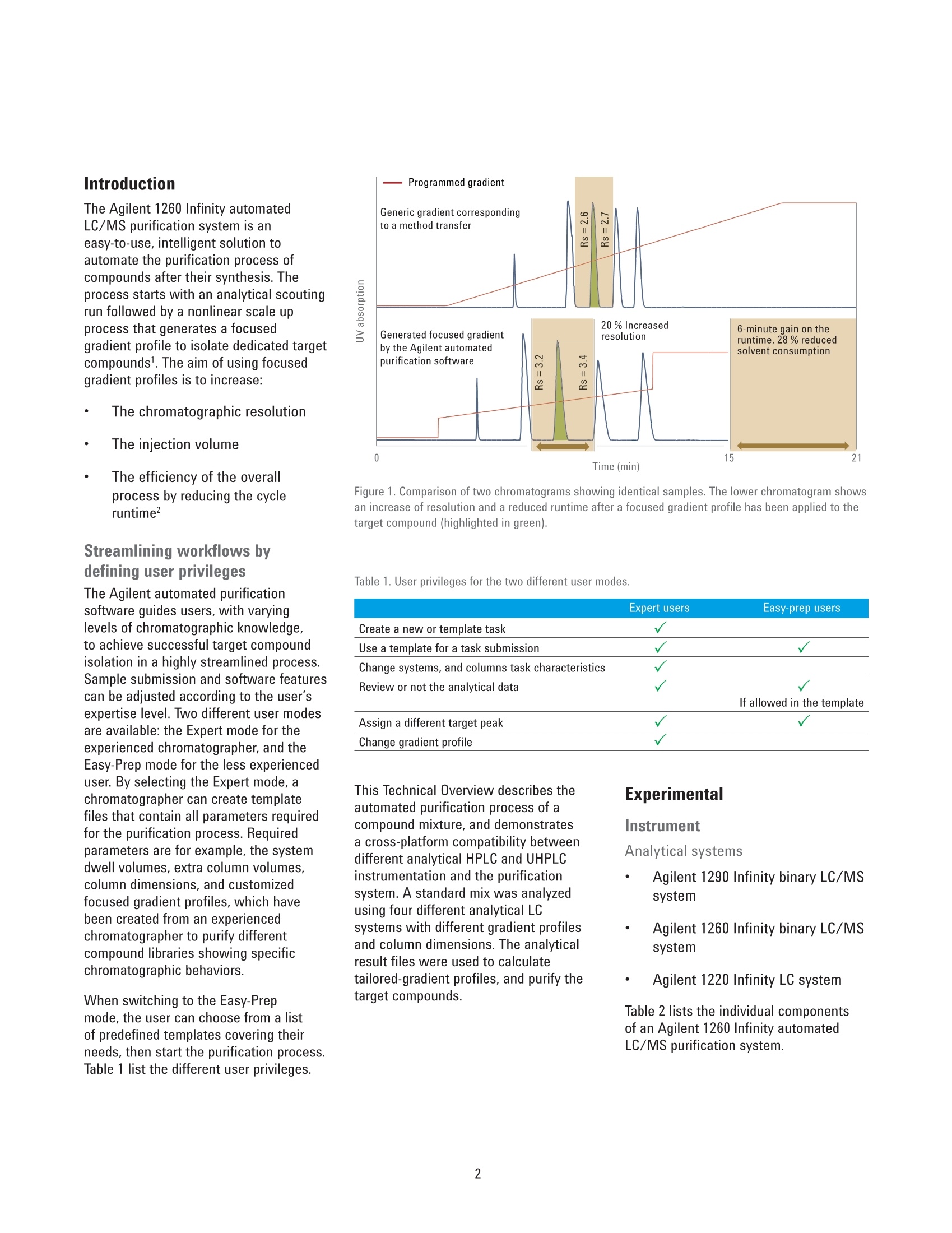
With the Agilent 1260 Infinity automated LC/MS purifi cation system, the purification process has reached a new level of automation. A tailored-gradient profile for each target compound was generated to increase the resolution, increase columnload, and reduce the cycle runtime. The Agilent automated purifi cation software automatically generated the different tailored-gradient profi les.
This Technical Overview describes an automated purifi cation process of a compound mixture, and shows cross-platform compatibility between different analytical LC systems and the Agilent 1260 Infi nity automated LC/MS purifi cation system. A standard mix was analyzed using four different analytical (U)HPLC systems, using different gradient profi les and different column dimensions. The four analytical result fi les were used to calculate gradient profi les, and purify target compounds. Through the use of this intelligent software algorithm, we obtained automated tailored-gradient profi les for all four samples using the purifi cation process. We purified 100 mg of a sample mix containing 24 mg of the target compound. We obtained 90 % recovery and 99 % purity of the target compound after purifi cation.
