方案详情
文
The Payne effect is assumed to arise from the same elementary mechanism of absorption-desorption of a macromolecular molecule from the sillica surface
方案详情

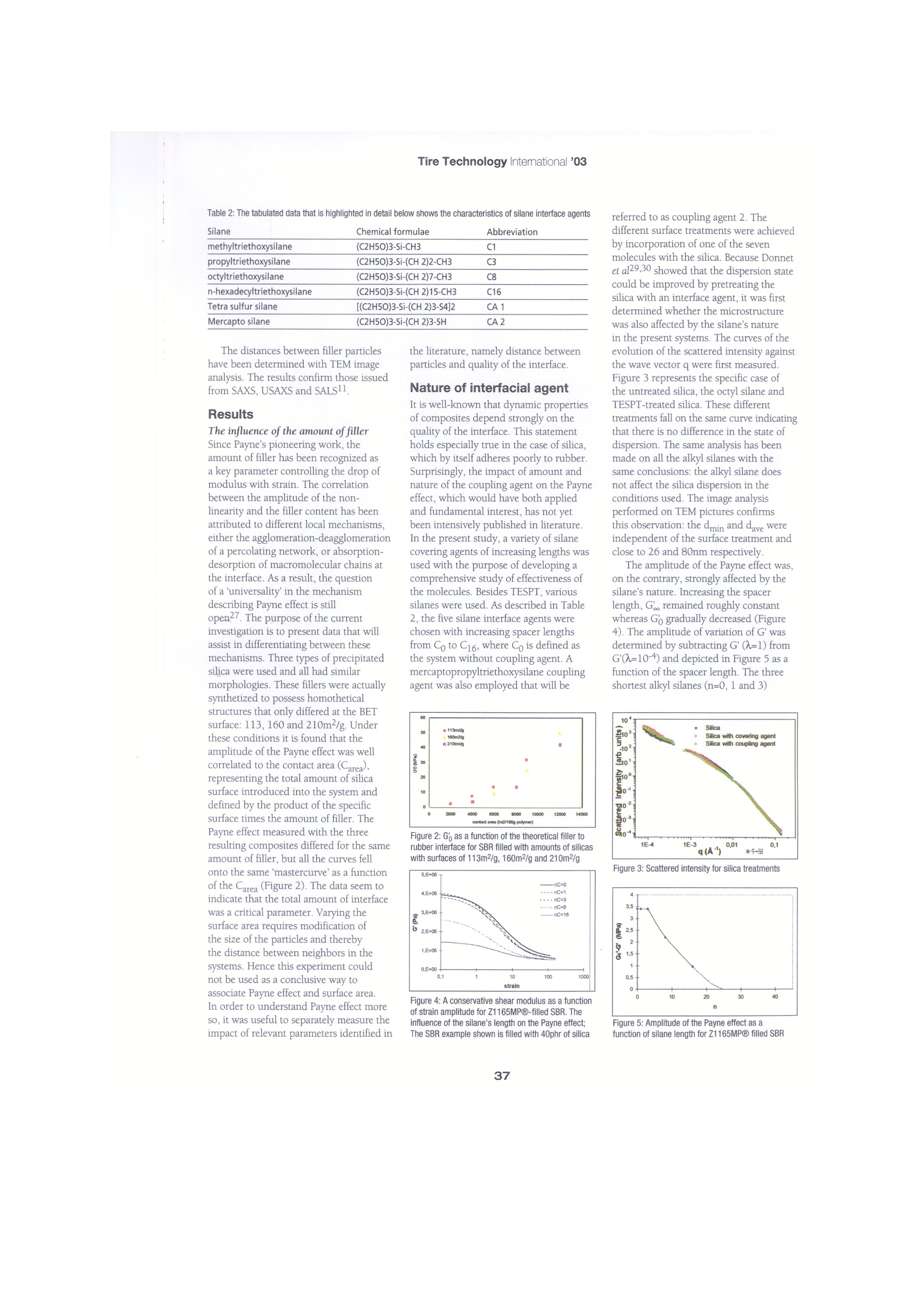
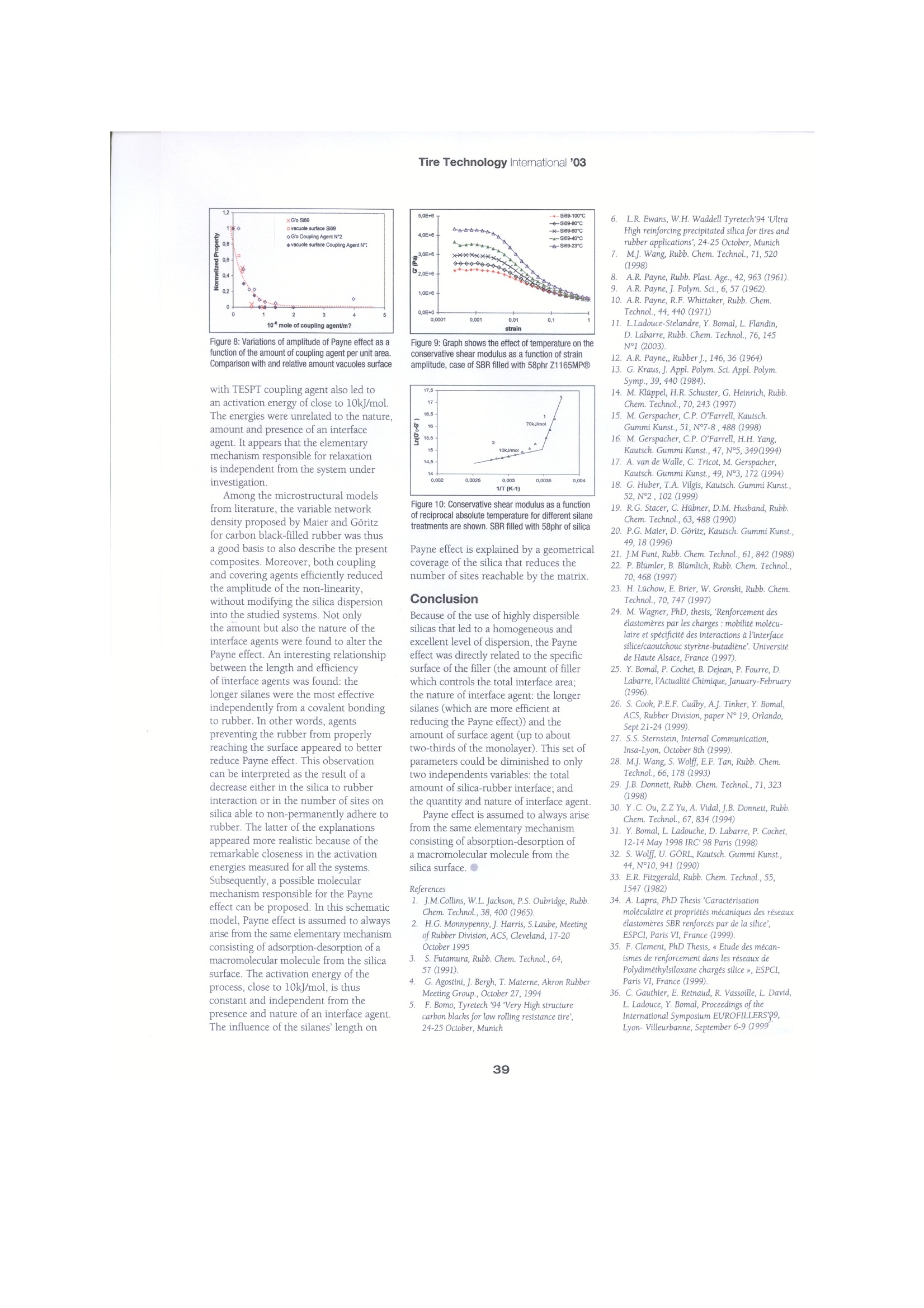
Tire Technology Intemational '03 Dynamic mechanicalproperties of highly dispersible silica-filled rubber The Payne effect is assumed to arise from the same elementary mechanism ofabsorption-desorption of a macromolecular molecule from the silica surface by L. Ladouce-Stelandre GY Bomal, Rhodia Research, P Cochet, Rhodia Silica Systems, J.Ramier GEMPPM ecause they are directly related to Table 1: Details of the formulation of the compounds rolling resistance of pneumatics1-6,the dynamic properties of filledelastomers have been extensivelyreported in literature. An excellent reviewon this matter was written by Wang7.The principal characteristics consist of adecrease in complex modulus (G*) withthe amplitude of deformation. Abundantlystudied by Payne in carbon black (CB)-filled elastomers8-10, this phenomenonwas named after him. The decrease in G’has been attributed to several mechanismsthat can be sorted in the three groupscorresponding to different localmechanisms, namely: the destruction-reformation of a percolating network offiller11-13 that can also involve polymerbounded to the filler14-18, adsorption-desorption of polymeric chain at theinterface19-20; disentanglement of bulkpolymer from the rubber bounded tothe surface21. The last two models arespecifically based on the existence of alow-mobility layer at the filler surface. Theexistence of the latter has been verified byNMR spectroscopy on carbon black22,23and silica24 filled rubber. Variouswell-defined silica fillers were employedto differentiate literature.Materials The formulation of compounds used inthis study are listed in Table 1. Zeosil1165MP@ 25 highly dispersible silica,commercialized by Rhodia, and otherhighly dispersible silicas that will bedescribed in each section were alsoemployed for comparison. The TESPT coupling agent was used asa reference. Other silanes were also usedfor comparison. Except where specified, a Ingredient PhrSBR(1) 137Filler (2) Variablesilane VariableStearic acid 1.5ZnO 2.5Antioxydant (2) 2S 1.5CBS (3) 1.8 (1) SBR5525-1 (2) Highly Dispersible Silicas utilized : ZEOSIL, 1115MP, 1165MP, 1205MP (3)Antioxydant : N-(1,3-dimethyl-butyl)-N'-phenyl-p-phenylenediamine. (4) CBS :N-cyclohexyl-2-benzothiazyl-sulphenamide constant ratio between interface agentand total silica surface was used in theformulations. The quantity of TESPT wasdetermined to ensure a monolayer, asdetermined by isothermal adsorption. Forthe other interface agents, the amount wasfixed to obtain the same number of activeends, i.e. moles of silicon. Iwo-step mixing was carried out in a1-liter Banbury internal mixer operating at80rpm with a fill factor adjusted to 0.8.Methods Dynamic mechanical measurements werecarried out at 5Hz and 23℃. Sampleswere tested in pure shear with a MetravibVA2000 setup. An innovative way to determine the adhesion of silica to