方案详情
文
The authors describe the characteristics and application of a 265 nm AlGaN light-emitting diode LED operated at 1 MHz repetition rate, 1.2 ns pulse duration, 1.32 μW average power, 2.3 mW peak power, and ?12 nm bandwidth. The LED enables the ?uorescence decay of weakly emitting phenylalanine to be measured routinely, even in dilute solution. For pH of 6–9.2, the authors ?nd evidence for a biexponential rather than monoexponential decay, providing direct evidence for the presence of phenylalanine rotamers with a photophysics closer to the other two ?uorescent amino acids tryrosine and tryptophan than has previously been reported. © 2006 American Institute of Physics. ?DOI: 10.1063/1.2245441?
方案详情

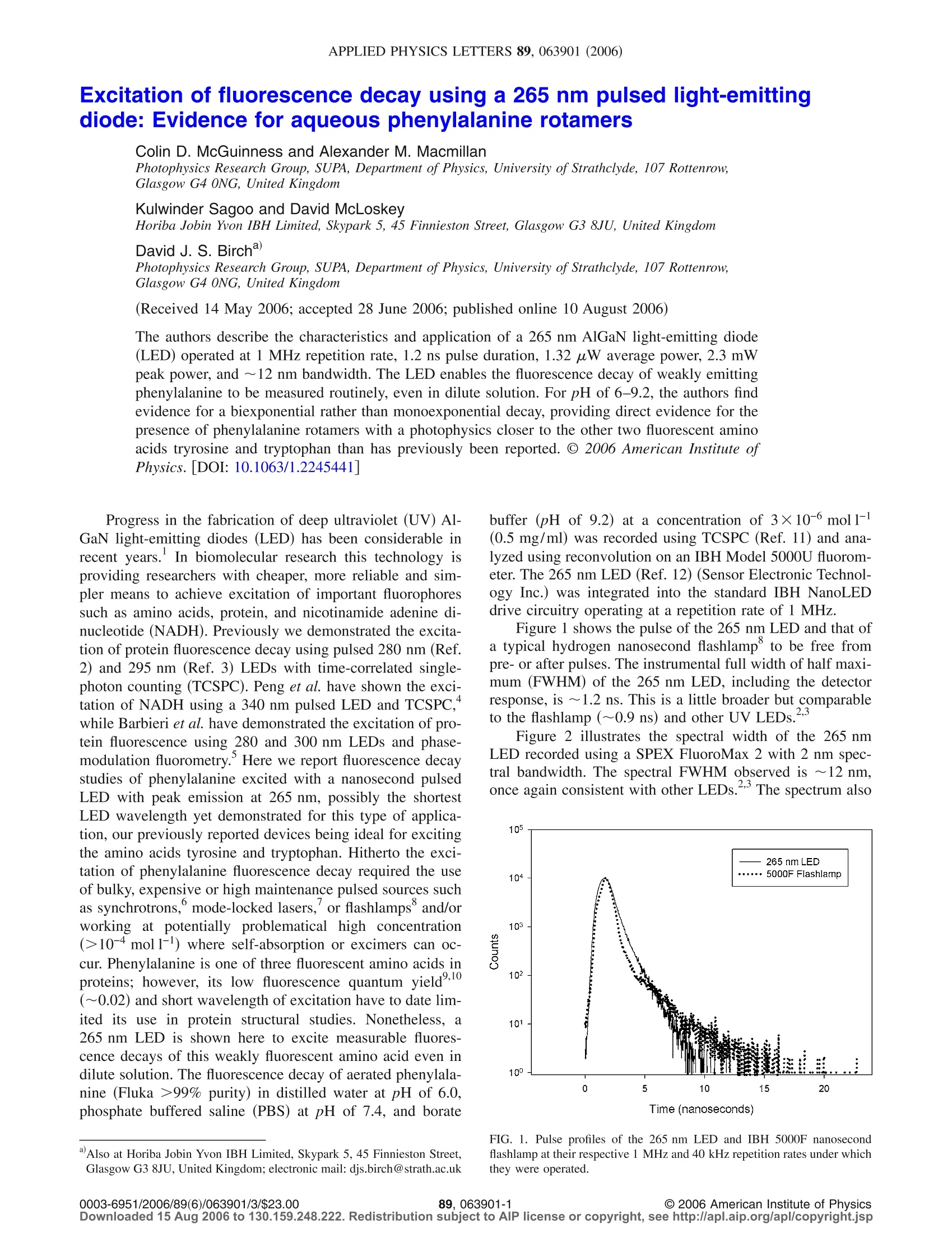
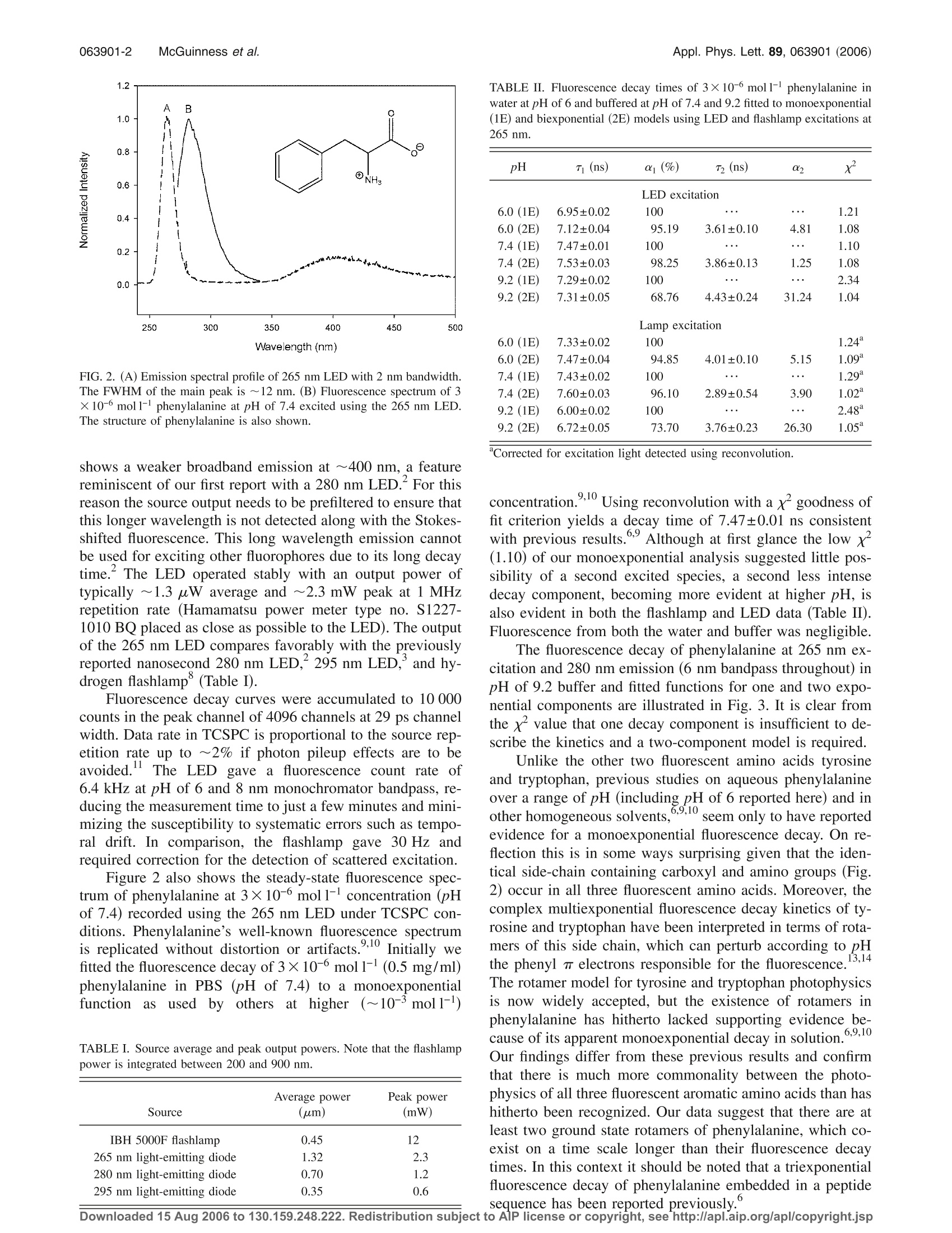
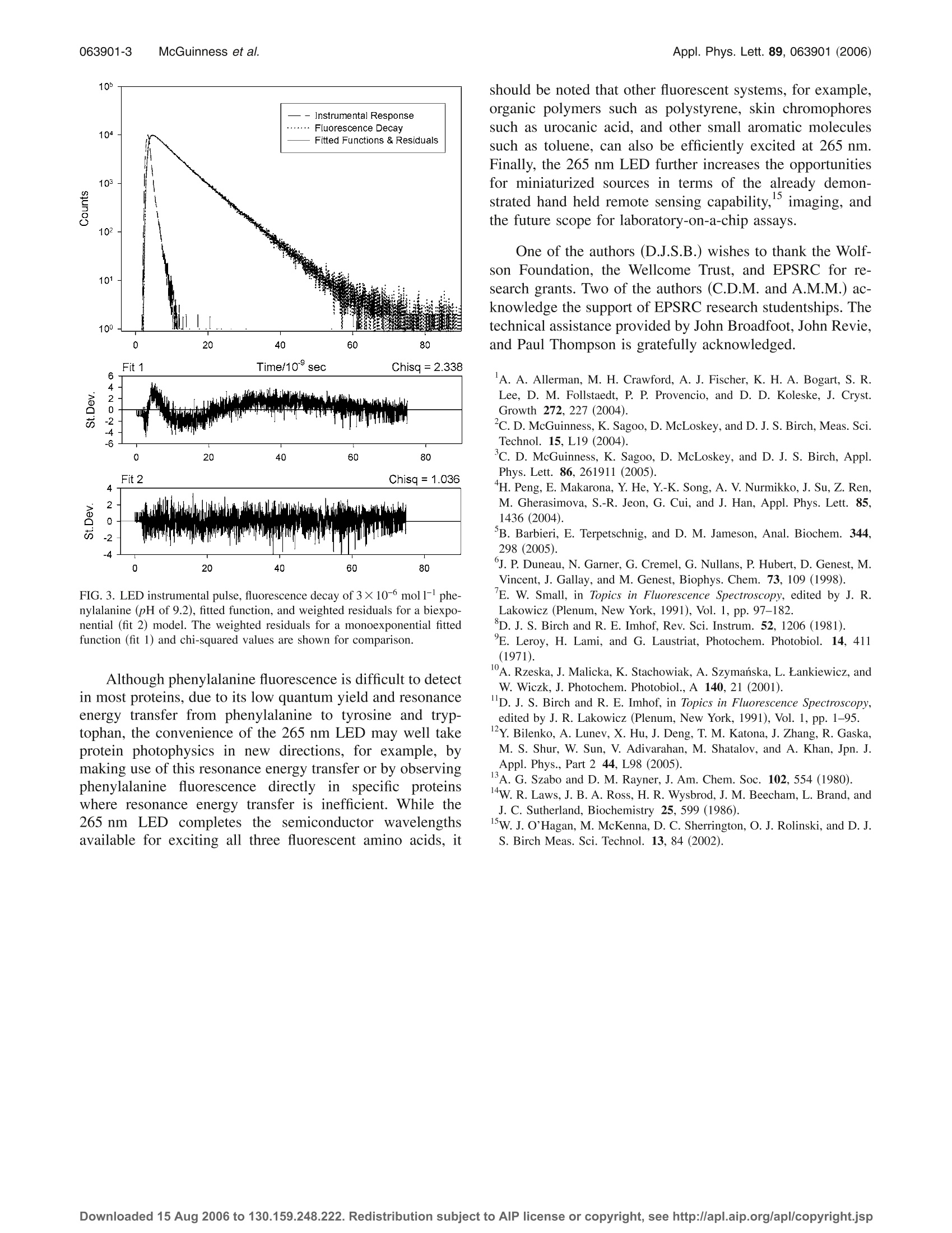
APPLIED PHYSICS LETTERS 89,063901(2006) 063901-2Appl. Phys. Lett. 89, 063901 (2006)McGuinness et al. Excitation of fluorescence decay using a 265 nm pulsed light-emittingdiode: Evidence for aqueous phenylalanine rotamers Colin D. McGuinness and Alexander M. Macmillan Photophysics Research Group, SUPA, Department of Physics, University of Strathclyde, 107 Rottenrow,Glasgow G4 0NG, United Kingdom Kulwinder Sagoo and David McLoskey Horiba Jobin Yvon IBH Limited, Skypark 5, 45 Finnieston Street, Glasgow G3 8JU, United KingdomDavid J.S. BirchdPhotophysics Research Group, SUPA, Department of Physics, University of Strathclyde, 107 Rottenrow,Glasgow G4 0NG, United Kingdom (Received 14 May 2006; accepted 28 June 2006; published online 10 August 2006) The authors describe the characteristics and application of a 265 nm AlGaN light-emitting diode(LED) operated at 1 MHz repetition rate, 1.2 ns pulse duration, 1.32 uW average power, 2.3 mWpeak power, and ~12 nm bandwidth. The LED enables the fluorescence decay of weakly emittingphenylalanine to be measured routinely, even in dilute solution. For pH of 6-9.2, the authors findevidence for a biexponential rather than monoexponential decay, providing direct evidence for thepresence of phenylalanine rotamers with a photophysics closer to the other two fluorescent aminoacids tryrosine and tryptophan than has previously been reported. o 2006 American Institute ofPhysics.