方案详情

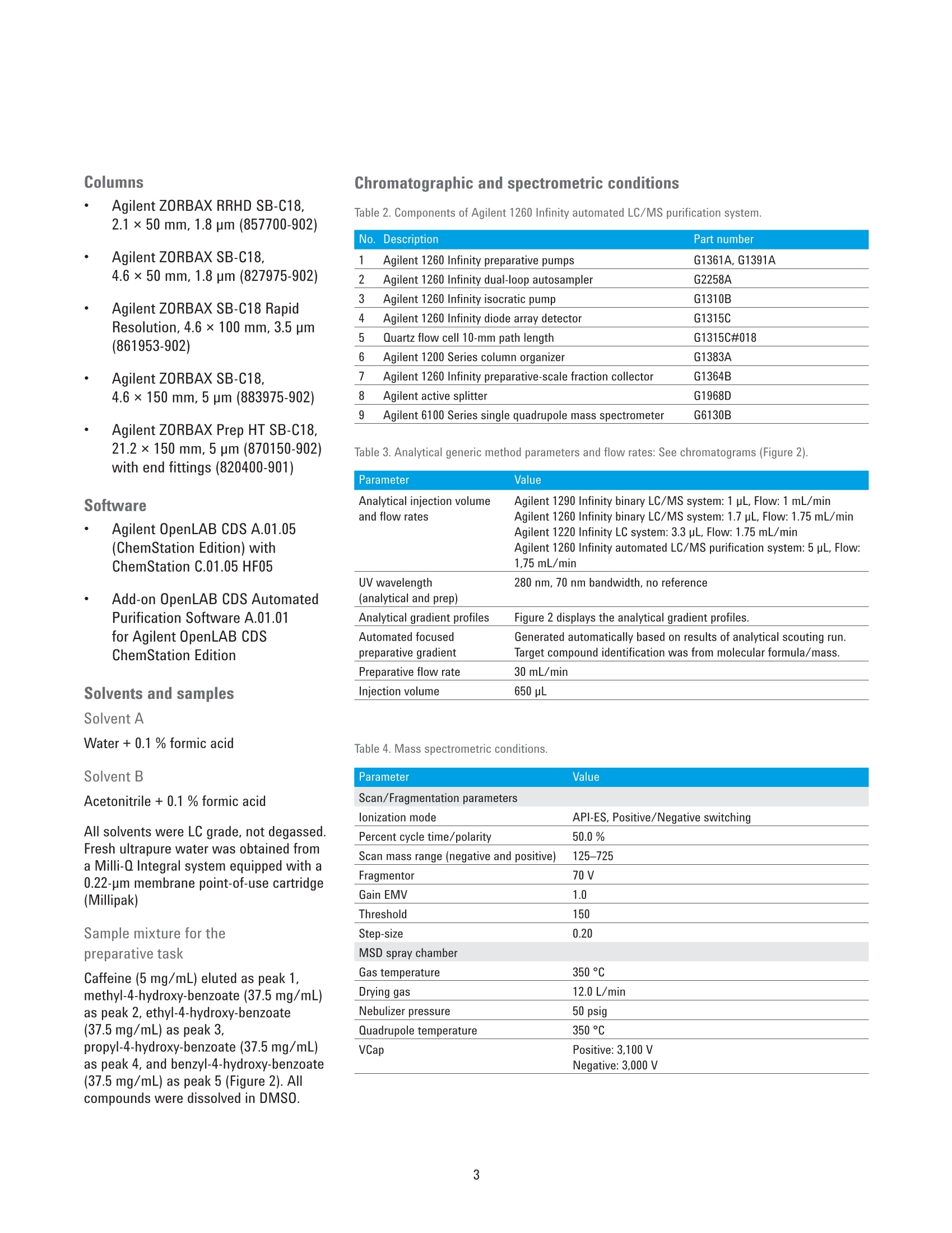
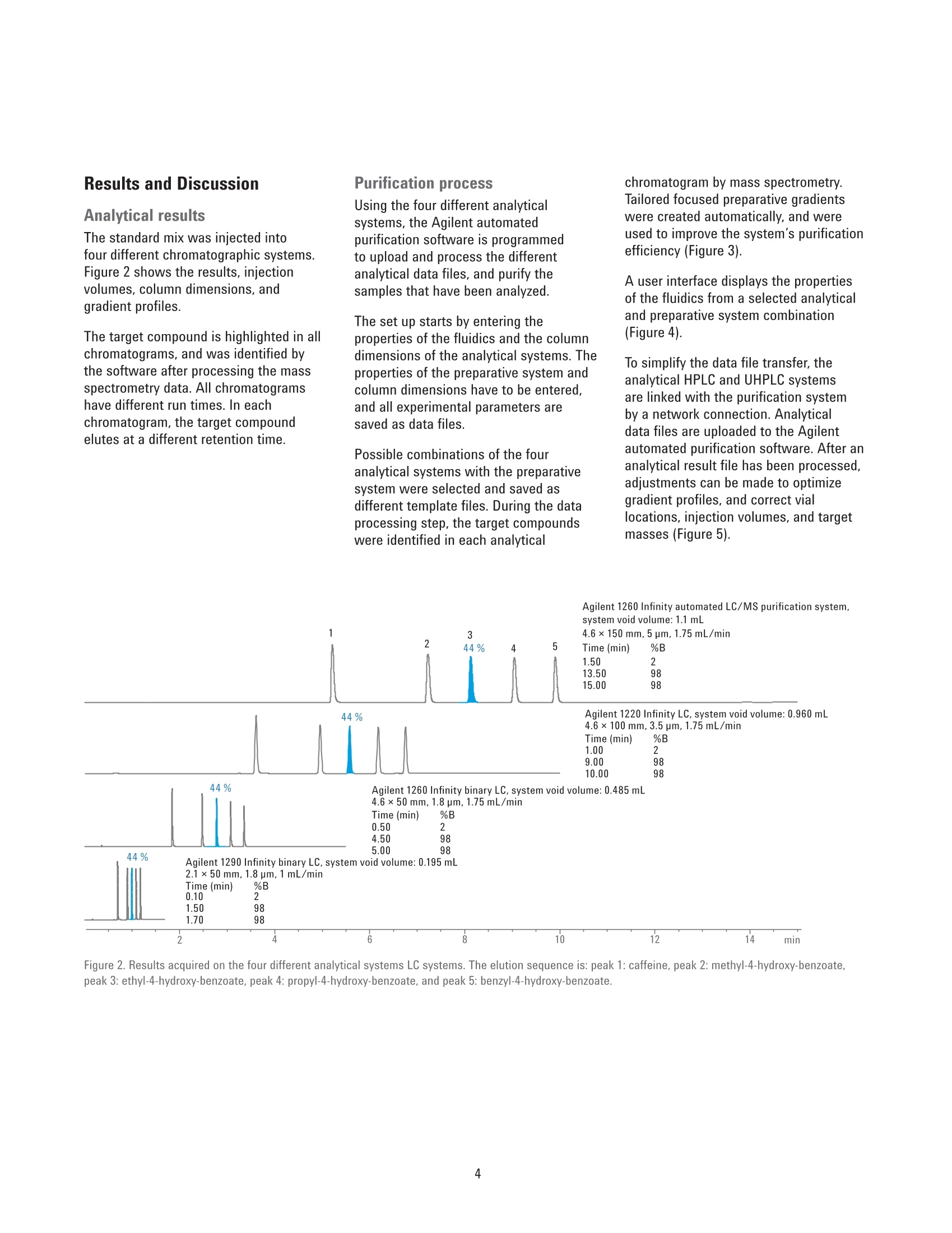
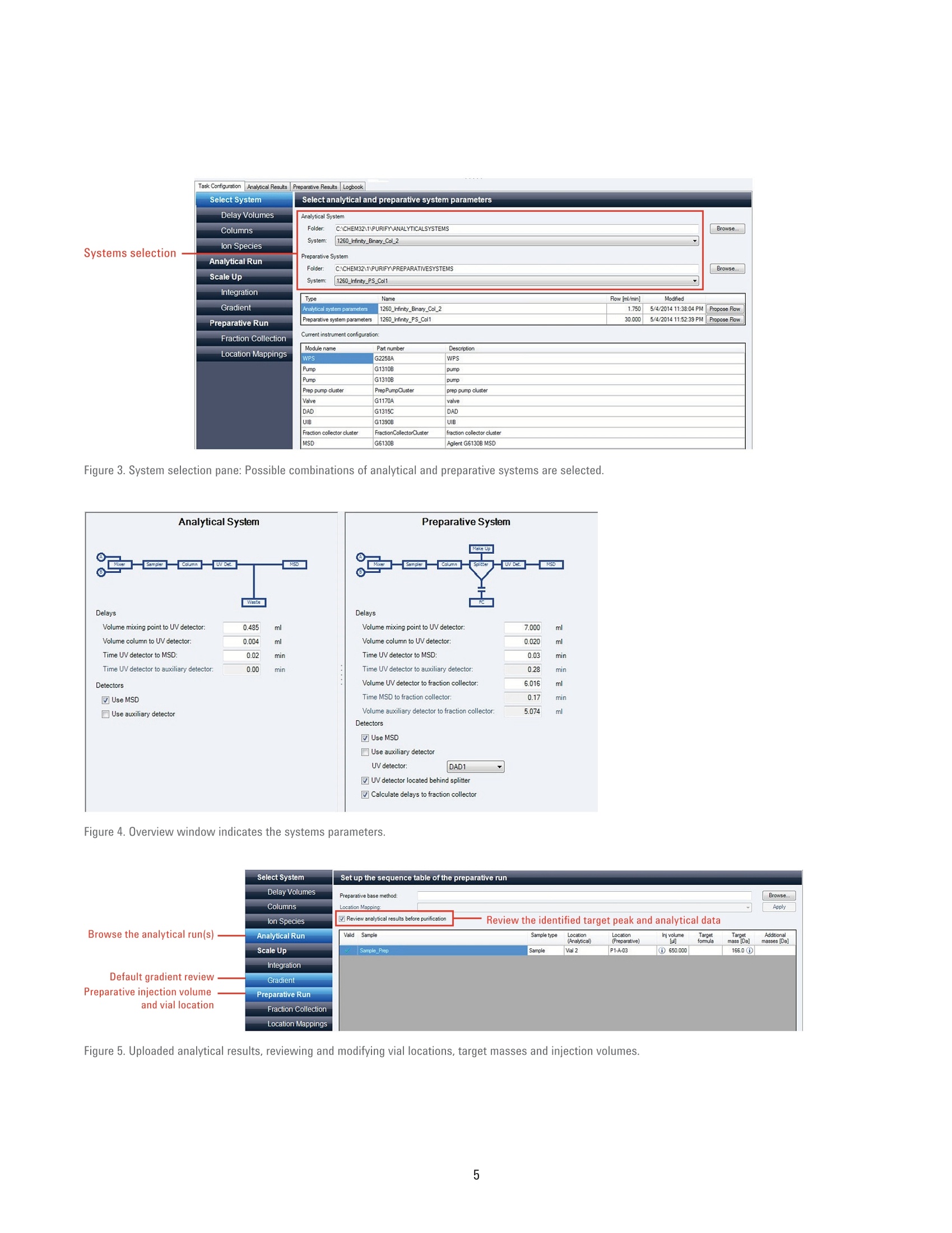
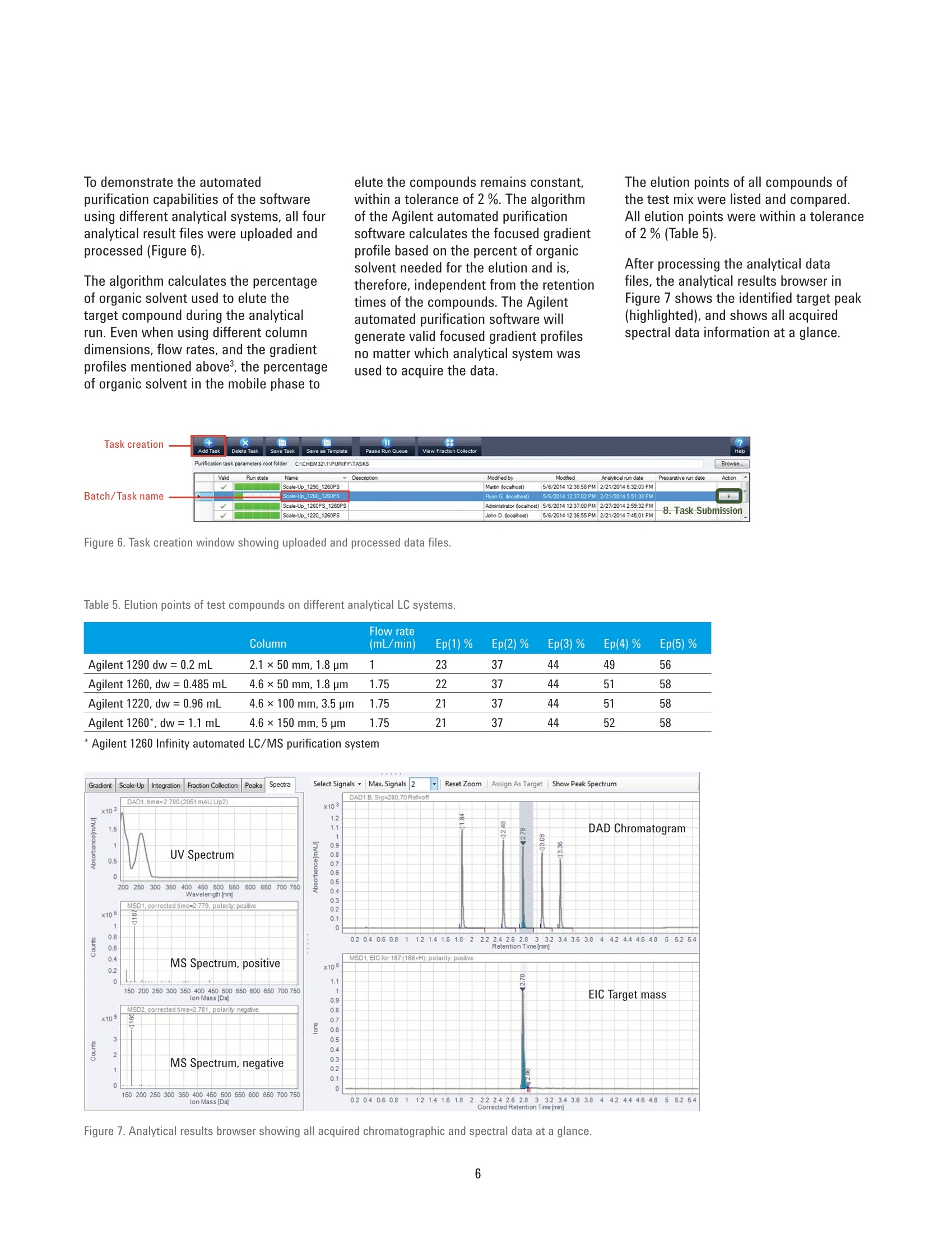
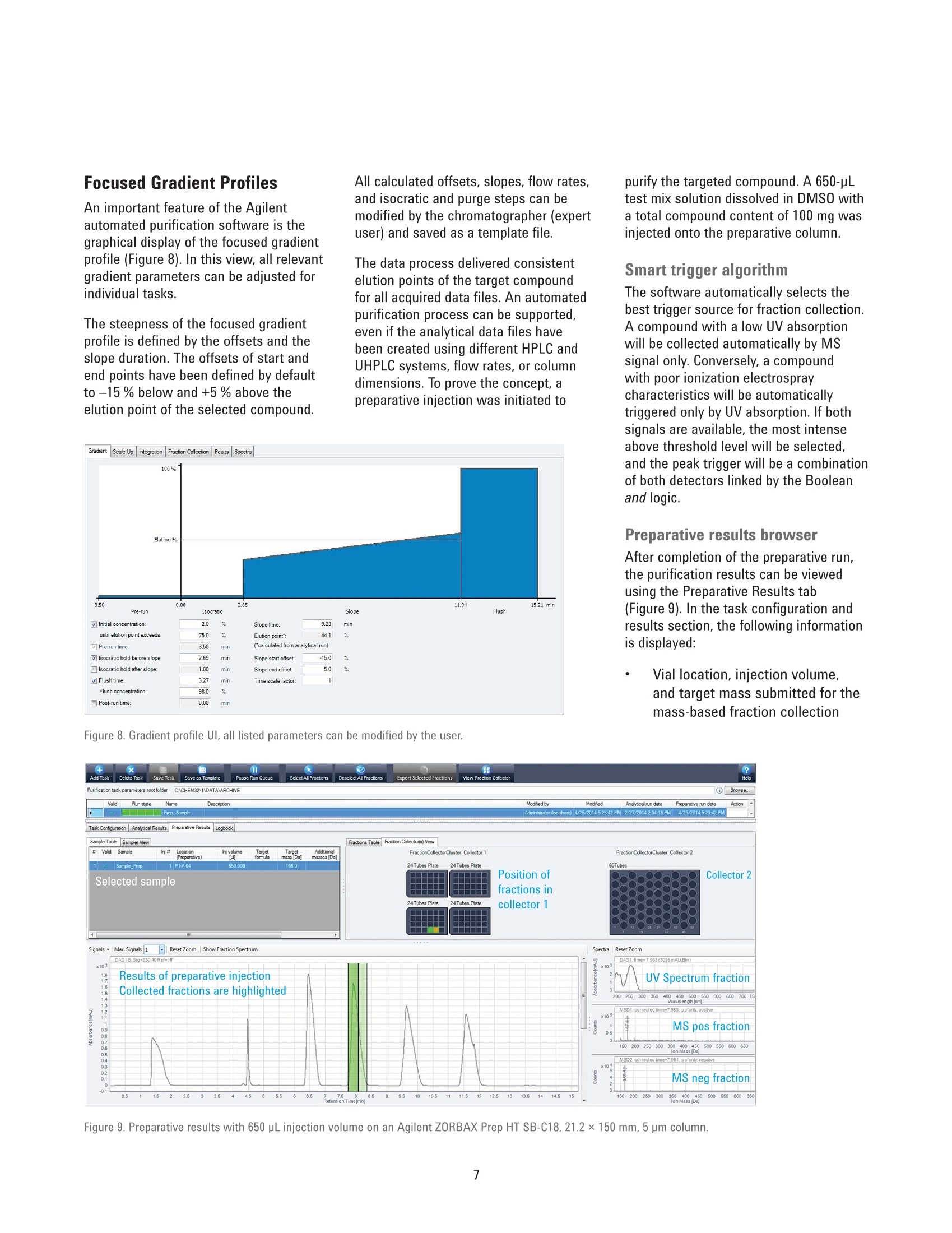
Authors Pierre Penduff and Andreas TeiAgilent Technologies, Inc. Waldbronn, Germany Automated Generation of FocusedGradient Profiles in Preparative LiquidChromatography Sample purification using the Agilent 1260 Infinityautomated LC/MS purification system Technical Overview Abstract With the Agilent 1260 Infinity automated LC/MS purification system, thepurification process has reached a new level of automation. A tailored-gradientprofile for each target compound was generated to increase the resolution,increase column load, and reduce the cycle runtime. The Agilent automatedpurification software automatically generated the different tailored-gradientprofiles. This Technical Overview describes an automated purification process of acompound mixture, and shows cross-platform compatibility between differentanalytical LC systems and the Agilent 1260 Infinity automated LC/MS purificationsystem. A standard mix was analyzed using four different analytical (U)HPLCsystems, using different gradient profiles and different column dimensions. Thefour analytical result files were used to calculate gradient profiles, and purifytarget compounds. Through the use of this intelligent software algorithm, weobtained automated tailored-gradient profiles for all four samples using thepurification process. We purified 100 mg of a sample mix containing 24 mg ofthe target compound. We obtained 90 % recovery and 99 % purity of the targetcompound after purification. Agilent Technologies Introduction The Agilent 1260 Infinity automatedLC/MS purification system is aneasy-to-use, intelligent solution toautomate the purification process ofcompounds after their synthesis. Theprocess starts with an analytical scoutingrun followed by a nonlinear scale upprocess that generates a focusedgradient profile to isolate dedicated targetcompounds1. The aim of using focusedgradient profiles is to increase: The chromatographic resolution The injection volume The efficiency of the overallprocess by reducing the cycleruntime² Streamlining workflows bydefining user privileges The Agilent automated purificationsoftware guides users, with varyinglevels of chromatographic knowledge,to achieve successful target compoundisolation in a highly streamlined process.Sample submission and software featurescan be adjusted according to the user’sexpertise level. Two different user modesare available: the Expert mode for theexperienced chromatographer, and theEasy-Prep mode for the less experienceduser. By selecting the Expert mode, achromatographer can create templatefiles that contain all parameters requiredfor the purification process. Requiredparameters are for example, the systemdwell volumes, extra