方案详情
文
锂电池的鼻祖J.-M. Tarascon的杰作——《Building better batteries》
方案详情

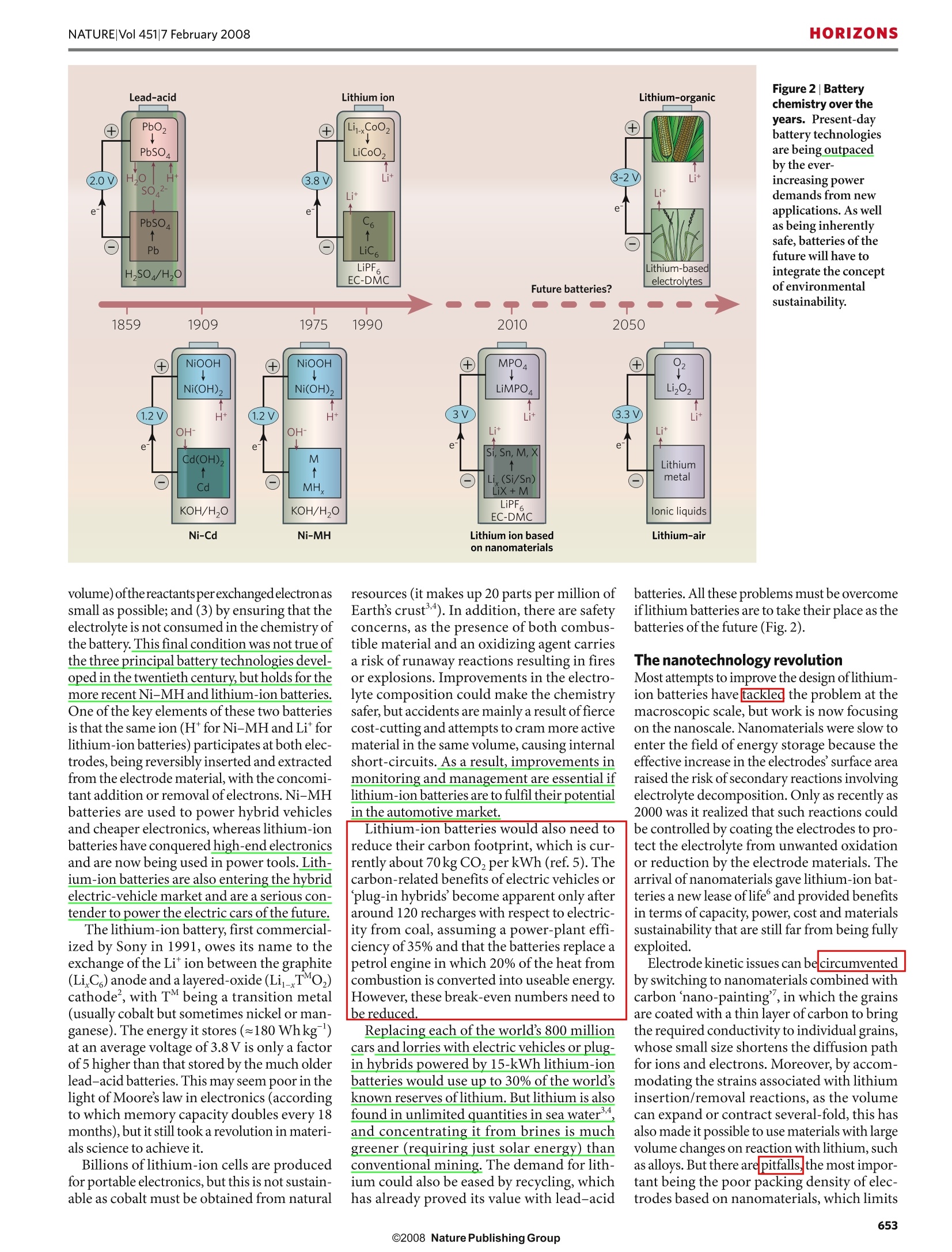
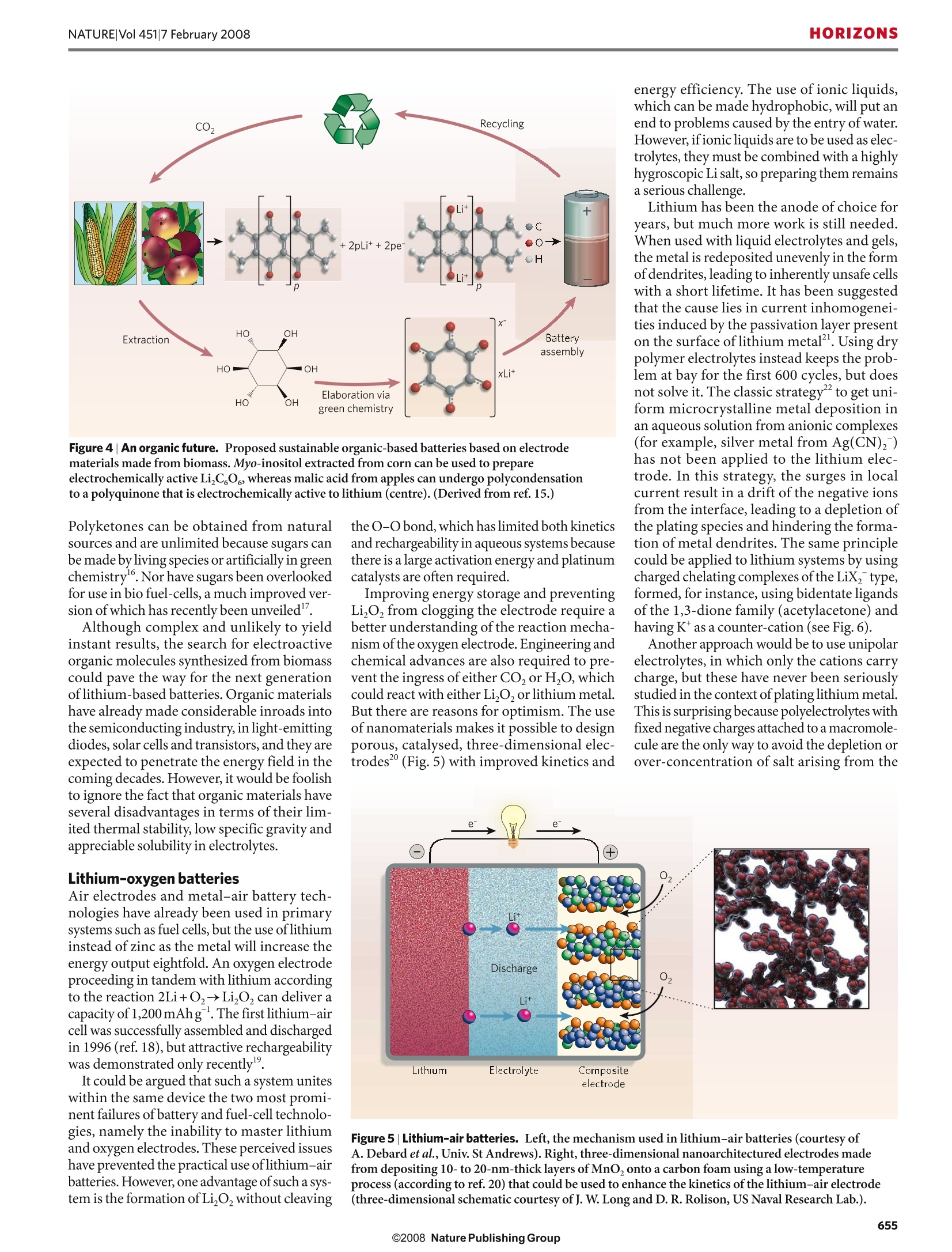
natureVol 451|7 February 2008 HORIZONSNATURE|Vol 451|7 February 2008 Building better batteries M. Armand andJ.-M. Tarascon Researchers must find a sustainable way of providing the power our modern lifestyles demand. Batteries are currentlybeing developed to poweran increasingly diverserange of applications, fromcars to microchips. Howcan scientists achieve theperformance that eachapplication demands?How will batteries be able to power the many other portable devices thatwill no doubt be developed in the comingyears? And how can batteries become a sus-tainable technology for the future? The technological revolution ofthe past fewcenturies has been fuelled mainly by varia-tions of the combustion reaction, the fire thatmarked the dawn of humanity. But this hascome at a price the resulting emissions of car-bon dioxide have driven global climate change.For the sake of future generations, we urgentlyneed to reconsider how we use energy in every-thing from barbecues to jet aeroplanes andpower stations. If a new energy economy is to emerge, itmust be based on a cheap and sustainableenergy supply. One of the most flagrantlywasteful activities is travel, and here batterydevices can potentially provide a solution,especially as they can be used to store energyfrom sustainable sources suchas the wind andsolar power. Because batteries are inherently simple inconcept, it is surprising that their developmenthas progressed much more slowly than otherareas ofelectronics. As a result, they are oftenseen as being the heaviest, costliest and least-green components of any electronic device. Itwas thelack of good batteries that slowed downthe deployment of electric cars and wirelesscommunication, which date from at least 1899and1920,respectively (Fig. 1). The slow prog-ress is due to the lack of suitable electrode mat-erials and electrolytes, together with difficultiesin mastering the interfaces between them. All batteries are composed of two electrodesconnected by an ionically conductive materialcalled an electrolyte. The two electrodes havedifferent chemical potentials, dictatedby thechemistry that occurs at each. When these elec-trodes are connected by means of an externaldevice,electrons spontaneously flow from themore negative to the more positive potential.Ions are transported through the electrolyte, Figure 1|Revisiting the past. In 1899 a Belgian car, La jamais contente (top left), equipped with lead-acid batteries, reached a speed of 30 metres per second (ref. 26). In the same year, at a car competitionin Paris, the only petrol-driven car was disqualified for having unpractically high consumption. Insidethe United States, between 1900 and 1920, the proportion of electrical cars produced fell from 60%to 4% of the total. One century later, fully electrical cars,such as the Tesla roadster (bottom left), arecoming back into the picture. Meanwhile,the first wireless communication took place in Pennsylvaniain 1920 (top right, after ref. 27). Nearly 100 years later, the latest mobile phones (bottom right) canperform a wide range of functions. neering but crucially on the chemicals the bat-tery contains. Hundreds of electrochemicalcouples were proposed during the nineteenthand early