[DOI: 10.1063/1.2245441] Progress in the fabrication of deep ultraviolet (UV) Al-GaN light-emitting diodes (LED) has been considerable inrecent years. In biomolecular research this technology isproviding researchers with cheaper, more reliable and sim-pler means to achieve excitation of important fluorophoressuch as amino acids, protein, and nicotinamide adenine di-nucleotide (NADH). Previously we demonstrated the excita-tion of protein fluorescence decay using pulsed 280 nm (Ref.2) and 295 nm (Ref. 3) LEDs with time-correlated single-photon counting (TCSPC). Peng et al. have shown the exci-tation of NADH using a 340 nm pulsed LED and TCSPC,while Barbieri et al. have demonstrated the excitation of pro-tein fluorescence using 280 and 300 nm LEDs and phase-modulation fluorometry. Here we report fluorescence decaystudies of phenylalanine excited with a nanosecond pulsedLED with peak emission at 265 nm, possibly the shortestLED wavelength yet demonstrated for this type ofapplica-tion, our previously reported devices being ideal for excitingthe amino acids tyrosine and tryptophan. Hitherto the exci-tation of phenylalanine fluorescence decay required the useof bulky, expensive or high maintenance pulsed sources suchas synchrotrons, mode-locked lasers, or flashlamps and/orworking at potentially problematical high concentration(>10-4mol1-1) where self-absorption or excimers can oc-cur. Phenylalanine is one of three fluorescent amino acids inproteins; however, its low fluoresc),10ence quantum yield(~0.02) and short wavelength of excitation have to date lim-ited its use in protein structural studies. Nonetheless, a265 nm LED is shown here to excite measurable fluores-cence decays of this weakly fluorescent amino acid even indilute solution. The fluorescence decay of aerated phenylala-nine (Fluka >99% purity) in distilled water at pH of 6.0,phosphate buffered saline (PBS) at pH of 7.4, and borate ( Also at H oriba Jobin Y von IBH Limited, Skypark 5 , 45 Finnieston S t reet,Glasgow G3 8JU, United Kingdom; electronic mail: djs.birch@strath.ac.uk ) buffer (pH of 9.2) at a concentration of 3×10-6 mol1-1(0.5 mg/ml) was recorded using TCSPC (Ref. 11) and ana-lyzed using reconvolution on an IBH Model 5000U fluorom-eter. The 265 nm LED (Ref. 12) (Sensor Electronic Technol-ogy Inc.) was integrated into the standard IBH NanoLEDdrive circuitry operating at a repetition rate of 1 MHz. Figure1 shows the pulse of the 265 nm LED and that ofa typical hydrogen nanosecond flashlamp° to be free frompre- or after pulses. The instrumental full width of half maxi-mum (FWHM) of the 265 nm LED, including the detectorresponse, is ~1.2 ns. This is a little broader but comparableto the flashlamp (~0.9 ns) and other UV LEDs. Figure 2 illustrates the spectral width of the 265 nmLED recorded using a SPEX FluoroMax 2 with 2 nm spec-tral bandwidth. The spectral FWHM observed is ~12 nm,once again consistent with other LEDs. The spectrum also FIG. 1. Pulse profiles of the 265 nm LED and IBH 5000F nanosecondflashlamp at their respective 1 MHz and 40 kHz repetition rates under whichthey were operated. Downloaded 15 Aug 2006 to 130.159.248.222. Redistribution subject to AlP license or copyright,see http://apl.aip.org/apl/copyright.jsp FIG. 2. (A) Emission spectral profile of 265 nm LED with 2 nm bandwidth.The FWHM of the main peak is ~12 nm. (B) Fluorescence spectrum of 3×10-6 moll- phenylalanine at pH of 7.4 excited using the 265 nm LED.The structure of phenylalanine is also shown. shows a weaker broadband emission at ~400 nm, a featurereminiscent of our first report with a 280 nm LED. For thisreason the source output needs to be prefiltered to ensure thatthis longer wavelength is not detected along with the Stokes-shifted fluorescence. This long wavelength emission cannotbe used for exciting other fluorophores due to its long decaytime. The LED operated stably with an output power oftypically ~1.3 uW average and ~2.3 mW peak at 1 MHzrepetition rate (Hamamatsu power meter type no. S1227-1010 BQ placed as close as possible to the LED). The outputof the 265 nm LED compares favorably with the previouslyreported nanosecond 280 nm LED, 295 nm LED,and hy-drogen flashlamp° (Table I). Fluorescence decay curves