方案详情
文
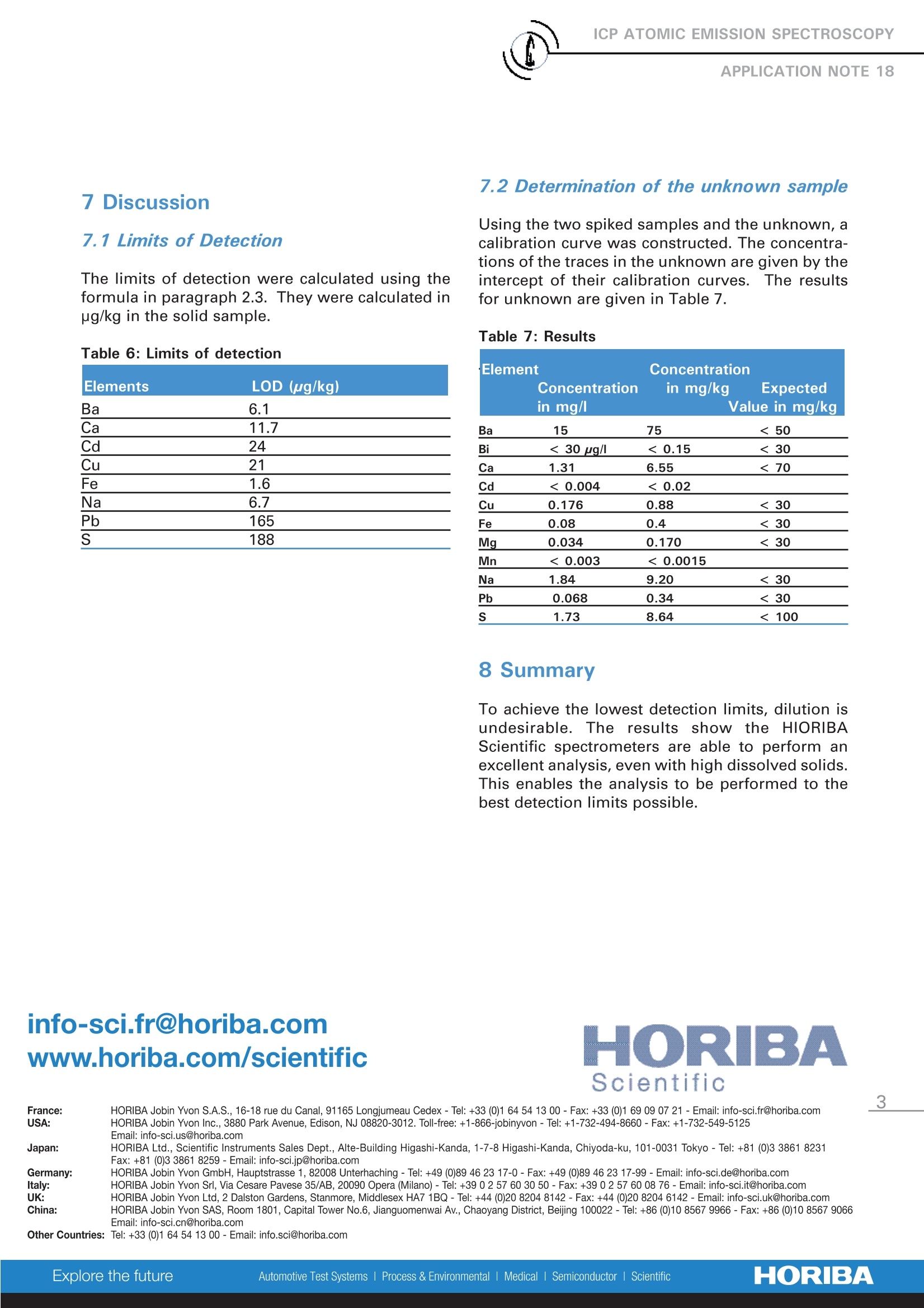
To achieve the lowest detection limits, dilution is undesirable. The results show the HORIBA Scientific spectrometers are able to perform an excellent analysis, even with high dissolved solids. This enables the analysis to be performed to the best detection limits possible.
