方案详情
文
postnova的场流分离仪,可以与各个厂家的ICP-MS、核磁NMR等仪器在线直接联用,广泛应用于纳米材料与环境科学领域的元素形态分析、聚合物和生物大分子材料的分子量/分子量分布和支化等分子构型分析表征。
方案详情

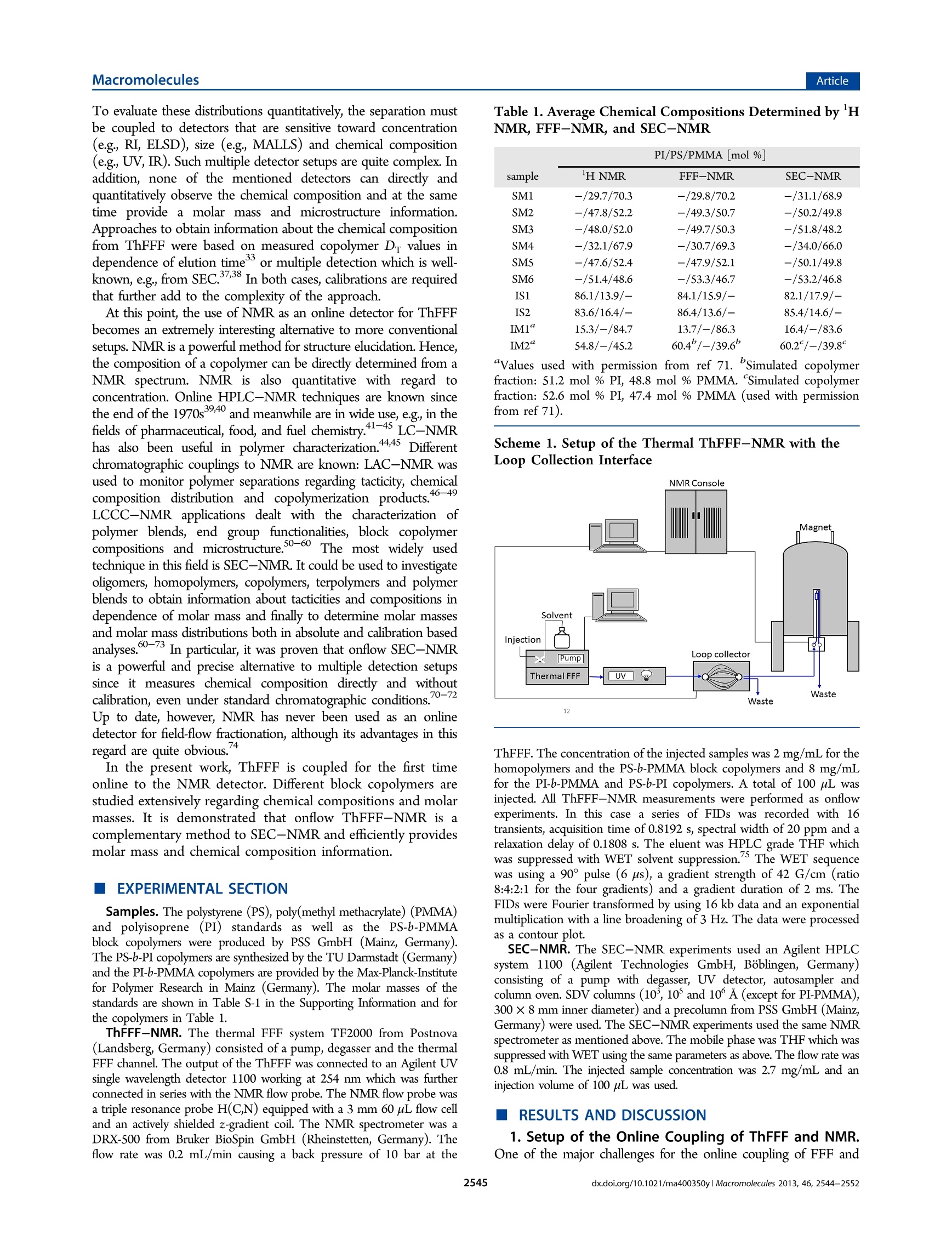
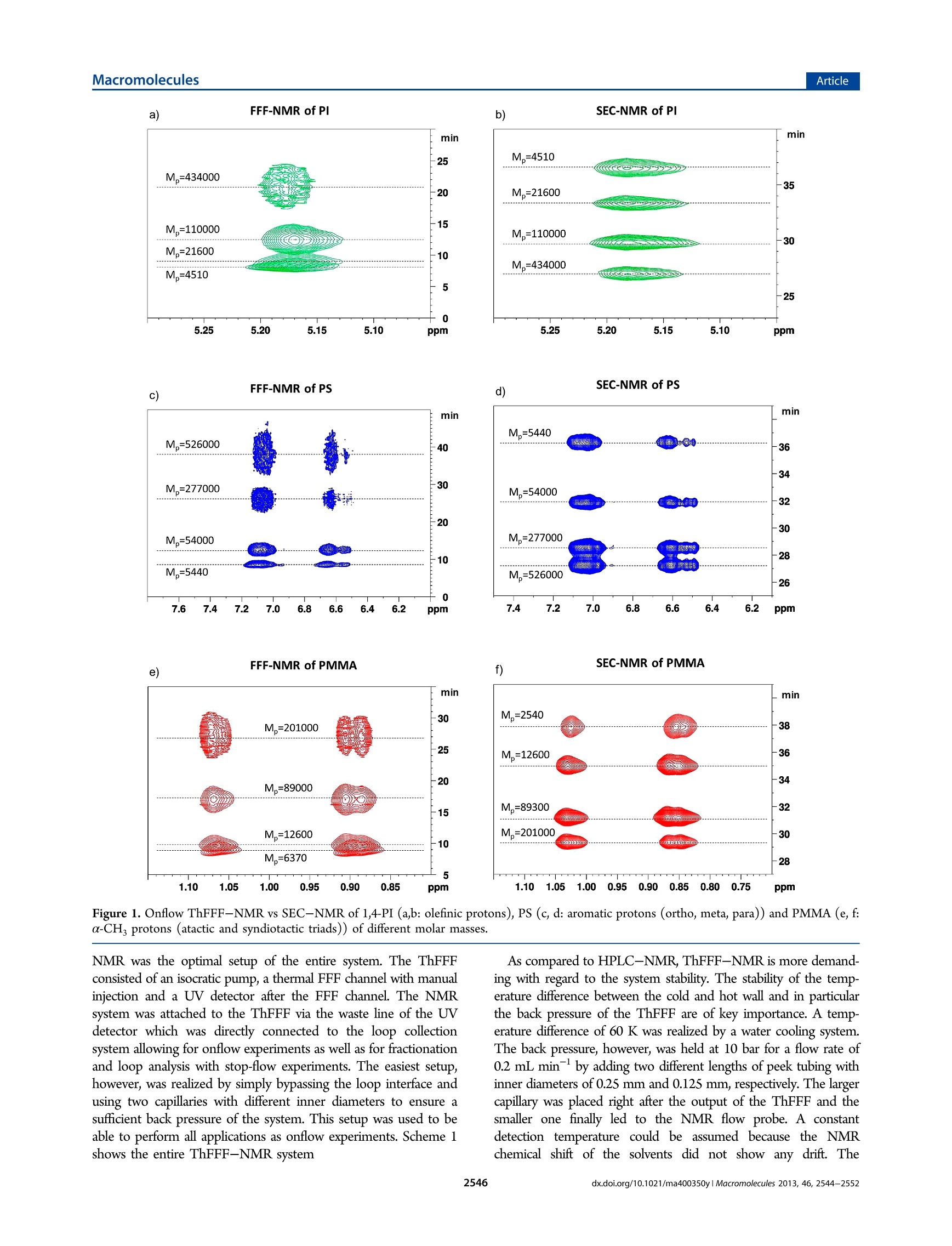
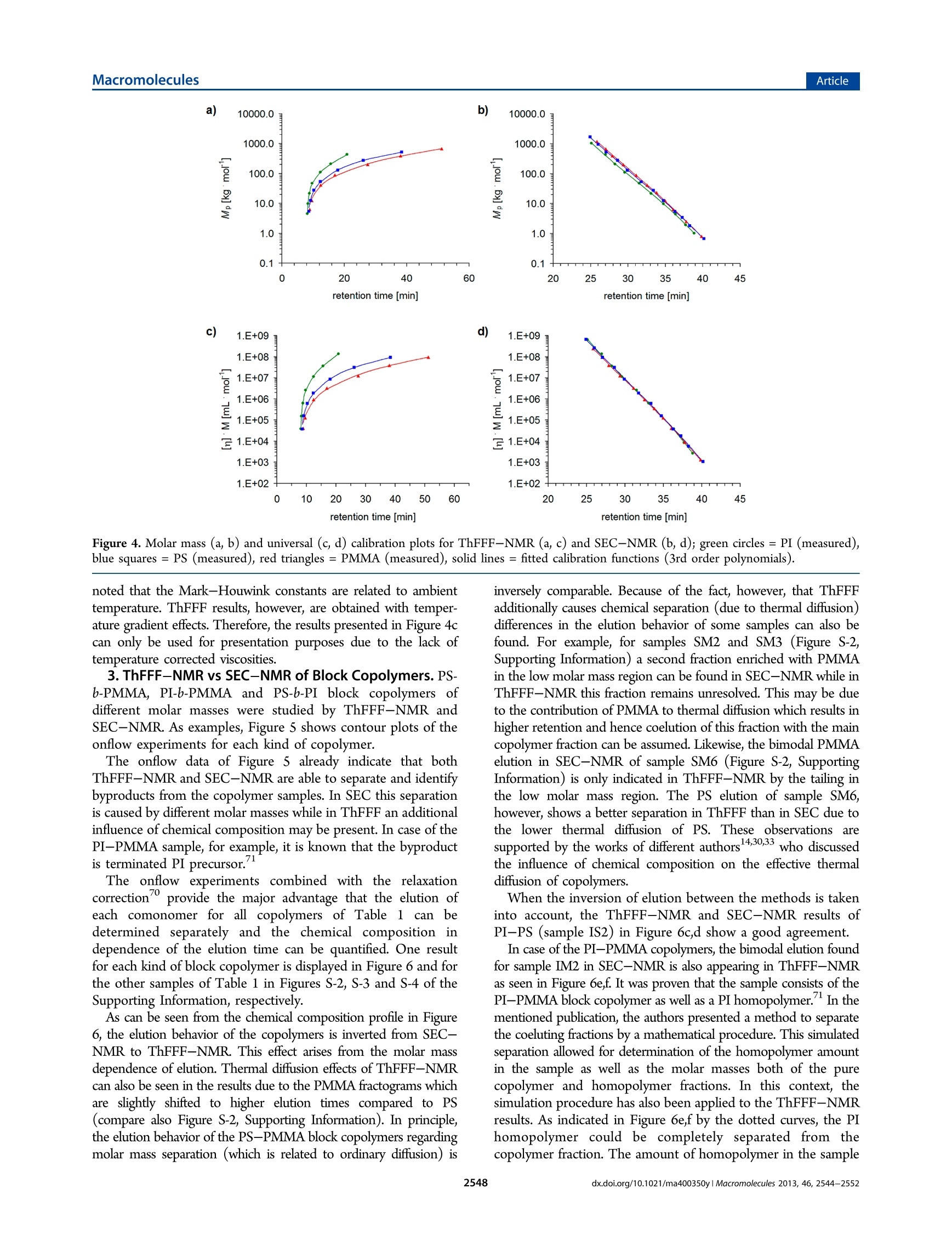
MacromoleculesArticlepubs.acs.org/Macromolecules ArticleMacromolecules Online ThFFF-NMR: A Novel Tool for Molar Mass and ChemicalComposition Analysis of Complex Macromolecules Wolf Hiller, * Werner van Aswegen, Mathias Hehn, and Harald Pasch* Faculty of Chemistry, TU Dortmund, Otto-Hahn-Strasse 6, 44227 Dortmund, Germany Department of Chemistry and Polymer Science, University of Stellenbosch, Private Bag X1, 7602 Matieland, South Africa Supporting Information ABSTRACT: For the first time, thermal field flow fractiona-tion (ThFFF) was coupled online with H NMR. ThFFF isone of the most important separation techniques for the analysis oflarge complex molecules and molecular assemblies. It providesinformation on molecular size and chemical composition in caseswhere multidetector setups are used. Multiple detection, however,is very complex. UsingH NMR as the sole detector offers anumber of significant benefits. First, NMR replaces the need to usemultidetector setups. Second,the structure and the microstructureof the molecules as well as the chemical composition of themolecules can be recorded. As the consequence, selective and quantitative information can be directly obtained from the NMR chromatograms. Third, the molar mass distributions of allseparated species can be derived directly from these NMR chromatograms. The new online ThFFF-NMR coupling was successfullyapplied to homopolymers and block copolymers of different molar masses and chemical compositions. INTRODUCTION Complex polymeric materials, e.g., copolymers of differentarchitectures and morphologies often represent a challenge toanalytics in particular for comprehensive separation and character-ization. Liquid adsorption chromatography (LAC), size exclusionchromatography (SEC), liquid chromatography at criticalconditions (LCCC), and multidimensional combinations arepopular tools for the elucidation of the molecular heterogeneityof complex polymers.