column volumes,column dimensions, and customizedfocused gradient profiles, which havebeen created from an experiencedchromatographer to purify differentcompound libraries showing specificchromatographic behaviors. When switching to the Easy-Prepmode, the user can choose from a listof predefined templates covering theirneeds, then start the purification process.Table 1 list the different user privileges. Programmed gradient Figure 1. Comparison of two chromatograms showing identical samples. The lower chromatogram showsan increase of resolution and a reduced runtime after a focused gradient profile has been applied to thetarget compound (highlighted in green). Table 1. User privileges for the two different user modes. Expert users Easy-prep users Create a new or template task Use a template for a task submission Change systems, and columns task characteristics Review or not the analytical data If allowed in the template Assign a different target peak Change gradient profile This Technical Overview describes theautomated purification process of acompound mixture, and demonstratesa cross-platform compatibility betweendifferent analytical HPLC and UHPLCinstrumentation and the purificationsystem. A standard mix was analyzedusing four different analytical LCsystems with different gradient profilesand column dimensions. The analyticalresult files were used to calculatetailored-gradient profiles, and purify thetarget compounds. Experimental Instrument Analytical systems Agilent 1290 Infinity binary LC/MSsystem Agilent 1260 Infinity binary LC/MSsystem Agilent 1220 Infinity LC system Table 2 lists the individual componentsof an Agilent 1260 Infinity automatedLC/MS purification system. ( Colu m ns ) Agilent ZORBAX RRHD SB-C18,2.1×50 mm, 1.8 pm (857700-902) Agilent ZORBAX SB-C18,4.6×50 mm, 1.8 pm (827975-902) Agilent ZORBAX SB-C18 RapidResolution, 4.6×100mm, 3.5 um(861953-902) Agilent ZORBAX SB-C18,4.6×150 mm, 5 pm (883975-902) Agilent ZORBAX Prep HT SB-C18,21.2×150 mm, 5 pm (870150-902)with end fittings (820400-901) Software Agilent OpenLAB CDS A.01.0(ChemStation Edition) withChemStation C.01.05 HF05 Add-on OpenLAB CDS AutomatedPurification Software A.01.01for Agilent OpenLAB CDSChemStation Edition Solvents and samples Solvent AWater + 0.1 % formic acid Solvent BAcetonitrile + 0.1 % formic acid All solvents were LC grade, not degassed.Fresh ultrapure water was obtained froma Milli-Q Integral system equipped with a0.22-um membrane point-of-use cartridge(Millipak) Sample mixture for thepreparative task Caffeine (5 mg/mL) eluted as peak 1,methyl-4-hydroxy-benzoate (37.5 mg/mL)as peak 2, ethyl-4-hydroxy-benzoate(37.5 mg/mL) as peak 3,propyl-4-hydroxy-benzoate (37.5 mg/mL)as peak 4,and benzyl-4-hydroxy-benzoate(37.5 mg/mL) as peak 5 (Figure 2). Allcompounds were dissolved in DMSO. Table 2. Components of Agilent 1260 Infinity automated LC/MS purification