twentieth centuries, the most nota-ble primary battery being Zn-MnO,withlead-acid and Ni-Cd being the most commonsecondaries. The stored energy content of a battery canbe maximized in three ways:(1) by havinga large chemical potential difference betweenthetwo electrodes; (2) by making the mass (or Billions of lithium-ion cells are producedfor portable electronics,but this is not sustain-able as cobalt must be obtained from natural resources (it makes up 20 parts per million ofEarth’s crust’4). In addition, there are safetyconcerns, as the presence of both combus-tible material and an oxidizing agent carriesa risk of runaway reactions resulting in firesor explosions. Improvements in the electro-lyte composition could make the chemistrysafer, but accidents are mainly a result of fiercecost-cutting and attempts to cram more activematerial in the same volume, causing internalshort-circuits.As a result, improvements inmonitoring and management are essential iflithium-ion batteries are to fulfil their potentialin the automotive market. volume) ofthereactantsperexchangedelectronassmall as possible; and (3) by ensuring that theelectrolyte is not consumed in the chemistry ofthe battery. This final condition was not true ofthe three principal battery technologies devel-oped in the twentieth century,but holds for themore recent Ni-MH and lithium-ion batteries.One of the key elements of these two batteriesis that the same ion (H*forNi-MH and Liforlithium-ion batteries) participates at both elec-trodes, being reversibly inserted and extractedfrom the electrode material, with the concomi-1entant addition or removal of electrons. Ni-MHbatteries are used to power hybrid vehiclesand cheaper electronics, whereas lithium-ion Lithium-ion batteries would also need tobatteries have conquered high-end electronics reduce their carbon footprint, which is cur-and are now being used in power tools. Lith-rently about 70kg COzperkWh (ref.5). Theium-ion batteries are also entering the hybrid carbon-related benefits of electric vehicles orelectric-vehicle market and are a serious con- plug-in hybrids become apparent only aftertender to power the electric cars of the future. around 120 recharges with respect to electric-The lithium-ion battery, first commercial- ity from coal, assuming a power-plant effi-ized by Sony in 1991,owes its name to the ciency of 35% and that the batteries replace aexchange of the Li ion between the graphite petrol engine in which 20% of the heat fromElectrode kinetic issues can be circumvented(LiC,) anode and a layered-oxide (Li_TMO,) combustion is converted into useable energy.by switching to nanomaterials combined withcathode’, with TM being a transition metal However, these break-even numbers need tocarbon nano-painting’,in which the grains(usually cobalt but sometimes nickel or man- be reduced.are coated with a thin layer of carbon to bringganese). The energy it stores (~180 Whkg) Replacing each of the world's 800 millionthe required conductivity to individual grains,at an average voltage of 3.8 V is only a factor cars and lorries with electric vehicles or plug-whose small size shortens the diffusion pathof 5 higher than that stored by the much older in hybrids powered by 15-kWh lithium-ionfor ions and electrons. Moreover, by accom-lead-acid batteries. This may seem poor in the batteries would use up to 30% of