-6 These methods, however, have theirlimitations when very high molar masses or polymer nano-composites have to be analyzed. Very strong forces in thechromatographic column may lead to shear degradation of themacromolecules. Field-flow fractionation (FFF) has been foundto be of increasing interest for polymer characterization andprotein analysis due to the fact that the shear forces in theseparation channel are significantly reduced.7-9 In FFF,separation is realized inside an empty channel using a singlesolvent or a mixture of solvents as mobile phase by applying anexternal field perpendicular to the laminar or axial flow. This fieldcauses the macromolecules to accumulate in different areas of thechannel. Depending on the nature of the field, differentsubtechniques of FFF can be used for the separation ofmacromolecules including asymmetrical flow field-flow fractiona-tion (AsFlFFF, AF4) where the external field is produced by across-flow, and thermal field-flow fractionation (ThFFF) whichworks with a temperature gradient that is established inside thechannel. The concepts and theory of FFF techniques are welldeveloped and documented.- Because of its sensitivity withregard to molar mass and chemical composition, ThFFF has proven to be a powerful tool in the characterization of complexpolymers. In ThFFF polymer retention is a function of thenormal diffusion coefficient D and the thermal diffusion coefficientD The former one is mainly influenced by the molecular size.Empirical findings indicate that Dr of macromolecules is practicallyindependent of molar mass. On the other hand it was foundexperimentally that the chemical composition of the macro-molecules influences Dr. In the case of copolymers, subject to thesegregation of different chain units, thermal diffusion appears to bedominated by the chain units that are located in the free-drainingregion of the solvated macromolecules. For copolymers that do notsegregate, thermal diffusion can be described by the molar fractionof the Dr values of the corresponding repeat units. Regardingnanoparticles, Shiundu and Giddings have found that the retentionof colloidal particles strongly depends on the chemical nature ofthe particle surface.In another study, Ratanathanawongs provedthat surface chemistry influences the