美国药典新章节<232>/<233>将于2018年1月1日正式发布。新方法将会取代当前的<231>方法,届时<231>方法也会失效。新方法将在赋形剂和原料药方面,给样品前处理及药物样品分析带来重大意义上的改变。一些形态的原料药是非常稳定的化合物,很难被消解。因此,消解这些原料药对传统的波消解方法来说无疑是一个巨大的挑战。
CEM最近推出了一款iPrep消解罐,具有双重密封专利,相对标准的消解罐,它可以承受更高的温度和压力。使用iPrep消解罐和iWave温度控制可以对难以消解的样品(原料药和凝胶胶囊)进行消解。
方案详情

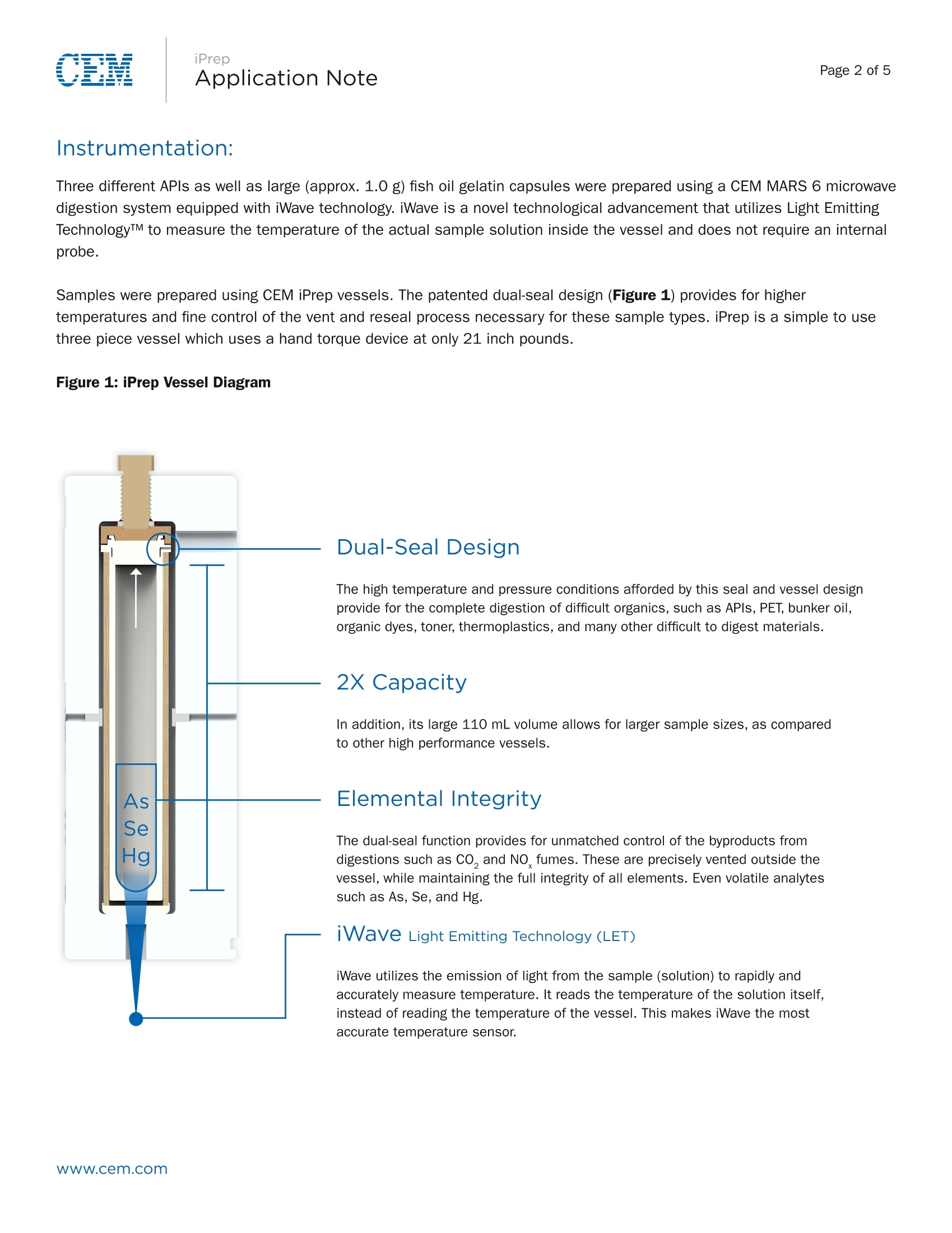
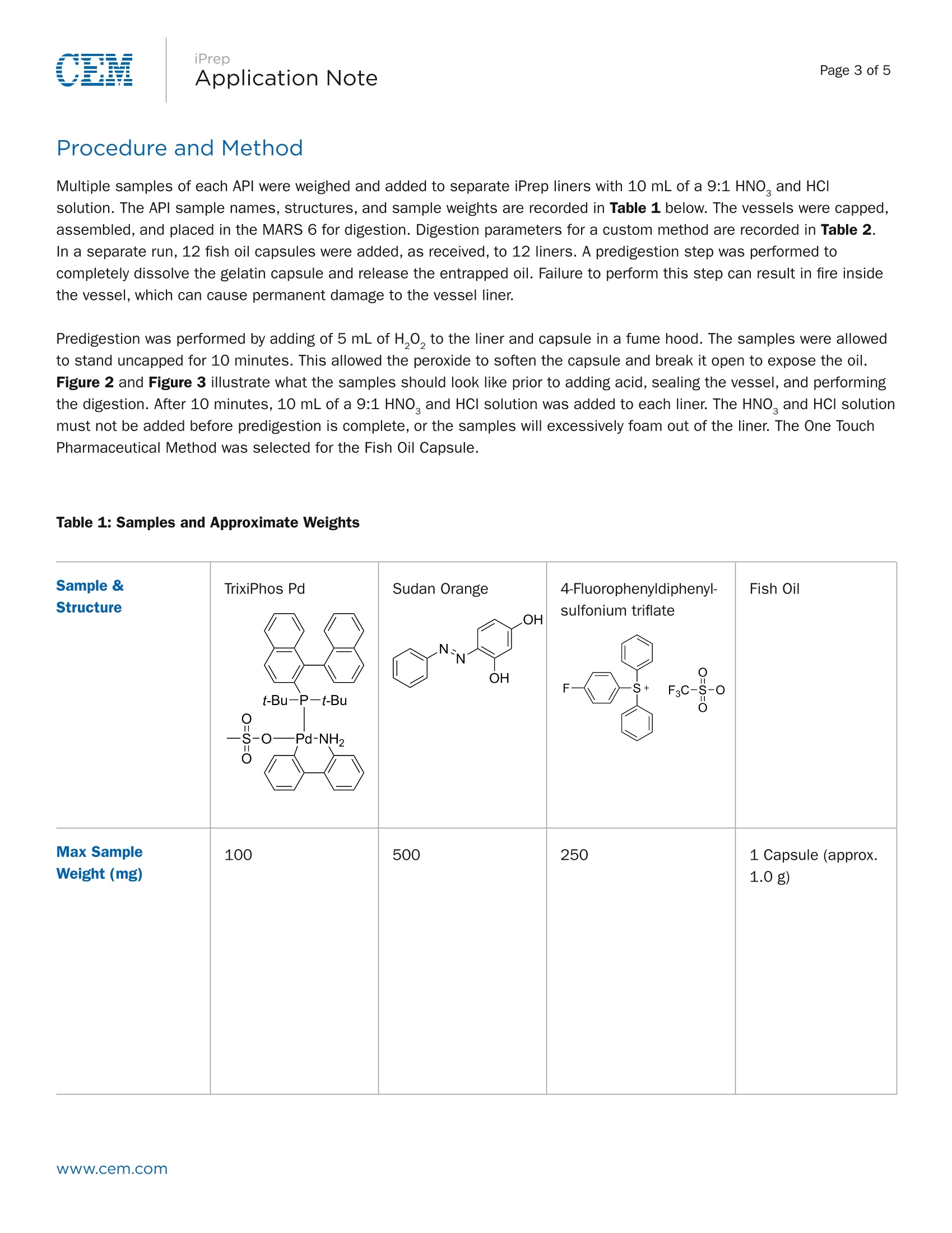
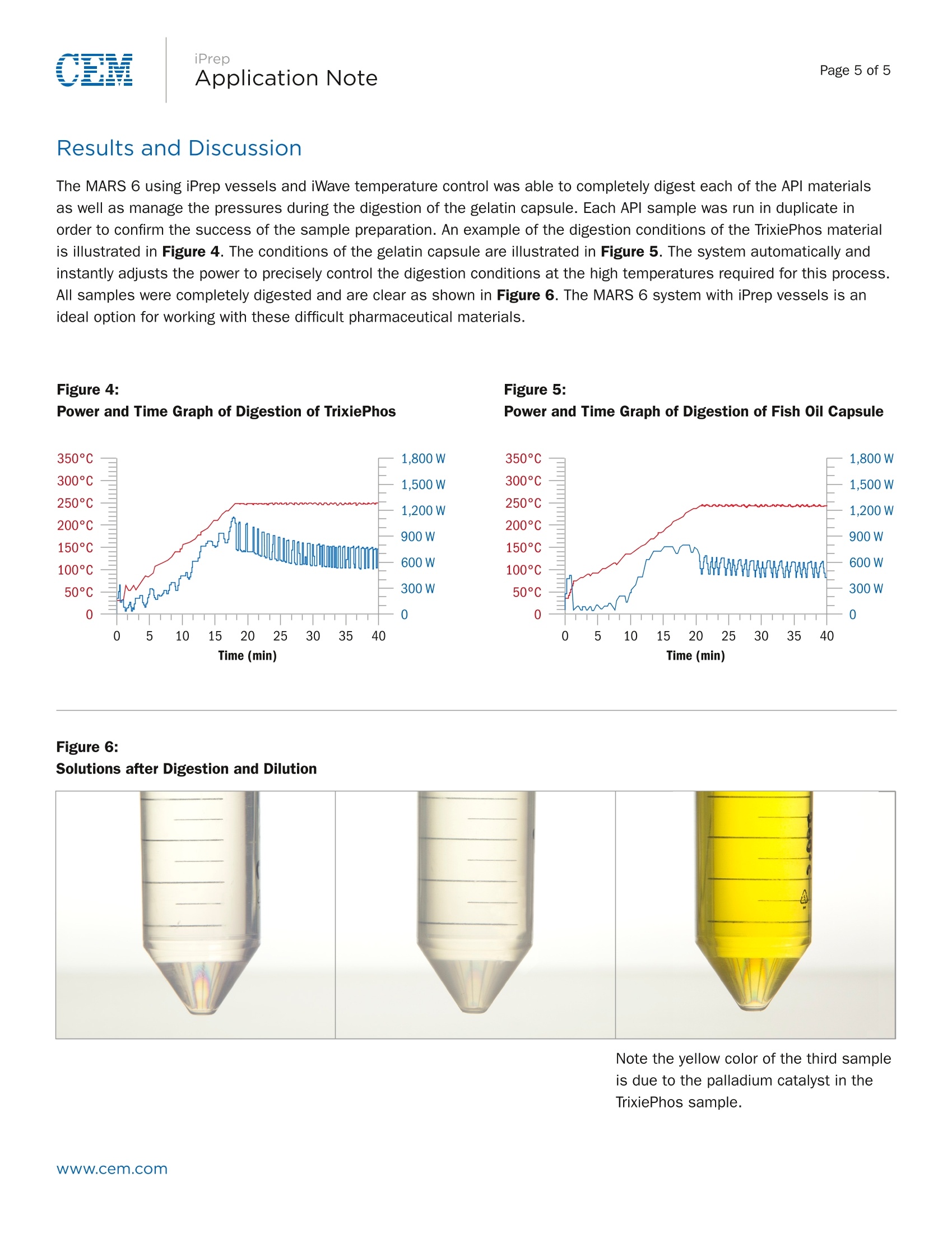
Page 1 of 5Application Note iPrepApplication NotePage 2 of 5 iPrep Digestion of Difficult APIs and Gel Capsules in Accordancewith USP <233> Abstract The new USP Chapters <232> and <233> will become official on January 1, 2018. At that time, they will supersede thecurrent Method <231>, which will no longer be valid. These new methods bring significant changes in sample preparationand analysis of pharmaceutical samples both in excipients and API’s. Certain APIs pose challenges to traditionalmicrowave digestion methods as they are very stable compounds and not easily broken down. CEM recently introduced the iPrep vessel with a patented dual-seal technology that allows the vessel to hold much highertemperatures and pressures than typical digestion vessels. Digestion of difficult API’s and large gelatin capsules wasachieved using this vessel and iWave advanced temperature control. Introduction The new USP Methods <232> and <233> call for total digestion of pharmaceutical samples and quantification of individualelements typically by ICP-OES or ICP-MS analysis. Many pharmaceutical materials