the world's modating the strains associated with lithiumlight of Moore's law in electronics (according known reserves of lithium. But lithium is also insertion/removal reactions, as the volumeto which memory capacity doubles every 18 found in unlimited quantities in sea water can expand or contract several-fold, this hasmonths), but it still took a revolution in materi- and concentrating it from brines is much also made it possible to use materials with largeals science to achieve it. greener (requiring just solar energy) than volume changes on reaction with lithium, suchconventional mining. The demand for lith- as alloys. But there are pitfalls, the most impor-ium could also be eased by recycling, which tant being the poor packing density of elec-has already proved its value with lead-acid trodes based on nanomaterials, which limits batteries.All these problems must be overcomeif lithium batteries are to take their place as thebatteries of the future (Fig.2). The nanotechnology revolution Most attempts to improve the design oflithium-ion batteries have tackled the problem at themacroscopic scale, but work is now focusingon the nanoscale. Nanomaterials were slow toenter the field of energy storage because theeffective increase in the electrodes' surface arearaised the risk of secondary reactions involvingelectrolyte decomposition. Only as recently as2000 was it realized that such reactions couldbe controlled by coating the electrodes to pro-tect the electrolyte from unwanted oxidationor reduction by the electrode materials. Thearrival of nanomaterials gave lithium-ion bat-teries a new lease of life° and provided benefitsin terms of capacity, power, cost and materialssustainability that are still far from being fullyexploited. Figure 3|Reaction mechanisms. Schematic representation showing the contrasting reactionmechanisms occurring during discharge for insertion (top) and conversion reactions (bottom).The insertion reaction demonstrates a maximum of 1 electron transfer per transition metal (heredesignated M), whereas the conversion reaction can transfer 2 to 6 electrons (derived from ref. 28). the energy that can be stored per unit volumeor mass because there is a larger proportion ofinert'components such as current collectorsor electrolyte. Another advantage of nanomaterials is thatthey can change the reaction pathway, afford-ing high capacities, rechargeability and gener-ality to a range of battery systems. One suchreaction pathway is referred to as a conversion’from transition-metal oxides: the final product consists of a homogeneousdistribution of metal nanoparticles ([TM],where the superscript 0 indicates the metal-lic form) embedded in a Li,O matrix (Fig.3).The drawback with this mechanism,however,is that the large voltage difference betweencharge and discharge results in poor energyefficiency. This problem is being addressedby studies of both the material chemistry andmorphology and the electrode configuration.The hunt is also under way for materials thatcan undergo conversion reactions involvingmultiple electrons at high potential, for use ascathode materials. However, the full impactof nanomaterials on living cells has yet to beappraised, although the risk is minimal whenthey are produced in situ, as they are for theconversion reaction. Solid electrolytes were quicker to benefitfrom the use of