retention of core-shellparticles in ThFFF.6Because of this complementarity, ThFFF canbe efficiently used for the characterization of various homopoly-mers, 17-28 polymer blends2.29 and complex polymeric materialssuch as copolymers,4,2830--333gel-containing samples, 4 polymericparticles,15.35 and aggregates.6 In particular, block copolymers arean extremely important species for the synthesis of biologicalproducts in medicine. In copolymer characterization, ThFFF fractionation isaccomplished by size and/or chemical composition distribution. ( Received: F ebruary 18, 2013 ) ( Revised: March 13, 2013 ) ( Published: March 26, 2 013 ) To evaluate these distributions quantitatively, the separation mustbe coupled to detectors that are sensitive toward concentration(e.g., RI, ELSD), size (e.g., MALLS) and chemical composition(e.g., UV, IR). Such multiple detector setups are quite complex. Inaddition, none of the mentioned detectors can directly andquantitatively observe the chemical composition and at the sametime provide a molar mass and microstructure information.Approaches to obtain information about the chemical compositionfrom ThFFF were based on measured copolymer Dr values independence of elution time’3or multiple detection which is well-known,e.g., from SEC.3738 In both cases, calibrations are requiredthat further add to the complexity of the approach. At this point, the use of NMR as an online detector for ThFFFbecomes an extremely interesting alternative to more conventionalsetups. NMR is a powerful method for structure elucidation. Hence,the composition of a copolymer can be directly determined from aNMR spectrum. NMR is also quantitative with regard toconcentration. Online HPLC-NMR techniques are known sincethe end of the 1970s3940 and meanwhile are in wide use, e.g., in thefields of pharmaceutical, food, and fuel chemistry.41-45 LC-NMRhas also been useful in polymer characterization.44.45 Differentchromatographic couplings to NMR are known: LAC-NMR wasused to monitor polymer separations regarding tacticity, chemicalcomposition distribution and copolymerization products.46-49LCCC-NMR applications dealt with the characterization ofpolymer blends, end group functionalities, block copolymercompositions