system. No. Description Part number 1 Agilent 1260 Infinity preparative pumps G1361A, G1391A 2 Agilent 1260 Infinity dual-loop autosampler G2258A 3 Agilent 1260 Infinity isocratic pump G1310B 4 Agilent 1260 Infinity diode array detector G1315C 5 Quartz flow cell 10-mm path length G1315C#018 6 Agilent 1200 Series column organizer G1383A 7 Agilent 1260 Infinity preparative-scale fraction collector G1364B 8 Agilent active splitter G1968D 9 Agilent 6100 Series single quadrupole mass spectrometer G6130B Table 3. Analytical generic method parameters and flow rates: See chromatograms (Figure 2). Parameter Value Analytical injection volume Agilent 1290 Infinity binary LC/MS system: 1 pL, Flow:1 mL/min and flow rates Agilent 1260 Infinity binary LC/MS system: 1.7 pL, Flow: 1.75 mL/min Agilent 1220 Infinity LC system: 3.3pL, Flow: 1.75 mL/min Agilent 1260 Infinity automated LC/MS purification system: 5 pL, Flow: 1,75 mL/min UV wavelength 280 nm, 70 nm bandwidth, no reference (analytical and prep) Analytical gradient profiles Figure 2 displays the analytical gradient profiles. Automated focused Generated automatically based on results of analytical scouting run. preparative gradient Target compound identification was from molecular formula/mass. Preparative flow rate 30 mL/min Injection volume 650 pL Table 4. Mass spectrometric conditions. Parameter Value Scan/Fragmentation parameters lonization mode API-ES, Positive/Negative switching Percent cycle time/polarity 50.0% Scan mass range (negative and positive) 125-725 Fragmentor 70 V Gain EMV 1.0 Threshold 150 Step-size 0.20 MSD spray chamber Gas temperature 350 °C Drying gas 12.0 L/min Nebulizer pressure 50 psig Quadrupole temperature 350 °C VCap Positive:3,100 V Negative: 3,000 V Results and Discussion Analytical results The standard mix was injected intofour different chromatographic systems.Figure 2 shows the results, injectionvolumes, column dimensions, andgradient profiles. The target compound is highlighted in allchromatograms, and was identified bythe software after processing the massspectrometry data. All chromatogramshave different run times. In eachchromatogram, the target compoundelutes at a different retention time. Purification process Using the four different analyticalsystems, the Agilent automatedpurification software is programmedto upload and process the differentanalytical data files, and purify thesamples that have been analyzed. The set up starts by entering theproperties of the fluidics and the columndimensions of the analytical systems. Theproperties of the preparative system andcolumn dimensions have to be entered.and all experimental parameters aresaved as data files. Possible combinations of the fouranalytical systems with the preparativesystem were selected and saved asdifferent template files. During the dataprocessing step, the target compoundswere identified in each analytical chromatogram