nanomaterials°.The additionofnano-fillers’ (nano-grains dispersed ina polymer, such as Al,O or TiO,) to simple polyether-based electrolytes increases the con-ductivity several-fold at 60-80°℃, but there isno advantage at room temperature. Organ-izing the polymer strands in such a way as toincrease the order locally (using crystallinewhorls, stretching or even chirality) can alsoprovide benefits, by increasing conductivity atlow temperatures,and further work is neededto assess the merits ofusing block co-polymers(AB or ABA)1. The phase separation inherentto these systems results in good mechanicalproperties, but also offers a way ofincreasingdissociation by partitioning anions and cationsin the two sub-phases. Giving the two phasesdifferent wetting or adhesion properties canhelp by avoiding grain growth as the polymer’snano-domains will determine the partitioningof space. True polymer batteries may still be someway off, but in the meantime we will see moreattempts to use ionic liquids as either solventsfor lithium salts or plasticizers for polyether-based electrolytes. Ionic liquids have exceed-ingly low vapour pressures, are non-flammableand have high conductivities, making themserious contenders for safer batteries. But itremains to be seen whether they can be pro-duced cheaply enough, at the desired purity, withsufficient conductivity at low temperature. Beyond nanomaterials The components of today's lithium-ionbatteries, such as LiCoO,and LiMn,O4,are notproduced from renewable energy resourcesbut from ores, and extracting the raw materials and manufacturing the electrodes will requireincreasing amounts of energy as they becomescarcer. Will the lithium-ion battery, which isso energetically expensive to fabricate, remainattractive and viable in the long term? In 50years, if all cars become electric and rely onthese scarce materials, might we face stag-gering price increases like those recentlyseen with fossil fuels? Not if we find a wayof making lithium-ion batteries sustainablewhile maintaining or exceeding the perform-ance of today's batteries. One option is touse renewable electrodes made from naturalresources, just as fuel cells can use hydrogenor(m)ethanol made from biomass. But whatwould these electrodes be like? Inspired by nature When scientists need new approaches, theyoften turn to the chemistry of life, with itsvirtually unlimited and incredible reactionmechanisms. The battery's insertion reac-tion may have no real equivalent in the livingworld, but the materials themselves could befabricated in living cells. Phosphate species aremanipulated to make DNA and ATP, so it isnot so hard to envisage an enzyme-mediatedsynthesis of LiFePO4, especially as the pH forthe precipitation is close to the physiologicalvalue of 7. The same outlook applies to con-version reactions, as preliminary work hasdemonstrated the synthesis of hydrated Co,Oand MnO, with the help of a virus and a bac-terium, respectively. Perhaps the ultimate in conversion reactionsalso comes from living systems. The proteinapoferritin, which encloses a small crystal ofiron oxide (Fe,O3.nH,O), can either grow ordissolve the particle according to the organism'scurrent need for iron .So could polymers withproperties rather similar to proteins, adsorbedon the surface of a conversion electrode, con-trol the growth or dissolution oftheTMO,andLizO crystals, the