rubber wasdeveloped in collaboration with TARRC(MRPRA)11. On the subsequent TEMphotomicrographs, three phases were Figure 1: TEM photomicrographs of some specimensthat have been swollen in styrene. Apparition of adecohesion between SBR rubber as well as silicaZ1165MPQ (compounded without coupling agent) differentiated that were assigned to silicafiller, matrix and polystyrene (Figure 1).These phases presumably arose from aphase separation26 between the styreneand the SBR during swelling that induceda non-homogeneous distribution ofstyrene around the silica. This mechanismwas confirmed by comparing compositeswith and without a coupling agent thatrespectively exhibited slight or significantvacuole areas. The global amount ofvacuoles was then quantified with animage analysis in order to indirectlyestablish the quality of filler to rubberinteraction. Samples were compared thatpossessed the same thermomechanicalhistory and formulation, except for thecoupling agent. Two other techniques are used todetermine the filler dispersion: the smallangle light scattering (l=632nm) and theangle and ultra small angle scattering.gx-ray. These technics give a 3D image ofdistribution and size. The combination ofthe three techniques makes it possible toconduct studies in the nanometer range.TEM has been also used Silane Chemical formulae Abbreviation methyltriethoxysilane (C2H50)3-Si-CH3 C1 propyltriethoxysilane (C2H5O)3-Si-(CH 2)2-CH3 C3 octyltriethoxysilane (C2H5O)3-Si-(CH 2)7-CH3 C8 n-hexadecyltriethoxysilane (C2H50)3-Si-(CH 2)15-CH3 C16 Tetra sulfur silane [(C2H5O)3-Si-(CH 2)3-S4]2 CA1 Mercapto silane (C2H50)3-Si-(CH 2)3-SH CA2 The distances between filler particles have been determined with TEM imageanalysis. The results confirm those issuedfrom SAXS, USAXS and SALS11 Results The influence of the amount of fillerSince Payne’s pioneering work, theamount of filler has been recognized asa key parameter controlling the drop ofmodulus with strain. The correlationbetween the amplitude of the non-linearity and the filler content has beenattributed to different local mechanisms.either the agglomeration-deagglomerationof a percolating network, or absorption-desorption of macromolecular chains atthe interface. As a result, the questionof a universality'in the mechanismdescribing Payne effect is stillopen27. The purpose of the currentinvestigation is to present data that willassist in differentiating between thesemechanisms. Three types of precipitatedsilica were used and all had similarmorphologies. These fillers were actuallysynthetized to possess homotheticalstructures that only differed at the BETsurface: 113, 160 and 210m²/g. Underthese conditions it is found that theamplitude of the Payne effect was wellcorrelated to the contact area (Carea),representing the total amount of silicasurface introduced into the system anddefined by the product of the specificsurface times the amount of filler. ThePayne effect measured with the threeresulting composites differed for the sameamount of filler, but all the curves fellonto the same ‘mastercurve'as a functionof the Carea (Figure 2). The data seem toindicate that the total amount of interfacewas a critical parameter. Varying thesurface area requires modification ofthe size of the particles and therebythe distance between neighbors in thesystems. Hence this experiment couldnot be used as a conclusive way toassociate Payne effect and surface area.In order to understand Payne effect moreso, it was useful to separately measure theimpact of relevant parameters identified in the literature, namely distance betweenparticles and quality of the interface. Nature of interfacial agent It is well-known that dynamic propertiesof composites depend strongly on thequality of the interface. This statementholds especially true in the case of silica,which by itself adheres poorly to rubber.Surprisingly, the impact of amount andnature of the coupling agent on the Payneeffect, which would have both appliedand fundamental interest, has not yetbeen intensively published in literature.In the present study, a variety of silanecovering agents of increasing lengths wasused with the purpose of developing acomprehensive study of effectiveness ofthe molecules. Besides TESPT, varioussilanes were used. As described in Table2, the five silane interface agents werechosen with increasing spacer lengthsfrom Co to C16, where Co is defined asthe system without coupling agent. Amercaptopropyltriethoxysilane couplingagent was also employed that will be referred to as coupling agent 2. Thedifferent surface treatments were achievedby incorporation of one of the sevenmolecules with the silica. Because Donnetet al29,30 showed that the dispersion statecould be improved by pretreating thesilica with an interface agent, it was firstdetermined whether the microstructurewas also affected by the silane’s naturein the present systems. The curves of theevolution of the scattered intensity againstthe wave vector q were first measured.Figure 3 represents the specific case ofthe untreated silica, the octyl silane andTESPT-treated silica. These differenttreatments fall on the same curve indicatingthat there is no difference in the state ofdispersion. The same analysis has beenmade on all the alkyl silanes with thesame conclusions: the alkyl silane doesnot affect the silica dispersion in theconditions used. The image analysisperformed on TEM pictures confirmsthis observation: the dmin and dave wereindependent of the surface treatment andclose to 26 and 80nm respectively. The amplitude of the Payne effect was,on the contrary, strongly affected by thesilane’s nature. Increasing the spacerlength, Gl remained roughly constantwhereas Go gradually decreased (Figure4). The amplitude of variation of G’wasdetermined by subtracting G'(A=1) fromG(A=10-4) and depicted in Figure 5 as afunction of the spacer length. The threeshortest alkyl silanes (n=0, l and 3) Figure 3: Scattered intensity for silica treatments Figure 5: Amplitude of the Payne effect as a Figure 6: Shear modulus as a function of strainamplitude for Z1165MP-filled SBR, with 80phr of silicaInfluenced coupling agents content on Payne effect exhibited very high AG. Further