by mass spectrometry.Tailored focused preparative gradientswere created automatically, and wereused to improve the system’s purificationefficiency (Figure 3). A user interface displays the propertiesof the fluidics from a selected analyticaland preparative system combination(Figure 4). To simplify the data file transfer, theanalytical HPLC and UHPLC systemsare linked with the purification systemby a network connection. Analyticaldata files are uploaded to the Agilentautomated purification software. After ananalytical result file has been processed,adjustments can be made to optimizegradient profiles, and correct viallocations, injection volumes, and targetmasses (Figure 5). 2 6 8 10 12 14 min Figure 2. Results acquired on the four different analytical systems LC systems. The elution sequence is: peak 1: caffeine, peak 2: methyl-4-hydroxy-benzoate,peak 3: ethyl-4-hydroxy-benzoate, peak 4: propyl-4-hydroxy-benzoate, and peak 5: benzyl-4-hydroxy-benzoate. Figure 3. System selection pane: Possible combinations of analytical and preparative systems are selected. Figure 4. Overview window indicates the systems parameters. Figure 5. Uploaded analytical results, reviewing and modifying vial locations, target masses and injection volumes. To demonstrate the automatedpurification capabilities of the softwareusing different analytical systems, all fouranalytical result files were uploaded andprocessed (Figure 6). The algorithm calculates the percentageof organic solvent used to elute thetarget compound during the analyticalrun. Even when using different columndimensions, flow rates, and the gradientprofiles mentioned above, the percentageof organic solvent in the mobile phase to elute the compounds remains constant,within a tolerance of 2 %. The algorithmof the Agilent automated purificationsoftware calculates the focused gradientprofile based on the percent of organicsolvent needed for the elution and is,therefore, independent from the retentiontimes of the compounds. The Agilentautomated purification software willgenerate valid focused gradient profilesno matter which analytical system wasused to acquire the data. The elution points of all compounds ofthe test mix were listed and compared.All elution points were within a toleranceof 2 % (Table 5). After processing the analytical datafiles, the analytical results browser inFigure 7 shows the identified target peak(highlighted), and shows all acquiredspectral data information at a glance. Figure 6. Task creation window showing uploaded and processed data files. Table 5. Elution points of test compounds on different analytical LC systems. Column Flow rate (mL/min) Ep(1)% Ep(2)% Ep(3)% Ep(4)% Ep(5)% Agilent 1290 dw=0.2mL 2.1×50 mm, 1.8 pm 1 23 37 44 49 56 Agilent 1260, dw=0.485 mL 4.6×50 mm, 1.8 pm 1.75 22 37 44 51 58 Agilent 1220, dw=0.96 mL 4.6 ×100 mm, 3.5 um 1.75 21 37 44 51 58 Agilent 1260*, dw=1.1 mL 4.6×150 