
方案详情
文
采用德国耶拿公司multi N/C系列TOC,TNb 分析仪,可以实现快速、可靠检测制药产品中的蛋白质。
方案详情

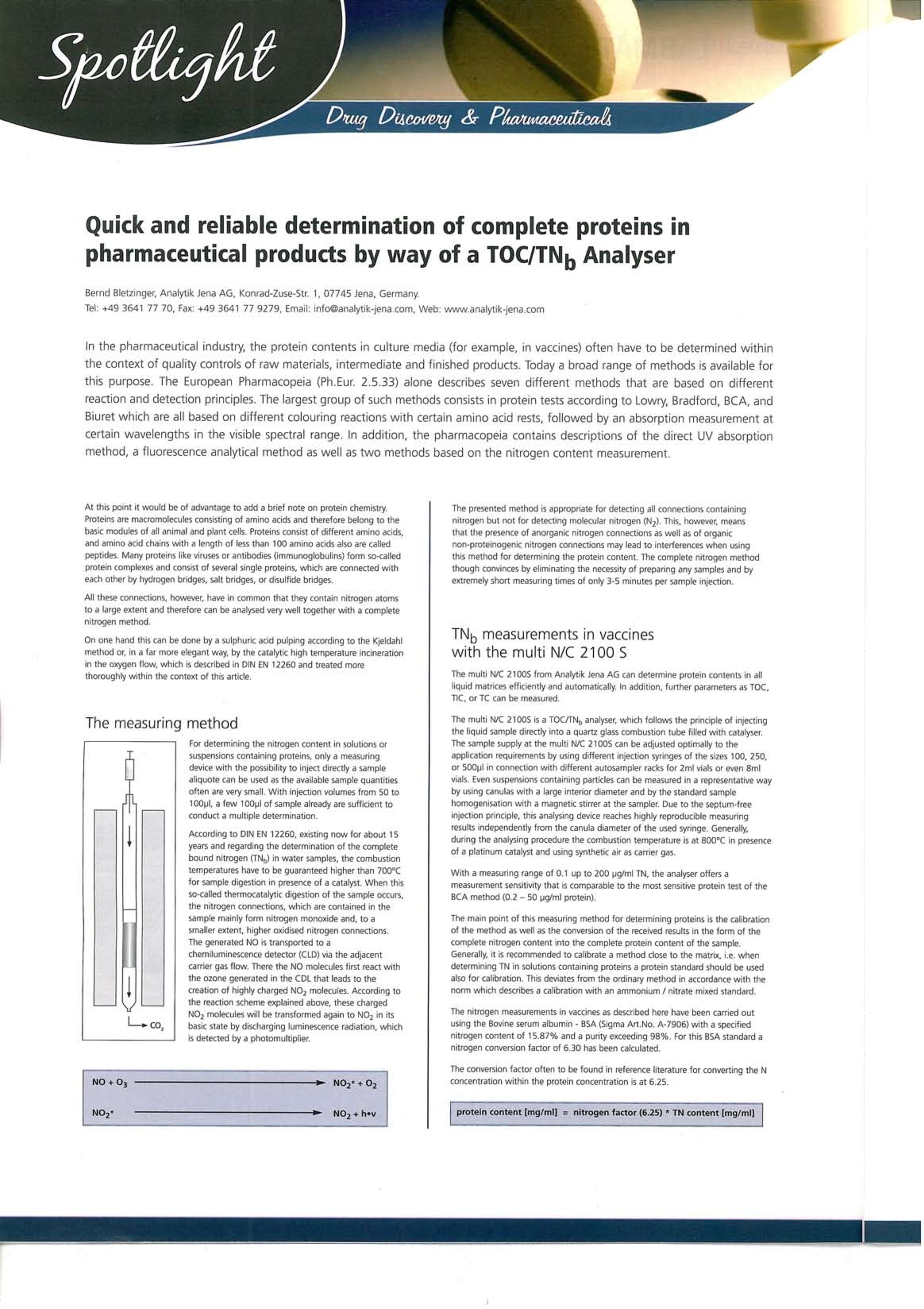
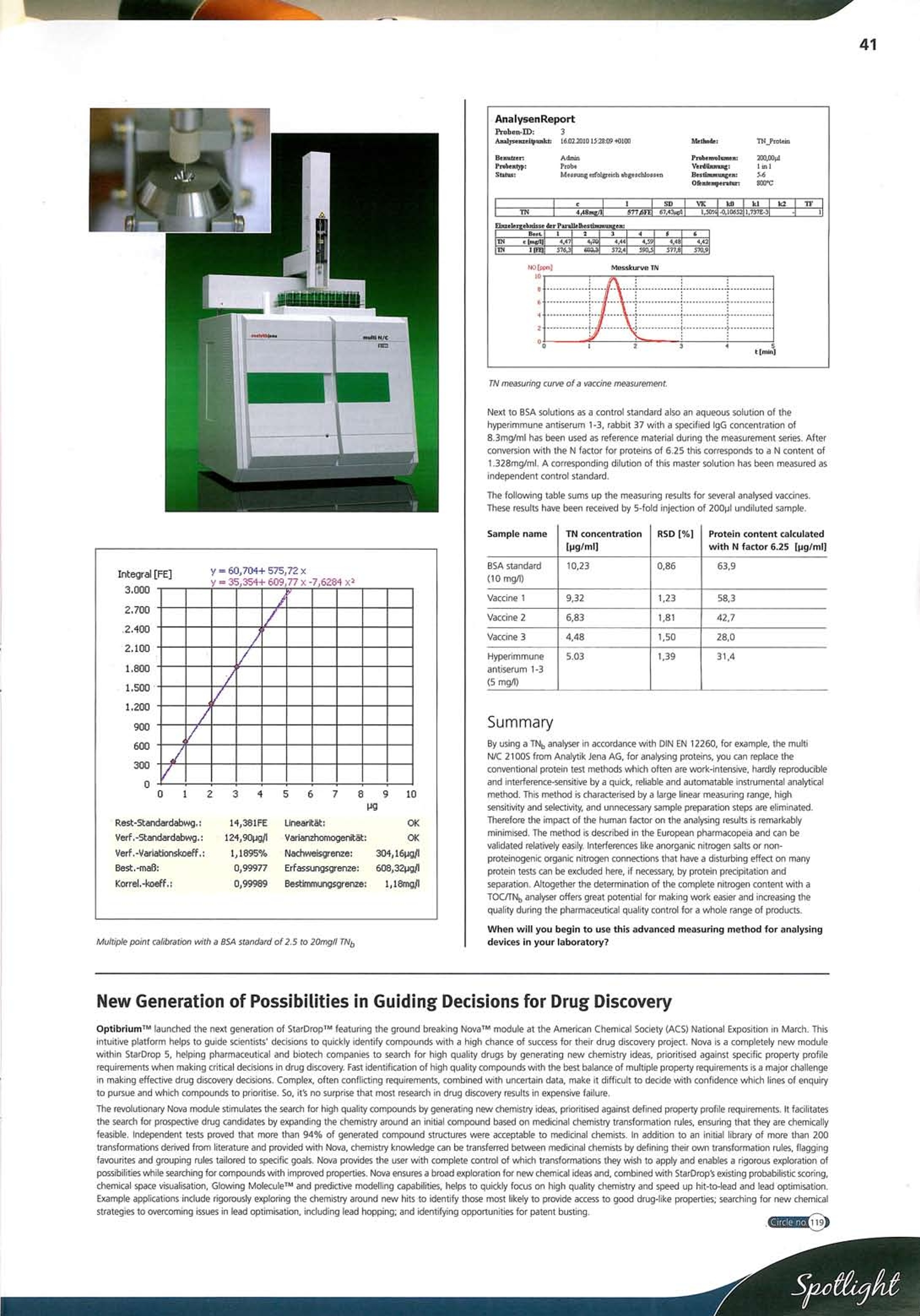
41 Application field /Industrybranch: Chemistry / Polymer Industry Clinical Chemistry /Medicine/Sanitation/ Health Care Electronics Semiconductor Technology Energy Environment/Water/Waste ■Geology / Mining Nutrition/ Agriculture Metallurgy/Electroplating Refineries / Petrochemistry ■Pharmacy ■Cosmetics Material Analysis Other Quick and reliable determination of complete proteins inpharmaceutical products by way of a TOC/TN Analyser Bernd Bletzinger, Analytik Jena AG, Konrad-Zuse-Str. 1, 07745 Jena, Germany. Tel: +49 3641 77 70, Fax: +49 3641 77 9279,Email: info@analytik-jena.com, Web: www.analytik-jena.com In the pharmaceutical industry, the protein contents in culture media (for example, in vaccines) often have to be determined withinthe context of quality controls of raw materials, intermediate and finished products. Today a broad range of methods is available forthis purpose. The European Pharmacopeia (Ph.Eur. 2.5.33) alone describes seven different methods that are based on differentreaction and detection principles. The largest group of such methods consists in protein tests according to Lowry, Bradford, BCA, andBiuret which are all based on different colouring reactions with certain amino acid rests, followed by an absorption measurement atcertain wavelengths in the visible spectral range. In addition, the pharmacopeia contains descriptions of the direct UV absorptionmethod, a fluorescence analytical method as well as two methods based on the nitrogen content measurement. At this point it would be of advantage to add a brief note on protein chemistry.Proteins are macromolecules consisting of amino acids and therefore belong to thebasic modules of all animal and plant cells. Proteins consist of different amino acids,and amino acid chains with a length of less than 100 amino acids also are calledpeptides. Many proteins like viruses or antibodies (immunoglobulins) form so-calledprotein complexes and consist of several single proteins, which are connected witheach other by hydrogen bridges, salt bridges, or disulfide bridges. All these connections, however, have in common that they contain nitrogen atomsto a large extent and therefore can be analysed very well together with a completenitrogen method. On one hand this can be done by a sulphuric acid pulping according to the Kjeldahlmethod or, in a far more elegant way, by the catalytic high temperature incinerationin the oxygen flow, which is described in DIN EN 12260 and treated morethoroughly within the context of this article. The measuring method For determining the nitrogen content in solutions orsuspensions containing proteins, only a measuringdevice with the possibility to inject directly a samplealiquote can be used as the available sample quantitiesoften are very small. With injection volumes from 50 to100pl, a few 100pl of sample already are sufficient toconduct a multiple