and microstructure.50-60 The most widely usedtechnique in this field is SEC-NMR. It could be used to investigateoligomers, homopolymers, copolymers, terpolymers and polymerblends to obtain information about tacticities and compositions independence of molar mass and finally to determine molar massesand molar mass distributions both in absolute and calibration basedanalyses.60-73 In particular, it was proven that onflow SEC-NMRis a powerful and precise alternative to multiple detection setupssince it measures chemical composition directly and withoutcalibration, even under standard chromatographic conditions. -72Up to date, however, NMR has never been used as an onlinedetector for field-flow fractionation, although its advantages in thisregard are quite obvious. In the present work, ThFFF is coupled for the first timeonline to the NMR detector. Different block copolymers arestudied extensively regarding chemical compositions and molarmasses. It is demonstrated that onflow ThFFF-NMR is acomplementary method to SEC-NMR and efficiently providesmolar mass and chemical composition information. EXPERIMENTAL SECTION Samples. The polystyrene (PS), poly(methyl methacrylate) (PMMA)and polyisoprene (PI) standards as well as the PS-b-PMMAblock copolymers were produced by PSS GmbH (Mainz, Germany).The PS-b-PI copolymers are synthesized by the TU Darmstadt (Germany)and the PI-b-PMMA copolymers are provided by the Max-Planck-Institutefor Polymer Research in Mainz (Germany). The molar masses of thestandards are shown in Table S-1 in the Supporting Information and forthe copolymers in Table 1. ThFFF-NMR. The thermal FFF system TF2000 from Postnova(Landsberg, Germany) consisted of a pump, degasser and the thermalFFF channel. The output of the ThFFF was connected to an Agilent UVsingle wavelength detector 1100 working at 254 nm which was furtherconnected in series with the NMR flow probe. The NMR flow probe wasa triple resonance probe H(C,N) equipped with a 3 mm 60 uL flow celland an actively shielded z-gradient coil. The NMR spectrometer was aDRX-500 from Bruker BioSpin GmbH (Rheinstetten, Germany). Theflow rate was 0.2 mL/min causing a back pressure of 10 bar at the Table 1. Average Chemical Compositions Determined