increasein spacer length (n=8 and 16) induced agradual decrease in the Payne effect. Therelative dependency of G and Go wassuch that only 30 per cent of the initialPayne effect remained with C16. Theoccurrence of a minimum silane lengthnecessary to alter the dynamic propertiesof the composites defined an apparentlength threshold'separating, as far asAG’is concerned,worthless and effectivemolecules. Assuming that no othermechanism would take place, anextrapolation of the data points revealedthat the Payne effect could eventuallyvanish by increasing n up to about 30(Figure 5). In summary, interface agent betweenthe filler and the matrix did not alter thesilica dispersion but strongly affected thenon-linearity. The variation in Payne effect01observed in these experiments could notbe associated with a change in distancesbetween particles, but to the spacerlength, that seemed to be efficient onlywhen the rubber molecules were repelled far enough from the silica particles. Coupling agent effects This section is devoted to the influenceof the amount of coupling agent on thestrength of the interface between SBRand silica. At present, the most commonlyused coupling agent employed for thesesystems is TESPT, and it was used invarying amounts ranging from 0-8 percent by weight of silica. As depicted in Figure 6, the amplitudeof the Payne effect was largely altered bythe coupling agent present in the system.This kind of behavior has already beendescribed for silica and CB31,32-filledrubbers. However, a saturation occurred for larger quantities and the non-linearity Table 3: The table shown below highlights in some detail the apparent activation energy for the Payne effect Figure 7: Shows a conservative low strain modulusthat is demonstrated as a function of silane contentnormalized to the TESPT coupling agent monolayer was no longer dependent upon the silanecontent, (Figure 7). The saturation tookplace for a silane amount correspondingto two thirds of the monolayer. The quality of the interface was also. investigated with the help of a proceduredeveloped in collaboration with theTARRC(MRPRA). This method, basedon styrene swelling, induces a solicitationof the whole composite but vacuolespreferentially developed when and whereweak interfaces are present. As depictedin Figure 8, the relative surface occupiedby vacuoles decreased gradually withincreasing silane content. In other words,less styrene was capable of damagingthe silica:rubber interface whenmore coupling agent was employed.Interestingly, an overlap between thecurves representing the variations ofGo and the total vacuoles’ surface wasevidenced after normalization of thequantities to those without the couplingagent. This overlap does not depend onthe nature of coupling agent. This mightseem surprising when one thinks of thetotally different methods used to obtainthe data: pure shear dynamic mechanicalanalysis on the one hand and isotropicswelling tests on the other. However, asindicated earlier, the styrene accumulatedwithin the weakest zones of the compositeoriginated from a de-wetting of the