were accumulated to 10 000counts in the peak channel of 4096 channels at 29 ps channelwidth. Data rate in TCSPC is proportional to the source rep-etition rate up to ~2% if photon pileup effects are to beavoided. The LED gave a fluorescence count rate of6.4 kHz at pH of 6 and 8 nm monochromator bandpass, re-ducing the measurement time to just a few minutes and mini-mizing the susceptibility to systematic errors such as tempo-ral drift. In comparison, the flashlamp gave 30 Hz andrequired correction for the detection of scattered excitation. Figure 2 also shows the steady-state fluorescence spec-trum of phenylalanine at 3×10-mol1-concentration (pHof 7.4) recorded using the 265 nm LED under TCSPC con-ditions. Phenylalanine’s well-known fluorescence spectrum9,10is replicated without distortion or artifacts.;.Initially wefitted the fluorescence decay of 3×10-mol1-1 (0.5 mg/ml)phenylalanine in PBS (pH of 7.4) to a monoexponentialfunction asused by others at higher (~10-mol1-) TABLE I. Source average and peak output powers. Note that the flashlamppower is integrated between 200 and 900 nm. Average power Peak power Source (um) (mW) IBH 5000F flashlamp 0.45 12 265 nm light-emitting diode 1.32 280 nm light-emitting diode 0.70 295 nm light-emitting diode 0.35 TABLE ⅡI. Fluorescence decay times of 3×10-mol1- phenylalanine inwater at pH of 6 and buffered at pH of 7.4 and 9.2 fitted to monoexponential(1E) and biexponential (2E) models using LED and flashlamp excitations at265 nm. pH Ti(ns) α(%) T2 (ns) 02 x2 LED excitation 6.0(1E) 6.95±0.02 100 1.21 6.0 (2E) 7.12±0.04 95.19 3.61±0.10 4.81 1.08 7.4 (1E) 7.47±0.01 100 mm ... 1.10 7.4 (2E) 7.53±0.03 98.25 3.86±0.13 1.25 1.08 9.2 (1E) 7.29±0.02 100 ... 2.34 9.2(2E) 7.31±0.05 68.76 4.43±0.24 31.24 1.04 Lamp excitation 6.0(1E) 7.33±0.02 100 1.24 6.0 (2E) 7.47±0.04 94.85 4.01±0.10 5.15 1.09" 7.4 (1E) 7.43±0.02 100 1.29° 7.4 (2E) 7.60±0.03 96.10 2.89±0.54 3.90 1.02° 9.2(1E) 6.00±0.02 100 ·.. ... 