reversible formation of whichis key to increasing the energy efficiency? Regarding the feasibility of using electro-chemically active organic molecules as cathodematerials, the use of polyanilineand otherredox polymers has been much hyped overthe years, but development has been disap-pointing. However, because lithium-exchang-ing materials do not involve the electrolyte intheir redox processes, substituting the cath-ode for an organic material might boost thecapacity. The feasibility of using active LiC,O,organic molecules that can be prepared fromnatural sugars common in living systems(Fig. 4) is currently under investigation. Inthe light of such findings, we can speculate onthe use of hypericine (apolyquinone-basedactive ingredient of St John's wort) or the con-densation polymers of malic acid as potentialhigh-capacity cathode materials. The close relationship between carbo-hydrates and their oxidized polyketone formsmakes the former the logical starting pointfor the design of new electrode materials. Figure 4|An organic future. Proposed sustainable organic-based batteries based on electrodematerials made from biomass.Myo-inositol extracted from corn can be used to prepareelectrochemically active Li,C,O, whereas malic acid from apples can undergo polycondensationto a polyquinone that is electrochemically active to lithium (centre). (Derived from ref.15.) energy efficiency. The use of ionic liquids,which can be made hydrophobic, will put anend to problems caused by the entry ofwater.However, ifionic liquids are to be used as elec-trolytes, they must be combined with a highlyhygroscopic Li salt, so preparing them remainsa serious challenge. Lithium has been the anode of choice foryears, but much more work is still needed.When used with liquid electrolytes and gels,the metal is redeposited unevenly in the formofdendrites, leading to inherently unsafe cellswith a short lifetime. It has been suggestedthat the cause lies in current inhomogenei-ties induced by the passivation layer presenton the surface of lithium metal. Using drypolymer electrolytes instead keeps the prob-lem at bay for the first 600 cycles, but doesnot solve it. The classic strategy" to get uni-form microcrystalline metal deposition inan aqueous solution from anionic complexes(for example, silver metal from Ag(CN))has not been applied to the lithium elec-trode. In this strategy, the surges in localcurrent result in a drift of the negative ionsfrom the interface, leading to a depletion ofthe plating species and hindering the forma-tion of metal dendrites. The same principlecould be applied to lithium systems by usingcharged chelating complexes ofthe LiX,type,formed, for instance, using bidentate ligandsof the 1,3-dione family (acetylacetone) andhaving K as a counter-cation (see Fig.6). Another approach would be to use unipolarelectrolytes, in which only the cations carrycharge, but these have never been seriouslystudied in the context of plating lithium metal.This is surprising because polyelectrolytes withfixed negative charges attached to a macromole-cule are the only way to avoid the depletion orover-concentration of salt arising from the Polyketones can be obtained from naturalsources and are unlimited because sugars canbe made by living species or artificially in greenchemistry. Nor have sugars been overlookedfor use in bio fuel-cells, a