rubberfrom silica. This phenomenon clearlyarose from a detachment of the macro-molecular chains from the filler. Hencethe data gathered in Figure 8 interrelates. the amplitude of the Payne effect with theamount of polymer detached from silicaduring solicitation. In conclusion, thisfact, illustrates as the previous one,that the interface between silica andrubber appears to be a key parameter in describing the Payne effect in our systems Ua(kJ/mol) 9,5 9,2 10,7 7,8 Ua (eV) 0,1 0,1 Because of the highly dispersible andnon-microporous fillers used in this study,the nature and amount of coupling agentdid not affect the final state of dispersionat the meso and microscale. The setof studied parameters affecting thePayne effect can be reduced to only twoindependents variables: the total amountof silica-rubber interface (a function of theamount of filler and its BET surface) andthe quantity and nature of interface agent.On the contrary, the distance betweenparticles and the particles'size had novisible effect on the non-linearity. Thisreduced set of relevant parameterssuggested that the Payne effect in thesecomposites should be directly related tothe amount of interface, however it did notgive information on the origin of the non-linearity. As described in the next section,a temperature study was useful to betterunderstand the molecular mechanisms. It is generally agreed11,33,34 that theamplitude of the Payne effect decreaseswith increasing temperature. Figure 9displays the effect of the temperature onsilica filled SBR. Go gradually decreasedwith temperature,whereas Gremainedroughly constant. To verify whetherthe variations of the low deformationconservative modulus originated from athermally activated process, the Arrheniandiagram of a composite without interfaceagent was built (Figure 10), and twodistinct mechanisms were observed. Thelow-temperature unit corresponded totemperatures below 330K and was mostlikely related to the o relaxation of SBR.This relaxation was associated with a muchhigher activation energy of 70kJ/mol. Thehigh-temperature relaxation also followedan Arrhenius type of behavior but wasassociated to a lower slope. This resultindicated that the amplitude of the Payneas a function of temperature followed: where the activation energy Ua was equal to 9.5kJ/mol in the present case.The magnitude of U was, as had alreadybeen noted, within the range of Van derWaals interactions. The results compiledin Table 3, were obtained by performingthe same analysis on systems containingvarious interface agents. The activationenergies in our systems were close tothose generally presented in literature,as for example, CB-filled SBR20, insilica-filled SBR34 and also silica-filledPDMS35. Moreover, composites processed 0.4 Figure 8: Variations of amplitude of Payne effect as afunction of the amount of coupling agent per unit area.Comparison with and relative amount vacuoles surface with TESPT coupling agent also led toan activation energy of close to 10kJ/mol.The energies were unrelated to the nature,amount and presence of an interfaceagent. It appears that