mm, 5 pm 1.75 21 37 44 52 58 * Agilent 1260 Infinity automated LC/MS purification system Focused Gradient Profiles An important feature of the Agilentautomated purification software is thegraphical display of the focused gradientprofile (Figure 8). In this view, all relevantgradient parameters can be adjusted forindividual tasks. The steepness of the focused gradientprofile is defined by the offsets and theslope duration. The offsets of start andend points have been defined by defaultto -15 % below and +5 % above theelution point of the selected compound. All calculated offsets,slopes, flow rates,and isocratic and purge steps can bemodified by the chromatographer (expertuser) and saved as a template file. The data process delivered consistentelution points of the target compoundfor all acquired data files. An automatedpurification process can be supported,even if the analytical data files havebeen created using different HPLC andUHPLC systems, flow rates, or columndimensions. To prove the concept, apreparative injection was initiated to Figure 8. Gradient profile Ul, all listed parameters can be modified by the user. Figure 9. Preparative results with 650 pL injection volume on an Agilent ZORBAX Prep HT SB-C18, 21.2×150 mm,5 pm column. purify the targeted compound. A 650-pLtest mix solution dissolved in DMSO witha total compound content of 100 mg wasinjected onto the preparative column. Smart trigger algorithm The software automatically selects thebest trigger source for fraction collection.A compound with a low UV absorptionwill be collected automatically by MSsignal only. Conversely, a compoundwith poor ionization electrospraycharacteristics will be automaticallytriggered only by UV absorption. If bothsignals are available, the most intenseabove threshold level will be selected,and the peak trigger will be a combinationof both detectors linked by the Booleanand logic. Preparative results browser After completion of the preparative run,the purification results can be viewedusing the Preparative Results tab(Figure 9). In the task configuration andresults section, the following informationis displayed: Vial location, injection volume,and target mass submitted for themass-based fraction collection Collected fractions on the fractioncollector diagram, or fractiondata using the fraction table tab.Locations, collected volumes,the main ion of the collectedcompound (m/z), start and stopcollection times, as well as thetype of collection (triggering UV orMS) By selecting a fraction on thechromatogram, the averagespectral data will be displayed onthe right side of the chromatogram. The fraction table information can beexported as a csv, Excel, or text file to aliquid handler for fraction pooling, or fordatabase import. For fraction re-analysis,a sequence file can be createdautomatically and exported to an Agilentanalytical LC/MS system or by using