much improved ver-sion of which has recently been unveiled". Although complex and unlikely to yieldinstant results, the search for electroactiveorganic molecules synthesized from biomasscould pave the way for the next generationoflithium-based batteries. Organic materialshave already made considerable inroads intothe semiconducting industry, in light-emittingdiodes, solar cells and transistors, and they areexpected to penetrate the energy field in thecoming decades. However, it would be foolishto ignore the fact that organic materials haveseveral disadvantages in terms of their lim-ited thermal stability, low specific gravity andappreciable solubility in electrolytes. Lithium-oxygen batteries Air electrodes and metal-air battery tech-nologies have already been used in primarysystems such as fuel cells, but the use of lithiuminstead of zinc as the metal will increase theenergy output eightfold. An oxygen electrodeproceeding in tandem with lithium accordingto the reaction 2Li+0z→Li,O can deliver acapacity of 1,200 mAhg.The first lithium-aircell was successfully assembled and dischargedin 1996 (ref. 18), but attractive rechargeabilitywas demonstrated only recently19. It could be argued that such a system uniteswithin the same device the two most promi-nent failures of battery and fuel-cell technolo-gies, namely the inability to master lithiumand oxygen electrodes. These perceived issueshave prevented the practical use oflithium-airbatteries. However, one advantage of such a sys-tem is the formation of Li,O, without cleaving Figure 5|Lithium-air batteries. Left, the mechanism used in lithium-air batteries (courtesy ofA. Debard et al.,Univ. St Andrews). Right, three-dimensional nanoarchitectured electrodes madefrom depositing 10-to 20-nm-thick layers of MnO, onto a carbon foam using a low-temperatureprocess (according to ref. 20) that could be used to enhance the kinetics of the lithium-air electrode(three-dimensional schematic courtesy of J. W. Long and D. R. Rolison, US Naval Research Lab.). to satisfy recent advances in microelectronics.These require miniature power sources, suchas solid-state, lithium-based, thin-film bat-teries. Much of the work has focused on flat,two-dimensional configurations, but theseare limited in terms of energy output, and theneed for greater performance has recently ledmicrobattery researchers to explore the thirddimension. This might seem relatively easy,given the spectacular three-dimensional cir-cuitry that the silicon microelectronic industrynow has to offer. However, microlithographyprocesses have proved both awkward andcostly to transfer to batteries. A combined chemical-electrochemicalapproach has much to offer as a way of man-ipulating materials at the atomic scale, andcould be used to develop skyscraper'batteries(Fig.7). Similarly, adding a third dimensionopens the way to a larger variety of configura-tions (such as the assembly of positive-negativeelectrodes and electrolyte) while maintaininga short diffusion length for electrodes andions, which is essential if a battery is to havethe required power. Conclusions It is not yet clear whether the next generationof batteries could be successfully integratedinto an energy market that is currently linkedto global warming. Fame and fortune certainlyawait anyone who can come up with a viablealternative to fossil fuels. Furthermore, it