can be easily digested but APIs withmultiple aromatic ring structures can be very difficult to completely break down and obtain a clear digestion, as prescribedin the new chapters. In addition, large gelatin capsules can prove challenging because of the amount of oil they typicallycontain. A large amount of gas is released once the capsule is dissolved and the acids begin to attack the contained oilwhich can lead to loss of volatile elements if not properly contained. This application note will focus on the use of the CEM MARS 6 microwave digestion system with iPrep vessels tocompletely digest both difficult active pharmaceutical ingredients and large gelatin capsules. Sample structures of APls areshown to illustrate complexity. Sample sizes are given as maximum allowable to achieve clear digest. Instrumentation: Three different APIs as well as large (approx. 1.0 g) fish oil gelatin capsules were prepared using a CEM MARS 6 microwavedigestion system equipped with iWave technology. iWave is a novel technological advancement that utilizes Light EmittingTechnologyTM to measure the temperature of the actual sample solution inside the vessel and does not require an internalprobe. Samples were prepared using CEM iPrep vessels. The patented dual-seal design (Figure 1) provides for highertemperatures and fine control of the vent and reseal process necessary for these sample types. iPrep is a simple to usethree piece vessel which uses a hand torque device at only 21 inch pounds. Figure 1: iPrep Vessel Diagram Dual-Seal Design The high temperature and pressure conditions afforded by this seal and vessel designprovide for the complete digestion of difficult organics, such as APls, PET, bunker oil,organic dyes, toner, thermoplastics, and many other difficult to digest materials. 2X Capacity In addition, its large 110 mL volume allows for larger sample sizes, as comparedto other high performance vessels. Elemental Integrity The dual-seal function provides for unmatched control of the byproducts fromdigestions such as CO, and NO fumes. These are precisely vented outside thevessel, while maintaining the full integrity of all elements. Even volatile analytessuch as As, Se, and Hg. iWaveLight Emitting Technology (LET) iWave utilizes the emission of light from the sample (solution) to rapidly andaccurately measure temperature. It reads the temperature of the solution itself,instead of reading the temperature of the vessel. This makes iWave the mostaccurate temperature sensor. iPrep Procedure and Method Multiple samples of each API were weighed and added to separate iPrep liners with 10 mL of a 9:1 HNO, and HCIsolution. The APl sample names, structures, and sample weights are recorded in Table 1 below. The vessels were capped,assembled, and placed in the MARS 6 for digestion. Digestion parameters for a custom method are recorded in Table 2.In a separate run, 12 fish oil capsules were added, as received, to 12 liners. A predigestion step was