2.48 9.2(2E) 6.72±0.05 73.70 3.76±0.23 26.30 1.05 Corrected for excitation light detected using reconvolution. concentration.Using reconvolution with a xgoodness offit criterion yields a decay time of 7.47±0.01 ns consistentwith previous results. Although at first glance the low x(1.10) of our monoexponential analysis suggested little pos-sibility of a second excited species, a second less intensedecay component, becoming more evident at higher pH, isalso evident in both the flashlamp and LED data (Table II).Fluorescence from both the water and buffer was negligible. The fluorescence decay of phenylalanine at 265 nm ex-citation and 280 nm emission (6 nm bandpass throughout) inpH of 9.2 buffer and fitted functions for one and two expo-nential components are illustrated in Fig. 3. It is clear fromthe xvalue that one decay component is insufficient to de-scribe the kinetics and a two-component model is required. Unlike the other two fluorescent amino acids tyrosineand tryptophan, previous studies on aqueous phenylalanineover a range of pH (including pH of 6 reported here) and in6,9,10other homogeneous solvents,seem only to have reportedevidence for a monoexponential fluorescence decay. On re-flection this is in some ways surprising given that the iden-tical side-chain containing carboxyl and amino groups (Fig.2) occur in all three fluorescent amino acids. Moreover, thecomplex multiexponential fluorescence decay kinetics of ty-rosine and tryptophan have been interpreted in terms of rota-mers of this side chain, which can perturb according to pHthe phenyl electrons responsible for the fluorescence.13,14The rotamer model for tyrosine and tryptophan photophysicsis now widely accepted, but the existence of rotamers inphenylalanine has hitherto lacked supporting evidence be-cause of its apparent monoexponential decay in solution.6,9,10Our findings differ from these previous results and confirmthat there is much more commonality between the photo-physics of all three fluorescent aromatic amino acids than hashitherto been recognized. Our data suggest that there are atleast two ground state rotamers of phenylalanine, which co-exist on a time scale longer than their fluorescence decaytimes. In this context it should be noted that a triexponentialfluorescence decay of phenylalanine embedded in a peptide FIG. 3. LED instrumental pulse, fluorescence