by HNMR, FFF-NMR, and SEC-NMR PI/PS/PMMA [mol %] sample H NMR FFF-NMR SEC-NMR SM -/29.7/70.3 -/29.8/70.2 -/31.1/68.9 SM2 -/47.8/52.2 -/49.3/50.7 -/50.2/49.8 SM3 -/48.0/52.0 -/49.7/50.3 -/51.8/48.2 SM4 -/32.1/67.9 -/30.7/69.3 -/34.0/66.0 SMS -/47.6/52.4 -/47.9/52.1 -/50.1/49.8 SM6 -/51.4/48.6 -/53.3/46.7 -/53.2/46.8 86.1/13.9/- 84.1/15.9/- 82.1/17.9/- 83.6/16.4/- 86.4/13.6/- 85.4/14.6/- IM1“ 15.3/-/84.7 13.7/-/86.3 16.4/-/83.6 IM2“ 54.8/-/45.2 60.4/-/39.6° 60.2/-/39.8 “Values used with permission from ref 71. simulated copolymerfraction: 51.2 mol % PI, 48.8 mol % PMMA. ‘Simulated copolymerfraction: 52.6 mol % PI, 47.4 mol % PMMA (used with permissionfrom ref 71). Scheme 1. Setup of the Thermal ThFFF-NMR with theLoop Collection Interface ThFFF. The concentration of the injected samples was 2 mg/mL for thehomopolymers and the PS-b-PMMA block copolymers and 8 mg/mLfor the PI-b-PMMA and PS-b-PI copolymers. A total of 100 uL wasinjected. All ThFFF-NMR measurements were performed as onflowexperiments. In this case a series of FIDs was recorded with 16transients, acquisition time of 0.8192 s, spectral width of 20 ppm and arelaxation delay of 0.1808 s. The eluent was HPLC grade THF whichwas suppressed with WET solvent suppression.The WET sequencewas using a 90° pulse (6 us), a gradient strength of 42 G/cm (ratio8:4:2:1 for the four gradients) and a gradient duration of 2 ms. TheFIDs were Fourier transformed by using 16 kb data and an exponentialmultiplication with a line broadening of 3 Hz. The data were processedas a contour plot. SEC-NMR. The SEC-NMR experiments used an Agilent HPLCsystem 1100 (Agilent Technologies GmbH, Boblingen, Germany)consisting of a pump with degasser, UV detector, autosampler andcolumn oven. SDV columns (10,10° and 10° A (except for PI-PMMA),300×8 mm inner diameter) and a precolumn from PSS GmbH (Mainz,Germany) were used. The SEC-NMR experiments used the same NMRspectrometer as mentioned above. The mobile phase was THF which wassuppressed with WET using the same parameters as above. The flow rate was0.8 mL/min. The injected sample concentration was 2.7 mg/mL and aninjection volume of 100 pL was used. RESULTS AND DISCUSSION 1. Setup of the Online Coupling of ThFFF and NMR.One of