determination. According to DIN EN 12260, existing now for about 15years and regarding the determination of the completebound nitrogen (TN) in water samples, the combustiontemperatures have to be guaranteed higher than 700℃for sample digestion in presence of a catalyst. When thisso-called thermocatalytic digestion of the sample occurs,the nitrogen connections, which are contained in thesample mainly form nitrogen monoxide and, to asmaller extent, higher oxidised nitrogen connections.The generated NO is transported to achemiluminescence detector (CLD) via the adjacentcarrier gas flow. There the NO molecules first react withthe ozone generated in the CDL that leads to thecreation of highly charged NOz molecules. According tothe reaction scheme explained above, these chargedNO2 molecules will be transformed again to NOz in itsbasic state by discharging luminescence radiation, which -DCO is detected by a photomultiplier. The presented method is appropriate for detecting all connections containingnitrogen but not for detecting molecular nitrogen (N2). This, however, meansthat the presence of anorganic nitrogen connections as well as of organicnon-proteinogenic nitrogen connections may lead to interferences when usingthis method for determining the protein content. The complete nitrogen methodthough convinces by eliminating the necessity of preparing any samples and by extremely short measuring times of only 3-5 minutes per sample injection. TNo measurements in vaccineswith the multi N/C 2100 S The multi N/C 2100S from Analytik Jena AG can determine protein contents in allliquid matrices efficiently and automatically. In addition, further parameters as TOC,TIC, or TC can be measured. The multi N/C 21005 is a TOC/TNg analyser, which follows the principle of injectingthe liquid sample directly into a quartz glass combustion tube filled with catalyser.The sample supply at the multi N/C 2100S can be adjusted optimally to theapplication requirements by using different injection syringes of the sizes 100, 250,or 500pl in connection with different autosampler racks for 2ml vials or even 8mlvials. Even suspensions containing particles can be measured in a representative wayby using canulas with a large interior diameter and by the standard samplehomogenisation with a magnetic stirrer at the sampler. Due to the septum-freeinjection principle, this analysing device reaches highly reproducible measuringresults independently from the canula diameter of the used syringe. Generally,during the analysing procedure the combustion temperature is at 800℃ in presenceof a platinum catalyst and using synthetic air as carrier gas. With a measuring range of 0.1 up to 200 pg/ml TN, the analyser offers ameasurement sensitivity that is comparable to the most sensitive protein test of theBCA method (0.2-50 pg/ml protein). The main point of this measuring method for determining proteins is the calibrationof the method as well as the conversion of the received results in the form of thecomplete nitrogen content into the