decay of 3×10-6mol1-l phe-nylalanine (pH of 9.2), fitted function, and weighted residuals for a biexpo-nential (fit 2) model. The weighted residuals for a monoexponential fittedfunction (fit 1) and chi-squared values are shown for comparison. Although phenylalanine fluorescence is difficult to detectin most proteins, due to its low quantum yield and resonanceenergy transfer from phenylalanine to tyrosine and tryp-tophan, the convenience of the 265 nm LED may well takeprotein photophysics in new directions, for example, bymaking use of this resonance energy transfer or by observingphenylalanine fluorescence directly in specific proteinswhere resonance energy transfer is inefficient. While the265 nm LED completesSthe semiconductor wavelengthsavailable for exciting all three fluorescent amino acids, it should be noted that other fluorescent systems, for example,organic polymers such as polystyrene, skin chromophoressuch as urocanic acid, and other small aromatic moleculessuch as toluene, can also be efficiently excited at 265 nm.Finally, the 265 nm LED further increases the opportunitiesfor miniaturized sources in terms of the already demon-strated hand held remote sensing capability,imaging, andthe future scope for laboratory-on-a-chip assays. One of the authors (D.J.S.B.) wishes to thank the Wolf-son Foundation, the Wellcome Trust, and EPSRC for re-search grants. Two of the authors (C.D.M. and A.M.M.) ac-knowledge the support of EPSRC research studentships. Thetechnical assistance provided by John Broadfoot, John Revie,and Paul Thompson is gratefully acknowledged. A. A. Allerman, M. H. Crawford, A. J. Fischer, K. H. A. Bogart, S. R.Lee, D. M. Follstaedt, P. P. Provencio, and D. D. Koleske, J. Cryst.Growth 272, 227 (2004). C. D. McGuinness, K. Sagoo, D. McLoskey, and D. J. S. Birch,Meas. Sci.Technol. 15,L19 (2004). C. D. McGuinness, K. Sagoo, D. McLoskey, and D. J. S. Birch, Appl.Phys. Lett. 86, 261911 (2005). "H. Peng, E. Makarona, Y. He, Y.-K. Song, A. V. Nurmikko, J. Su, Z. Ren,M. Gherasimova, S.-R. Jeon, G. Cui, and J. Han, Appl. Phys. Lett. 85,1436 (2004). B. Barbieri, E. Terpetschnig, and D. M. Jameson, Anal. Biochem. 