the major challenges for the online coupling of FFF and SEC-NMR of PS FFF-NMR of PMMA SEC-NMR of PMMA e) Figure 1. Onflow ThFFF-NMR vs SEC-NMR of 1,4-PI (a,b: olefinic protons), PS (c, d: aromatic protons (ortho, meta, para)) and PMMA (e, f:a-CH, protons (atactic and syndiotactic triads)) of different molar masses. NMR was the optimal setup of the entire system. The ThFFFconsisted of an isocratic pump, a thermal FFF channel with manualinjection and a UV detector after the FFF channel. The NMRsystem was attached to the ThFFF via the waste line of the UVdetector which was directly connected to the loop collectionsystem allowing for onflow experiments as well as for fractionationand loop analysis with stop-flow experiments. The easiest setup,however, was realized by simply bypassing the loop interface andusing two capillaries with different inner diameters to ensure asufficient back pressure of the system. This setup was used to beable to perform all applications as onflow experiments. Scheme 1shows the entire ThFFF-NMR system As compared to HPLC-NMR, ThFFF-NMR is more demand-ing with regard to the system stability. The stability of the temp-erature difference between the cold and hot wall and in particularthe back pressure of the ThFFF are of key importance. A temp-erature difference of 60 K was realized by a water cooling system.The back pressure, however, was held at 10 bar for a flow rate of0.2 mL minby adding two different lengths of peek tubing withinner diameters of 0.25 mm and 0.125 mm, respectively. The largercapillary was placed right after the output of the ThFFF and thesmaller one finally led to the NMR flow probe. A constantdetection temperature could be assumed because the NMRchemical shift of the solvents did not show any drift. The Figure 2. ThFFF-NMR fractograms of PMMA homopolymersextracted from the syndio- and atactic a-CHs signals (0.8-1.1 ppm)of the onflow runs. ThFFF-NMR was performed with protonated THF without locksolvent. Figure S-1 (in the Supporting Information) demonstratesthe quality of the solvent suppression. It shows the H spectrum ofprotonated THF (Figure S-1a, Supporting Information) at a highervertical scale and solvent suppression with WET (Figure S-1b,Supporting