performed tocompletely dissolve the gelatin capsule and release the entrapped oil. Failure to perform this step can result in fire insidethe vessel, which can cause permanent damage to the vessel liner. Predigestion was performed by adding of 5 mL of H,0, to the liner and capsule in a fume hood. The samples were allowedto stand uncapped for 10 minutes. This allowed the peroxide to soften the capsule and break it open to expose the oil.Figure 2 and Figure 3 illustrate what the samples should look like prior to adding acid, sealing the vessel, and performingthe digestion. After 10 minutes, 10 mL of a 9:1 HNO, and HCI solution was added to each liner. The HNO, and HCl solutionmust not be added before predigestion is complete, or the samples will excessively foam out of the liner. The One TouchPharmaceutical Method was selected for the Fish Oil Capsule. Table 1: Samples and Approximate Weights Sample & Structure TrixiPhos Pd t-Bu-P-t-Bu SO ·Pd-NH Sudan Orange OH N`NNOH 4-Fluorophenyldiphenyl-sulfonium triflate F S+ F3C--S--O O Fish Oil Max SampleWeight (mg) 100 500 250 1 Capsule (approx.1.0g) iPrep Table 2: MARS 6 Digestion Parameters for APIs Stage Ramp Time (min) Hold Time (min) Temperature (C) 1 25 30 250 Acid used: 9 mL HN0,1 mL HCI per sample Figure 2:Photos of Fish Oil Capsule after Peroxide Addition Figure 3: Photos of Fish Oil Capsule after Nitric Acid Addition iPrep Results and Discussion The MARS 6 using iPrep vessels and iWave temperature control was able to