方案详情

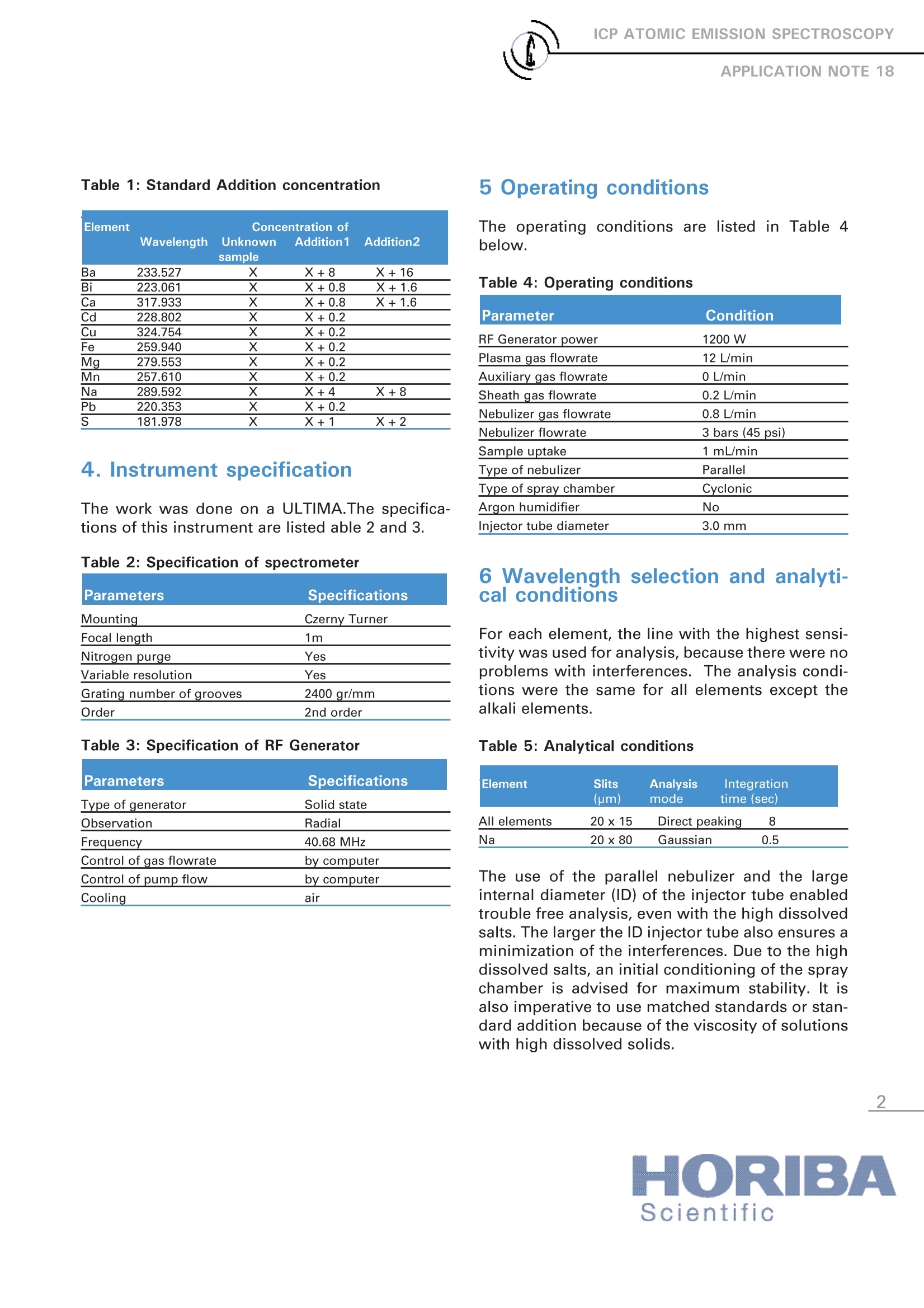
ICP ATOMIC EMISSION SPECTROSCOPY Analysis of metals in 200 g/l Strontium Nitrate Nathalie Le CorreHORIBA Scientific Longjumeau, France Keywords: chemicals, inorganic 1 Introduction A sample with 200 g/L strontium nitrate waspresented to this laboratory for trace determina-tion. As there was only one sample to be ana-lyzed and it had a complex matrix, the standardaddition method was used foranalysis. 2 Principle 2.1 Technique used The elemental analysis of a 200 g/L strontiumnitrate solution was undertaken by InductivelyCoupled Plasma Atomic Emission Spectrometry(ICP-OES). The sample is nebulized then trans-ferred to an argon plasma. It is decomposed,atomized and ionized whereby the atoms andions are excited. We measure the intensity ofthe light emitted when the atoms or ions returnto lower levels of energy. Each element emitslight at characteristic wavelengths and theselines can be used for quantitative analysis aftera calibration. 2.2 Wavelength choice The choice of the wavelength in a given matrixcan be made using the "profile"function, or byusing Win-IMAGE, which is rapid semi-quantita-tive analysis mode using multiple wavelengths.The principle is the same in either case: recordthe scans of analytes at low concentration, andof the matrix. By superimposing the spectra, wesee possible interferences. 2.3 Limits of detection estimation The limits of detection are calculated using thefollowing formula: With: LOD = limits of detection, k=3 for the normal 3-sigma values, BEC=Background equivalent concentration, RSDo= relative standard deviation of the blank. To calculate the LOD, a calibration curve is con-structed using two points, 0 ppm and 5 ppm, orsome concentration where the calibration is lin-ear; this gives the BEC. The RSD is evaluatedby running the blank ten times. 3 Sample preparation 19.9968 g of sample was weighed and dissolvedwith 5 mL 69% HNO3 and diluted up to 100 mLwith deionized water. Two spiked samples were prepared for the stan-dard addition method.Addition 1: 0.1 mL of standard "A" and 0.2 mL ofstandard "B", with 25 mL of Sr(OH)2.8H2O.Addition 2: 0.2 mL of standard "A" with 25 mL ofSr(OH)2.8H2O. With sample "A" containing :2 g/L of Ba,1 g/L of Na,0.25 g/L of S,0.2 g/L of Bi, Ca and sample "B" containing 25 mg/L of Cd, Cu,Fe, Mg, Mn, Pb. The concentrations of the spiked samples arelisted in Table 1. Table 1: Standard Addition concentration Element Concentration of Wavelength Unknown Addition1 Addition2 sample Ba 233.527 X X+8 X+16 Bi 223.061 X X+0.8 X+1.6 Ca 317.933 X X+0.8 X+1.6 Cd 228.802 X X+0.2 Cu 324.754 X X+0.2 Fe 259.940 X X+0.2 Mg 279.553 X+0.2 Mn 257.610 X X+0.2 Na 289.592 X X+4 X+8 Pb 220.353 X+0.2 181.978 X X+1 X+2 4. Instrument specification The work was done on a ULTIMA.The specifica-tions of this instrument are listed able 2 and 3. Table 2: Specification of spectrometer Parameters Specifications Mounting Czerny Turner Focal length 1m Nitrogen purge Yes Variable resolution Yes Grating number of grooves 2400 gr/mm Order 2nd order Table 3: Specification of RF Generator Parameters Specifications Type of generator Solid state Observation Radial Frequency 40.68 MHz Control of gas flowrate by computer Control of pump flow by computer