the elementarymechanism responsible for relaxationis independent from the system underinvestigation. Among the microstructural modelsfrom literature, the variable networkdensity proposed by Maier and Goritzfor carbon black-filled rubber was thusa good basis to also describe the presentcomposites. Moreover, both couplingand covering agents efficiently reducedthe amplitude of the non-linearity,without modifying the silica dispersioninto the studied systems. Not onlythe amount but also the nature of theinterface agents were found to alter thePayne effect. An interesting relationshipbetween the length and efficiencyof interface agents was found: thelonger silanes were the most effectiveindependently from a covalent bondingto rubber. In other words, agentspreventing the rubber from properlyreaching the surface appeared to betterreduce Payne effect. This observationcan be interpreted as the result of adecrease either in the silica to rubberinteraction or in the number of sites onsilica able to non-permanently adhere torubber. The latter of the explanationsappeared more realistic because of theremarkable closeness in the activationenergies measured for all the systems.Subsequently, a possible molecularmechanism responsible for the Payneeffect can be proposed. In this schematicmodel, Payne effect is assumed to alwaysarise from the same elementary mechanismconsisting of adsorption-desorption of amacromolecular molecule from the silicasurface. The activation energy of theprocess, close to 10kJ/mol, is thusconstant and independent from thepresence and nature of an interface agent.The influence of the silanes length on -+-Si69-100*C Figure 9:Graph shows the effect of temperature on theconservative shear modulus as a function of strainamplitude, case of SBR filled with 58phr Z1165MPQ 1615.5· 215 10kJ/m14,5 0.002 0.0025 0.003 0.0035 0,0041/T(K-1) Figure 10: Conservative shear modulus as a functionof reciprocal absolute temperature for different silanetreatments are shown. SBR filled with 58phr of silica Payne effect is explained by a geometricalcoverage of the silica that reduces the. number of sites reachable by the matrix. Conclusion Because of the use of highly dispersiblesilicas that led to a homogeneous and. excellent level of dispersion, the Payneeffect was directly related to the specificsurface of the filler (the amount of fillerwhich controls the total interfacearea:the nature of interface agent: the longersilanes (which are more efficient atreducing the Payne effect)) and theamount of surface agent (up to abouttwo-thirds of the monolayer). This set ofparameters could be diminished to onlytwo independents variables: the totalamount of silica-rubber interface; and the quantity and nature of interface agent.Payne effect is assumed to always arisefrom the same elementary mechanismconsisting of absorption-desorption ofa macromolecular molecule from thesilica surface.● ( R efe r ences ) 1. J.M.Collins, W.L Jackson, P.S. Oubridge, RubbChem. Technol.,38, 400(1965). 2. H.G. Monnypenny,J. Harris, S.Laube, MeetingofRubber Division, ACS, Cleveland, 17-20October 1995 3.S. Futamura, Rubb. Chem. Technol.,64, 57 (1991) 4.