complete protein content of the sample.Generally, it is recommended to calibrate a method close to the matrix, i.e. whendetermining TN in solutions containing proteins a protein standard should be usedalso for calibration. This deviates from the ordinary method in accordance with thenorm which describes a calibration with an ammonium / nitrate mixed standard. The nitrogen measurements in vaccines as described here have been carried outusing the Bovine serum albumin-BSA (Sigma Art.No.A-7906) with a specifiednitrogen content of 15.87% and a purity exceeding 98%. For this BSA standard anitrogen conversion factor of 6.30 has been calculated. The conversion factor often to be found in reference literature for converting the Nconcentration within the protein concentration is at 6.25. protein content [mg/ml]= nitrogen factor(6.25)* TN content [mg/ml] Multiple point calibration with a BSA standard of 2.5 to 20mg// TN, TN measuring curve of a vaccine measurement. Next to BSA solutions as a control standard also an aqueous solution of the hyperimmune antiserum 1-3, rabbit 37 with a specified IgG concentration of8.3mg/ml has been used as reference material during the measurement series. Afterconversion with the N factor for proteins of 6.25 this corresponds to a N content of1.328mg/ml. A corresponding dilution of this master solution has been measured asindependent control standard. The following table sums up the measuring results for several analysed vaccines.These results have been received by 5-fold injection of 200pl undiluted sample. Sample name TN concentration [pg/ml] RSD [%] Protein content calculatedwith N factor 6.25 pg/ml] BSA standard(10 mg/l) 10,23 0.86 63,9 Vaccine 1 9,32 1,23 58,3 Vaccine 2 6,83 1.81 42.7 Vaccine 3 4,48 1.50 28,0 Hyperimmuneantiserum 1-3(5 mg/l) 5.03 1,39 31,4 Summary By using a TNg analyser in accordance with DIN EN 12260, for example, the multiN/C 2100S from Analytik Jena AG, for analysing proteins, you can replace theconventional protein test methods which often are work-intensive, hardly reproducibleand interference-sensitive by a quick, reliable and automatable instrumental analyticalmethod. This method is characterised by a large linear measuring range, highsensitivity and selectivity, and unnecessary sample preparation steps are eliminated.Therefore the impact of the human factor on the analysing results is remarkablyminimised. The method is described in the European pharmacopeia and can bevalidated relatively easily. Interferences like anorganic nitrogen salts or non-proteinogenic organic nitrogen connections that have a disturbing effect on manyprotein tests can be excluded here, if necessary, by protein precipitation andseparation. Altogether the determination of the complete nitrogen content with aTOC/TN, analyser offers great potential for making work easier and increasing thequality during the pharmaceutical quality control for a whole range of products. When will you begin to use this advanced measuring method for analysingdevices in your laboratory? New