thecapabilites of the Agilent 1260 Infinityautomated LC/MS purification system. Conclusion This Technical Overview shows theanalysis of a crude sample usingfour different HPLC and UHPLC systemswith different column dimensions,flow rates, and gradient lengths. Allanalytical data files were processedusing Agilent automated purificationsoftware to generate tailored focusedgradient profiles. To demonstrate themethodology, a preparative injection wasperformed to purify the target compound.In this Technical Overview, 22 mg ofthe target compound, corresponding to90 % of recovery, were recovered. Afterfraction re-analysis, a purity level of99 % purity was obtained.A streamlinedand automated purification process issupported, even when the analyticalresult files have been acquired ondifferent HPLC and UHPLC systems. References 1. Analytical to Preparative HPLCMethod Transfer. An easy way toscale up from UHPLC to preparativeHPLC using focused gradients,Agilent Technical Overview,publication number 5991-2013EN 2. Automated Workflow Solution forPreparative Chromatography fromAnalytical Scouting to FractionReanalysis, Agilent TechnicalOverview, publication number5991-4115EN 3. Guillarme, D; et al. Method transferfor fast liquid chromatography inpharmaceutical analysis: Applicationto short columns packed with smallparticle. Part II: Gradient experiments.European Journal of Pharmaceuticsand Biopharmaceutics 2008, 68,430-440 Abstract With the Agilent 1260 Infinity automated LC/MS purifi cation system, the purification process has reached a new level of automation. A tailored-gradient profile for each target compound was generated to increase the resolution, increase columnload, and reduce the cycle runtime. The Agilent automated purifi cation software automatically generated the different tailored-gradient profi les. This Technical Overview describes an automated purifi cation process of a compound mixture, and shows cross-platform compatibility between different analytical LC systems and the Agilent 1260 Infi nity automated LC/MS purifi cation system. A standard mix was analyzed using four different analytical (U)HPLC systems, using different gradient profi les and different column dimensions. The four analytical result fi les were used to calculate gradient profi les, and purify target compounds. Through the use of this intelligent software algorithm, we obtained automated tailored-gradient profi les for all four samples using the purifi cation process. We purified 100 mg of a sample mix containing 24 mg of the target compound. We obtained 90 % recovery and 99 % purity of the target compound after purifi cation.Introduction The Agilent 1260 Infi nity automated LC/MS purifi cation system is an easy-to-use, intelligent solution to automate the purifi cation process of compounds after their synthesis. The process starts with an analytical scouting run followed by a nonlinear