方案详情
文
利用氢能实现清洁、健康的环境是当今世界迫切解决的问题。当前最关键的问题是如何解决氢的储存,即储氢(hydrogen storage)。
Hiden公司的智能重量分析仪IGA是世界储氢研究者广为选择的研究工具。
方案详情

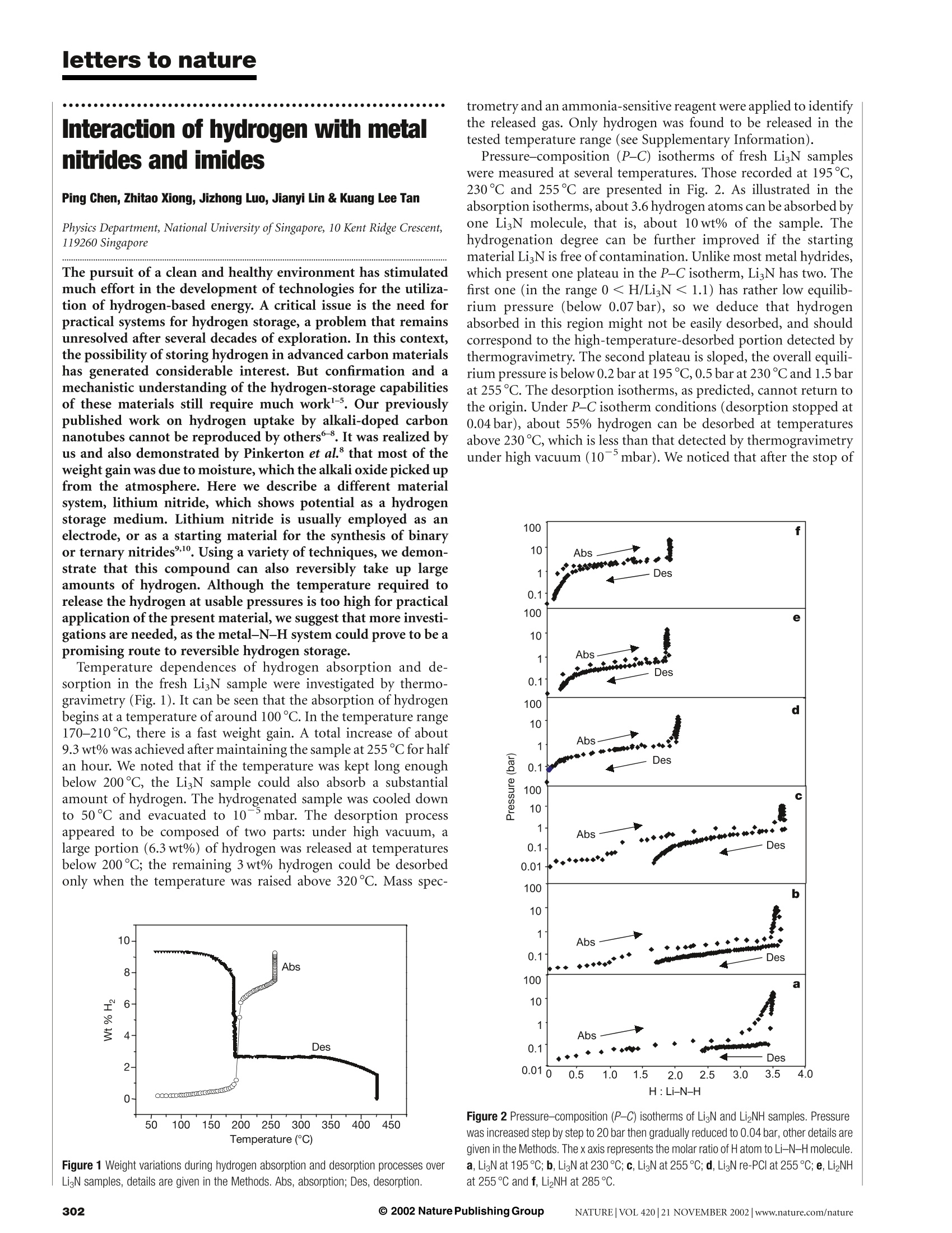
letters to nature Interaction of hydrogen with metalnitrides and imides Ping Chen, Zhitao Xiong, Jizhong Luo, Jianyi Lin & Kuang Lee Tan Physics Department, National University ofSingapore, 10 Kent Ridge Crescent,119260 Singapore The pursuit of a clean and healthy environment has stimulated much effort in the development of technologies for the utiliza-tion of hydrogen-based energy. A critical issue is the need forpractical systems for hydrogen storage, a problem that remainsunresolved after several decades of exploration. In this context,the possibility of storing hydrogen in advanced carbon materialshas generated considerable interest. But confirmation and amechanistic understanding of the hydrogen-storage capabilitiesof these materials still require much work -5. Our previouslypublished work on hydrogen uptake by alkali-doped carbonnanotubes cannot be reproduced by others8. It was realized byus and also demonstrated by Pinkerton et al. that most of theweight gain was due to moisture, which the alkali oxide picked upfrom the atmosphere. Here we describe a different materialsystem, lithium nitride, which shows potential as a hydrogenstorage medium. Lithium nitride is usually employed as anelectrode, or as a starting material for the synthesis of binaryor ternary nitrides,. Using a variety of techniques, we demon-strate that this compound can also reversibly take up largeamounts of hydrogen. Although the temperature required torelease the hydrogen at usable pressures is too high for practicalapplication of the present material, we suggest that more investi-gations are needed, as the metal-N-H system could prove to be apromising route to reversible hydrogen storage. Temperature dependences of hydrogen absorption and de-sorption in the fresh LigN sample were investigated by thermo-gravimetry (Fig.1). It can be seen that the absorption of hydrogenbegins at a temperature of around 100℃. In the temperature range170-210℃, there is a fast weight gain. A total increase of about9.3 wt% was achieved after maintaining the sample at 255℃ for halfan hour. We noted that if the temperature was kept long enoughbelow 200℃, the LigN sample could also absorb a substantialamount of hydrogen. The hydrogenated sample was cooled downto 50℃ and evacuated to 10mbar. The desorption processappeared to be composed of two parts: under high vacuum, alarge portion (6.3 wt%) of hydrogen was released at temperaturesbelow 200C; the remaining 3wt% hydrogen could be desorbedonly when the temperature was raised above 320℃. Mass spec- Figure 1 Weight variations during hydrogen absorption and desorption processes overLigN samples, details are given in the Methods. Abs, absorption; Des, desorption. trometry and an ammonia-sensitive reagent were applied to identifythe released gas. Only hydrogen was found to be released in thetested temperature range (see Supplementary Information). Pressure-composition (P-C) isotherms of fresh LigN sampleswere measured at several temperatures. Those recorded at 195°℃,230℃ and 255℃ are presented in Fig. 2. As illustrated in theabsorption isotherms, about 3.6 hydrogen atoms can be absorbed byone LigN molecule, that is, about 10wt% of the sample. Thehydrogenation degree can be further improved if the startingmaterial Li N is free of contamination. Unlike most metal hydrides,which present one plateau in the P-C isotherm, LigN has two. Thefirst one (in the range 0
确定


还剩1页未读,是否继续阅读?
北京英格海德分析技术有限公司为您提供《氢气中与金属氢化物的交互作用检测方案(蒸汽吸附仪)》,该方案主要用于工业气体中与金属氢化物的交互作用检测,参考标准--,《氢气中与金属氢化物的交互作用检测方案(蒸汽吸附仪)》用到的仪器有Hiden IGA 智能重量法吸附分析仪
相关方案
更多
该厂商其他方案
更多










