three different incubation extracts are shown in Figure 7b.These results demonstrate the formation of trans-5'-hydroxytha-lidomide as a primary metabolite (5 min). As an induction periodin this microsomal preparation system should be very unlikely,the cis isomer must be a product of the pH-dependent epimer-ization exclusively. The known absolute configuration of theseenantiomeric thalidomide derivatives assigned by means of CDspectroscopy enabled us to determine that an inversion occurs atthe chiral center C-3'of the molecule: (3'R,5'R)-trans-5'-hydroxy-thalidomide epimerizes to the thermodynamically more stable(3'S, 5'R)-cis isomer (Figure 8). Previous hypotheses15 weredisproved by the present investigations. Stereochemical Aspects of the in Vitro Biotransformationof Thalidomide. The capillary electrophoresis method using adual cyclodextrin system provided the first example for theenantioselective resolution of thalidomide and its hydroxylatedmetabolites in a single run. The electropherogram of this chiralseparation is illustrated in Figure 9. The analysis of referencecompounds as well as incubation extracts of thalidomide enantio-mers by circular dichroism spectroscopy presents the opportunity to assign the CD spectra to the enantiomers of each metaboliteseparated by CE. The known absolute configuration of these chiralmolecules allowed us to follow the stereo- and enantioselectiveeffects of the thalidomide metabolism in detail. The current resultsare in agreement with previous observations and demonstrate that(R)- and (S)-thalidomide were predominantly hydroxylated atdifferent moieties of the molecule. The phenolic 5-hydroxymetabolite of (S)-thalidomide was unambiguously identified tohave the (S) absolute configuration (peak 1). The evidence forthe formation of an intermediate arene oxide has been presentedpreviously. The optical antipode (R)-5-hydroxythalidomide wasformed from (R)-thalidomide but in substantially lower amounts.Hydroxylation in the glutarimide moiety leading to diastereomericproducts was proved to be the major metabolic pathway of thisenantiomer. The primary metabolite trans-5'-hydroxythalidomidewas assigned to have (3'R,5'R) absolute configuration (peak 4).This stereoisomer epimerizes nonenzymatically by inversion atthe chiral center C-3'. Both incubation time and pH of theincubation medium have a determining influence on this epimer-ization. The (3'S,5'R) absolute stereochemistry of this secondaryproduct (peak 5) follows from the known relative configurationand the negative CD band at 250 nm. The major and minor metabolites of each thalidomide enan-tiomer as well as their secondary products are shown in Figure10. The present investigations confirm the former assumptionthat no inversion occurs at the asymmetric center C-3'of thesubstrate during the cytochrome P-450-catalyzed biotransforma-tion. CONCLUSIONS Former investigations of the in vitro biotransformation ofthalidomide using chiral capillary electrophoresis in combinationwith X-ray crystallography for the stereochemical characterization of metabolites were extended to the application of circulardichroism spectroscopy. This CD-based detection system enablesthe direct analysis of chiral molecules during HPLC separationon a nonracemic sorbent. The sensitivity of the CD instrumentwas found to be sufficient for the direct determination of evenlow concentrations of metabolites in biological matrixes, e.g., inincubation extracts of rat liver microsomes. The CD data of thenonracemic metabolites of (R)-and (S)-thalidomide were obtainedduring chromatographic resolution on the achiral sorbent RP-18silica and compared to the calculated CD spectra. These resultstogether with previous observations15 allowed an unequivocalassignment of the absolute configurations of the hydroxylatedthalidomide metabolites as well as a reliable determination of theenantioselective effects in metabolism. This study represents an important step toward a wider useof chiroptical detection during liquid chromatography and pointsout some features that are unique to a detection system based oncircular dichroism. ACKNOWLEDGMENT The authors thank the Deutsche Forschungsgemeinschaft andthe Fonds der Chemischen Industrie for financial support and Prof.K. Eger for providing the reference compounds. We are gratefulto R. Wiirdinger, Jasco GmbH Deutschland, Gross-Umstadt, forthe opportunity to use the CD-1595 instrument. Furthermore, wethank Prof.J. Fleischhauer (Rheinisch-Westfalische TechnischeHochschule Aachen), Prof. J. Michl, and Dr. J. W. Downing (bothUniversity of Colorado) for providing the program packageBDZDO/MCDSPD, and Dr. K.-P. Gulden for porting it to Linux. Received for review May 8, 2002. Accepted May 9, 2002.AC0203138 nalytical Chemistry, Vol. No. August , acCCC: $ American Chemical SocietyPublished on Web nalytical Chemistry, Vol. No. August ,
确定


还剩8页未读,是否继续阅读?
华洋科仪为您提供《(R)-和(S)-沙利度胺(反应停)体外羟基化代谢产物中立体化学研究检测方案(液相色谱仪)》,该方案主要用于其他中立体化学研究检测,参考标准--,《(R)-和(S)-沙利度胺(反应停)体外羟基化代谢产物中立体化学研究检测方案(液相色谱仪)》用到的仪器有
相关方案
更多