Information). The solvent suppression can be evaluatedby comparing the impurity at 2.46 ppm which is smaller than thesize of the l3c satellites of the THF signals. In Figure S-1b,Supporting Information, however, this impurity is significantlyhigher than the remaining THF solvent signals. This resultdemonstrates the high quality of the solvent suppression. 2. ThFFF-NMR vs SEC-NMR of Homopolymers. TheThFFF-NMR coupling shall be applied for the separation ofblock copolymers. As a first step a thermal FFF method was devel-oped and tested for the related PS, PMMA and PI homopoly-mers of different molar masses. By using THF as the eluent at aflow rate of 0.2 mL min=, 100 uL injections of a concentrationof 2 mg mL and a temperature difference of AT= 60 K(Tcold=28℃) the following separations of homopolymers wereachieved. Figure 1 shows the separately recorded ThFFF-NMRand SEC-NMR onflow runs of 4 PI, 4 PS, and 4 PMMAstandards. The FFF onflow spectra in Figure 1 show that the homopolymerselute in the expected order from small to high molar masses with increasing elution time. SEC shows the typical inverted order.However, the NMR elution plots show a difference. Whereas SEC-NMR provides narrow elution profiles according to the polydisper-sities below 1.1, the ThFFF-NMR fractograms show increasingpeak widths with increasing molar masses. This behavior is illustra-ted by the projections of 7 PMMA standards in Figure 2. TheThFFF separation is clearly improving with increasing molar masses.In this respect the ThFFF-NMR is providing significant betterseparation than SEC-NMR for the higher molar mass region. Itshould be noted, however, that the ThFFF separation for molarmasses below 10 kg molis not as efficient. In this case SEC is theappropriate technique. In the following PS, PMMA and PI of similar hydrodynamicsizes were compared. Figure 3 shows the corresponding ThFFF-NMR and SEC-NMR experiments. It is seen that ThFFF coversan elution range of 30 min for the separation of these threepolymers with the elution order PI < PS
确定






还剩7页未读,是否继续阅读?
产品配置单
上海积利科学仪器有限公司为您提供《postnova各型场流仪与ICP-MS和核磁NMR均可在线直接联用》,该方案主要用于其他中--检测,参考标准--,《postnova各型场流仪与ICP-MS和核磁NMR均可在线直接联用》用到的仪器有非对称流动场场流分离仪、离心场场流分离仪、热场场流分离仪
相关方案
更多