Generation of Possibilities in Guiding Decisions for Drug Discovery OptibriumTM launched the next generation of StarDropTM featuring the ground breaking NovaTM module at the American Chemical Society (ACS) National Exposition in March. Thisintuitive platform helps to guide scientists’ decisions to quickly identify compounds with a high chance of success for their drug discovery project. Nova is a completely new modulewithin StarDrop 5, helping pharmaceutical and biotech companies to search for high quality drugs by generating new chemistry ideas, prioritised against specific property profilerequirements when making critical decisions in drug discovery. Fast identification of high quality compounds with the best balance of multiple property requirements is a major challengein making effective drug discovery decisions. Complex, often conflicting requirements, combined with uncertain data, make it difficult to decide with confidence which lines of enquiryto pursue and which compounds to prioritise. So, it’s no surprise that most research in drug discovery results in expensive failure. The revolutionary Nova module stimulates the search for high quality compounds by generating new chemistry ideas, prioritised against defined property profile requirements. It facilitatesthe search for prospective drug candidates by expanding the chemistry around an initial compound based on medicinal chemistry transformation rules, ensuring that they are chemicallyfeasible. Independent tests proved that more than 94% of generated compound structures were acceptable to medicinal chemists. In addition to an initial library of more than 200transformations derived from literature and provided with Nova,chemistry knowledge can be transferred between medicinal chemists by defining their own transformation rules, flaggingfavourites and grouping rules tailored to specific goals. Nova provides the user with complete control of which transformations they wish to apply and enables a rigorous exploration ofpossibilities while searching for compounds with improved properties. Nova ensures a broad exploration for new chemical ideas and, combined with StarDrops existing probabilistic scoring,chemical space visualisation, Glowing MoleculeTM and predictive modelling capabilities, helps to quickly focus on high quality chemistry and speed up hit-to-lead and lead optimisation.Example applications include rigorously exploring the chemistry around new hits to identify those most likely to provide access to good drug-like properties; searching for new chemicalstrategies to overcoming issues in lead optimisation, including lead hopping; and identifying opportunities for patent busting. Quick and reliable determination of complete proteins in pharmaceutical products by way of a TOC/TNb analyzerLit _SP TOC d|Ble Circle no
确定

还剩1页未读,是否继续阅读?
耶拿分析仪器(北京)有限公司为您提供《化学药中特殊物质和基团检测方案 》,该方案主要用于化药制剂中含量测定检测,参考标准--,《化学药中特殊物质和基团检测方案 》用到的仪器有德国耶拿 multi N/C 3100 TOC总有机碳/总氮分析仪、multi N/CPharma HT制药专用干法总有机碳/总氮分析仪
相关方案
更多
该厂商其他方案
更多