completely digest each of the API materialsas well as manage the pressures during the digestion of the gelatin capsule. Each API sample was run in duplicate inorder to confirm the success of the sample preparation. An example of the digestion conditions of the TrixiePhos materialis illustrated in Figure 4. The conditions of the gelatin capsule are illustrated in Figure 5. The system automatically andinstantly adjusts the power to precisely control the digestion conditions at the high temperatures required for this process.All samples were completely digested and are clear as shown in Figure 6. The MARS 6 system with iPrep vessels is anideal option for working with these difficult pharmaceutical materials. Figure 4:Power and Time Graph of Digestion of TrixiePhos Figure 5:Power and Time Graph of Digestion of Fish Oil Capsule Figure 6:Solutions after Digestion and Dilution Note the yellow color of the third sampleis due to the palladium catalyst in theTrixiePhos sample. Www.cem.com 根据药典新方法<232>/<233>,通过ICP-OES或ICP-MS可分析出药物样品的总消化量以及各个代表性元素的含量。根据药典的方法,很多药物材料都很容易地被消解,得到消解完全的消解液体。但是具有多重芳环结构的原材料很难完全地被消化。此外,由于较大的明胶胶囊含有大量的鱼油,因此其消解具有很大的挑战性。一旦胶囊溶解会产生并释放大量的气体,溶剂中的酸与胶囊中包含的鱼油作用,如控制不当将导致挥发性元素的丢失。 这篇文章主要介绍利用MARS 6 微波消解系统的iPrep消解罐消解难以消化的药物活性成分和较大的明胶胶囊。原料药样品的结构非常复杂。样品的尺寸是允许消解的最大尺寸。
确定


还剩3页未读,是否继续阅读?
培安有限公司为您提供《原料药、明胶胶囊中微波消解检测方案(微波消解)》,该方案主要用于原料药中含量测定检测,参考标准--,《原料药、明胶胶囊中微波消解检测方案(微波消解)》用到的仪器有CEM Mars6 高通量密闭高压微波消解仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