344,298(2005). J. P. Duneau, N. Garner, G. Cremel, G. Nullans, P. Hubert, D. Genest, M.Vincent, J. Gallay, and M. Genest, Biophys. Chem. 73, 109 (1998). E. W. Small, in Topics in Fluorescence Spectroscopy, edited by J. R.Lakowicz (Plenum, New York, 1991), Vol. 1, pp. 97-182. °D. J.S. Birch and R. E. Imhof, Rev. Sci. Instrum. 52, 1206 (1981). E. Leroy, H. Lami, and G. Laustriat, Photochem. Photobiol. 14, 411(1971). A.Rzeska, J. Malicka, K. Stachowiak, A. Szymanska, L. Lankiewicz, andW. Wiczk, J. Photochem. Photobiol., A 140,21(2001). D. J. S. Birch and R. E. Imhof, in Topics in Fluorescence Spectroscopy,edited by J. R. Lakowicz (Plenum, New York, 1991), Vol. 1, pp. 1-95. Y. Bilenko, A. Lunev, X. Hu, J. Deng, T.M. Katona, J. Zhang, R. Gaska, M. S. Shur, W. Sun, V. Adivarahan, M. Shatalov, and A. Khan, Jpn. J.Appl. Phys., Part 2 44,L98 (2005). A. G. Szabo and D. M. Rayner, J. Am. Chem. Soc. 102, 554 (1980). “W.R. Laws, J. B. A. Ross, H. R. Wysbrod, J. M. Beecham, L. Brand, andJ. C. Sutherland, Biochemistry 25,599 (1986). W. J. O'Hagan, M. McKenna, D. C. Sherrington, O. J. Rolinski, and D. J.S. Birch Meas. Sci. Technol. 13, 84 (2002). /$ American Institute of Physics Downloaded Aug to Redistribution subject to AlP license or copyright, see http://apl.aip.org/apl/copyright.jsp The authors describe the characteristics and application of a 265 nm AlGaN light-emitting diode LED operated at 1 MHz repetition rate, 1.2 ns pulse duration, 1.32 μW average power, 2.3 mW peak power, and ϳ12 nm bandwidth. The LED enables the fluorescence decay of weakly emitting phenylalanine to be measured routinely, even in dilute solution. For pH of 6–9.2, the authors find evidence for a biexponential rather than monoexponential decay, providing direct evidence for the presence of phenylalanine rotamers with a photophysics closer to the other two fluorescent amino acids tryrosine and tryptophan than has previously been reported. © 2006 American Institute of Physics. ͓DOI: 10.1063/1.2245441͔
确定
还剩1页未读,是否继续阅读?
HORIBA(中国)为您提供《苯基丙氨基酸中荧光衰减检测方案(分子荧光光谱)》,该方案主要用于其他中荧光衰减检测,参考标准--,《苯基丙氨基酸中荧光衰减检测方案(分子荧光光谱)》用到的仪器有HORIBA DeltaPro超快时间分辨荧光光谱仪
相关方案
更多
该厂商其他方案
更多