(G. Agostini, J. Bergh, T. Materne, Akron RubberT.Meeting Group., October 27, 1994 5.).F. Bomo, Tyretech '94 Very High structurecarbon blacks for low rolling resistance tire’,24-25October, Munich L.R. Ewans, W.H. Waddell Tyretech'94 Ultra High reinforcing precipitated silica for tires and rubber applications, 24-25 October, Munich 7..M.J. Wang,Rubb. Chem. Technol., 71, 520 (1998) 8.A.R. Payne, Rubb. Plast. Age., 42,963 (1961). 9A..R. Payne, J. Polym. Sci., 6, 57 (1962).10. A.R. Payne, R.F. Whittaker, Rubb. Chem. Technol., 44,440 (1971) 11. LLadouce-Stelandre, Y. Bomal, L. Flandin,D. Labarre, Rubb. Chem. Technol., 76, 145N°1 (2003). 12. A.R. Payne,, Rubber J., 146,36 (1964)13. G. Kraus,J. Appl. Polym. Sci. Appl. Polym. Symp.,39, 440 (1984). 14. M.Kluppel, H.R. Schuster, G. Heinrich, Rubb. Chem. Technol., 70, 243 (1997) 15. M. Gerspacher, C.P. O'Farrell, Kautsch. Gummi Kunst., 51,N°7-8, 488 (1998) 16. M. Gerspacher, C.P.OFarrell, H.H. Yang, Kautsch.Gummi Kunst., 47, N°5, 349(1994) 17. A. van de Walle, C. Tricot, M. Gerspacher, Kautsch. Gummi Kunst., 49,N°3, 172 (1994) 18. G. Huber, T.A. Vilgis, Kautsch. Gummi Kunst., 52,N°2,102 (1999) 19. R.G. Stacer, C. Hubner, D.M. Husband, Rubb. Chem. Technol., 63, 488 (1990) 20. P.G.Maier, D. Goritz, Kautsch. Gummi Kunst., 49,18(1996) 21. J.M Funt,Rubb. Chem. Technol., 61,842 (1988) 22. P. Blumler, B. Blumlich, Rubb. Chem. Technol., 70, 468 (1997) 23. H. Luchow, E. Brier, W. Gronski, Rubb. Chem. . Technol, 70, 747 (1997) 24. M. Wagner, PhD, thesis, Renforcement des elastomeres par les charges : mobilite molecu- laire et specificite des interactions a l'interface silice/caoutchouc styrene-butadiene. Universite de Haute Alsace, France (1997). 25. Y. Bomal, P. Cochet, B. Dejean, P. Fourre, D.Labarre, PActualite Chimique, January-February(1996). 26. S. Cook, P.E.F. Cudby, A.J. Tinker, Y. Bomal, ACS, Rubber Division, paper N°19, Orlando, Sept 21-24(1999). 27. S.S. Sternstein, Internal Communication, Insa-Lyon, October 8th (1999) 28. M.J. Wang, S.Wolff, E.F. Tan, Rubb. Chem Technol., 66,178(1993) 29. J.B.Donnett, Rubb. Chem. Technol., 71, 323 (1998) 30. Y.C. Ou, Z.Z Yu, A. Vidal, J.B. Donnett, Rubb Chem. Technol., 67, 834 (1994) . 31. Y. Bomal, L. Ladouche, D. Labarre, P. Cochet, 12-14 May 1998 IRC' 98 Paris (1998) 32. S. Wolff, U. GORL, Kautsch. Gummi Kunst., 44,N°10, 941 (1990) 33. E.R. Fitzgerald, Rubb. Chem. Technol., 55, 1547(1982) 34. A. Lapra, PhD Thesis Caracterisationmoleculaire et proprietes mecaniques des reseauxelastomeres SBR renforces par de la silice,ESPCI, Paris VI, France (1999). 35. F. Clement, PhD Thesis, 《 Etude des mecan-ismes de renforcement dans les reseaux dePolydimethylsiloxane charges silice x, ESPCI,Paris VI, France (1999). 36. C. Gauthier, E. Retnaud, R. Vassoille, L. David,L Ladouce, Y. Bomal, Proceedeieangs of theInternational Symposium EUROFILLERS'99,Lyon-Villeurbanne, September 6-9 (1999
确定


还剩2页未读,是否继续阅读?
仪尊科技有限公司为您提供《DMA中应用文章检测方案(热机械分析仪)》,该方案主要用于其他中应用文章检测,参考标准--,《DMA中应用文章检测方案(热机械分析仪)》用到的仪器有高级动态热机械分析仪
相关方案
更多