is dif-ficult to see how the performance gap betweenthe internal-combustion engine and lithium-ion batteries will be filled using only new bat-tery technology; other approaches, such asfuel cells, will be needed, but here a completeoverhaul of present systems will probably berequired. In our journey into the future we havereinvestigated existing systems and suggestednew trends and ideas that require much workto become a reality. Designing green and sus-tainable battery systems is essential, so criteriasuch as life cycle, abundance of raw materialsand electrode recycling are becoming cru-cial. For these reasons, much is expected ofthe lithium-air system, which offers a greatimprovement in energy density, and lithium- mobility of the anions. These two strategies(anioniclithium salts and unipolar conductivity)should be further explored to ensure that allavenues towards making the lithium-metalelectrode viable have been exhausted. Alternatives to lithium Although we have focused on lithium, thereare several alternatives for use as electrodes(Table 1). The metals worth considering aremagnesium (ref. 23) and aluminium (ref. 24)because of their light weight, but they deliverless voltage, undermining their use as anodes,and repetitive plating of these metals is dif-ficult using most electrolytes. Similarly, onlyhigh-capacity cathode materials can be consid-ered, which narrows it down to oxygen or sul-phur for magnesium, or graphite fluoride foraluminium, to harness the metals high affinityfor fluorine. However, little is known about thekinetics of electrode reactions involving themotion of multivalent species, and addressingthese challenges would need extensive collabo-ration between organometal researchers andelectrochemists. Proton-based battery technologies havebeen well studied, but do they still have any-thing to offer? Even with the best air electrode,to be competitive with lithium-ion batteries, ahydrogen system, with a voltage of 1.0-1.5 V,requires the anode to have an extremely lowequivalent mass, far below that of conventional Figure 6| Wild cards. Lithium-bearing anionic complexes that could be explored for efficient lithiumplating (left) and a contender for a high-capacity proton-exchanging polymer (right). Figure 7|Entering the third dimension. Schematic representation of athree-dimensional, integrated,solid-state lithium-ion battery. The surfacearea of the battery has increased 25-fold compared with a two-dimensionalthin-film battery with the same footprint surface area, and will therefore be able to provide enough energy to power smart autonomous network devicesrelated to sensing applications. (Courtesy of P. H. L. Notten, R. A. H. Niessenand L. Baggetto, Philips Research Laboratories,and Technical University ofEindhoven, the Netherlands.) based systems that use electroactive organicmolecules,which could be obtained frombiomass using green chemistry. Yet it seemsincongruous to insist that batteries are sus-tainable while the car or appliance they driveis not. The next generation oflithium-ion batter-ies fully based on nanomaterials will soon behere, followed by lithium-air batteries andothers using organic materials. And there isplenty to inspire us in the living world, as longas we can capture the function of each moleculein a cumulative sequential process, which is notan easy task. Both biofuel cells and high-volt-age liquid-electrolyte microbatteries inspiredby electric eels have already been demon-strated. We all live on organic-based energy,so why shouldn’t our appliances and vehiclesuse it too? One thing is clear, however. Solving theremaining challenges will require researchersfrom a range of disciplines, and their successwill depend on the efficiency of their cross-fertilization. M. Armand and J.