scale up process that generates a focused gradient profi le to isolate dedicated target compounds. The aim of using focused gradient profi les is to increase: • The chromatographic resolution • The injection volume • The effi ciency of the overall process by reducing the cycle runtime Streamlining workfl ows by defi ning user privileges The Agilent automated purifi cation software guides users, with varying levels of chromatographic knowledge, to achieve successful target compound isolation in a highly streamlined process. Sample submission and software features can be adjusted according to the user’s expertise level. Two different user modes are available: the Expert mode for the experienced chromatographer, and the Easy-Prep mode for the less experienced user. By selecting the Expert mode, a chromatographer can create template fi les that contain all parameters required for the purifi cation process. Required parameters are for example, the system dwell volumes, extra column volumes, column dimensions, and customized focused gradient profiles, which have been created from an experienced chromatographer to purify different compound libraries showing specifi c chromatographic behaviors.When switching to the Easy-Prep mode, the user can choose from a list of predefi ned templates covering their needs, then start the purifi cation process. Table 1 list the different user privileges.This Technical Overview describes the automated purifi cation process of a compound mixture, and demonstrates a cross-platform compatibility between different analytical HPLC and UHPLC instrumentation and the purifi cation system. A standard mix was analyzed using four different analytical LC systems with different gradient profiles and column dimensions. The analytical result files were used to calculate tailored-gradient profiles, and purify the target compounds.Conclusion This Technical Overview shows the analysis of a crude sample using four different HPLC and UHPLC systems with different column dimensions, fl ow rates, and gradient lengths. All analytical data fi les were processed using Agilent automated purifi cation software to generate tailored focused gradient profi les. To demonstrate the methodology, a preparative injection was performed to purify the target compound. In this Technical Overview, 22 mg of the target compound, corresponding to 90 % of recovery, were recovered. After fraction re-analysis, a purity level of 99 % purity was obtained. A streamlined and automated purifi cation process is supported, even when the analytical result fi les have been acquired on different HPLC and UHPLC systems.
确定


还剩6页未读,是否继续阅读?
安捷伦科技(中国)有限公司为您提供《混合标样中咖啡因,4-羟基苯甲酸甲酯等检测方案(制备液相色谱)》,该方案主要用于其他中其他检测,参考标准--,《混合标样中咖啡因,4-羟基苯甲酸甲酯等检测方案(制备液相色谱)》用到的仪器有Agilent 1290 Infinity II 制备型液相色谱、Agilent 6470 三重四极杆液质联用系统、Agilent 1290 Infinity II Multisampler、OpenLAB 软件
推荐专场
相关方案
更多
该厂商其他方案
更多