Cooling air 5 0perating conditions The operating conditions are Ilisted in Table 4below. Table 4: Operating conditions Parameter Condition RF Generator power 1200W Plasma gas flowrate 12 L/min Auxiliary gas flowrate 0 L/min Sheath gas flowrate 0.2 L/min Nebulizer gas flowrate 0.8 L/min Nebulizer flowrate 3 bars (45 psi) Sample uptake 1 mL/min Type of nebulizer Parallel Type of spray chamber Cyclonic Argon humidifier No Injector tube diameter 3.0 mm 6 Wavelength selection and analyti-cal conditions For each element, the line with the highest sensi-tivity was used for analysis, because there were noproblems with interferences. The analysis condi-tions were the same for all elements except thealkali elements. Table 5: Analytical conditions Element Slits Analysis Integration (um) mode time (sec) All elements 20x15 Direct peaking 8 Na 20x80 Gaussian 0.5 The use of the parallel nebulizer and the largeinternal diameter (ID) of the injector tube enabledtrouble free analysis, even with the high dissolvedsalts. The larger the ID injector tube also ensures aminimization of the interferences. Due to the highdissolved salts, an initial conditioning of the spraychamber is advised for maximum stability. It isalso imperative to use matched standards or stan-dard addition because of the viscosity of solutionswith high dissolved solids. 7 Discussion 7.1 Limits of Detection The limits of detection were calculated using theformula in paragraph 2.3. They were calculated inug/kg in the solid sample. Table 6: Limits of detection Elements LOD (ug/kg) Ba 6.1 Ca 11.7 Cd 24 Cu 21 Fe 1.6 Na 6.7 Pb 165 S 188 7.2 Determination of the unknown sample Using the two spiked samples and the unknown, acalibration curve was constructed. The concentra-tions of the traces in the unknown are given by theintercept of their calibration curves. The resultsfor unknown are given in Table 7. Table 7: Results Element Concentration Concentration in mg/kg Expected in mg/l Value in mg/kg Ba 15 75 <50 Bi < 30 ug/l <0.15 <30 Ca 1.31 6.55 <70 Cd <0.004 <0.02 Cu 0.176 0.88 <30 Fe 0.08 0.4 <30 Mg 0.034 0.170 <30 Mn <0.003 < 0.0015 Na 1.84 9.20 <30 Pb 0.068 0.34 <30 S 1.73 8.64 <100 8 Summary To achieve the lowest detection limits, dilution isundesirable. Thee results show the HIORIBAScientific spectrometers are able to perform anexcellent analysis, even with high dissolved solids.This enables the analysis to be performed to thebest detection limits possible. Scientific France: HORIBA Jobin Yvon S.A.S., 16-18 rue du Canal, 91165 Longjumeau Cedex- Tel:+33 (0)1 64 54 13 00- Fax: +33 (0)1 69 09 07 21-Email: info-sci.fr@horiba.com Other Countries: Tel: +33 (0)1 64 54 13 00-Email: info.sci@horiba.com ORIBAScientific ORIBAExplore the futureAutomotive Test Systems Process & Environmental Medical Semiconductor Scientific To achieve the lowest detection limits, dilution is undesirable. The results show the HORIBA Scientific spectrometers are able to perform an excellent analysis, even with high dissolved solids. This enables the analysis to be performed to the best detection limits possible.
确定

还剩1页未读,是否继续阅读?
HORIBA(中国)为您提供《硝酸锶溶液中金属元素检测方案(ICP-AES)》,该方案主要用于无机盐中金属元素检测,参考标准--,《硝酸锶溶液中金属元素检测方案(ICP-AES)》用到的仪器有HORIBA Ultima Expert高性能ICP光谱仪
相关方案
更多
该厂商其他方案
更多