-M. Tarascon*are at the LRCS,CNRS UMR-6007, Universite de Picardie JulesVerne, Amiens, France. ( * Corresponding author: ) ( e-mail:jean-marie.tarascon@sc.u-picardie.fr ) 1.Linden, D. & Reddy, T. B. (eds) Handbook of Batteries, 3rdedn(McGraw-Hill,2002). 2.Nagaura, T. & Tozawa, K. Prog. Batteries Sol. Cells 9,209(1990). 3.Lutgens, F. K. & Tarbuck,E.J. Essentials of Geology 7th edn(Prentice Hall, New York,2000). 4.Ward, R.D. & Brownlee, D. Rare Earth (Copernicus,NewYork,2000). ( I shihara,K . 5th Int. Conf. Ecobalance, Tsukuba (2002). A rico, A. S., B ruce, P ., Scrosati, B., Tarascon,J.-M.& ) van Schalkwijk, W. Nature Mater. 4,366-377(2005).7.Ravet,N.et al. Electrochemical Society Meeting, Hawaii ( (1999) . ) 8. Poizot, P., Laruelle, S., Grugeon, S., Dupont, L. & Tarascon,J.-M. Nature407,496-499(2000). ( 9. Croce, F.,Appetecchi, G. B. , Persi,L . & Scrosati, B . Nature394,456-458(1998). ) ( ) . Sadoway, D. R. et a l .J. Power Sources 97-98,621-623(2001). 10. ) ( . Nam,K . T. et al. Science 312,885-888(2006). ) ( 12. Hoare,R. J . ,Harrison, P . M. & H oy, T . G. N ature 255,653-654(1975). ) ( 13. MacDiarmid, A. G., Y ang, L. S ., Huang, W. S. & Humphrey, B . D. Synth. Metals 18, 393-398(1987). ) ( 14. Novak, P., Muller , K.,Santhanam,K. S. V. & Haas,O. Chem. Rev.97,207-282(1997). ) 15. Chen, H.et al. ChemSusChem (in the press). ( 1 6. A nastas,P. & Warner, J. C. Green Chemistry (Oxford Univ. P ress,New York,1998). ) ( 17. http://www . sony.net/Sonylnfo/News/Press/200708/07-074E/index.html ) ( 1 8. Abraham, K.M.& Jiang,Z. J.Electrochem. Soc.1 4 3,N01 ( 1996). ) 19..Ogasawara,T., Debart, A., Holzapfel, M., Novak, P. & Bruce, P. J. Am. Chem. Soc. 128,1390-1393(2006). 20. Fischer, A. E., Pettigrew, K. A., Rolison, D. R., Stroud,R.M& Long, J.W. Nano Lett. 7,281-286(2007). 21. Rosso,M. et al.Electrochim. Acta 51,5334-5340(2006). 22. Glasstone,S. The Fundamentals ofElectrochemistry andElectrodeposition (American Electroplaters'Society, NewYork, 1943). 23. Aurbach,D. et al. J.Electrochem. Soc. 149,A115-A121(2002). ( 24. Kamavaram, V. & Reddy, R. G. Electrochemical Studies o f Aluminum Deposition in lonic Liquids at Ambient T emperatures: Light Metals (Warrendale,2002). ) ( 2 5. L ong,J. W.,Dunn, B., Rolison, D. R. & White, H. S. Chem. R ev.104,4463-4492(2004). ) ( 26. A travers le monde (ed. Hachette)213-214(1899). ) ( 27. La Science et la Vie N°50, (May 1920). ) ( 2 8.Amatucci,G. G . & P e reira,N. J. Fl u orine Chem. 128, 243-262(2007). ) Acknowledgements The authors wish to thank thescientific community in the field of energy storage andconversion for laying the foundations for these views.They are also grateful to G. Amatucci,D. Murphy andP. Poizot for valuable comments. NaturePublishing Group
确定




还剩4页未读,是否继续阅读?
武汉科思特仪器股份有限公司为您提供《Building better batteries》,该方案主要用于其他中--检测,参考标准--,《Building better batteries》用到的仪器有CS2350H双单元电化学工作站(双恒电位仪)、CS350M电化学工作站/电化学测试系统、CS310M电化学工作站/电化学测试系统
相关方案
更多
该厂商其他方案
更多












