方案详情
文
锂电池鼻祖J.-M. Tarascon——《Nanostructured materials for advanced energy conversion and storage devices》
方案详情

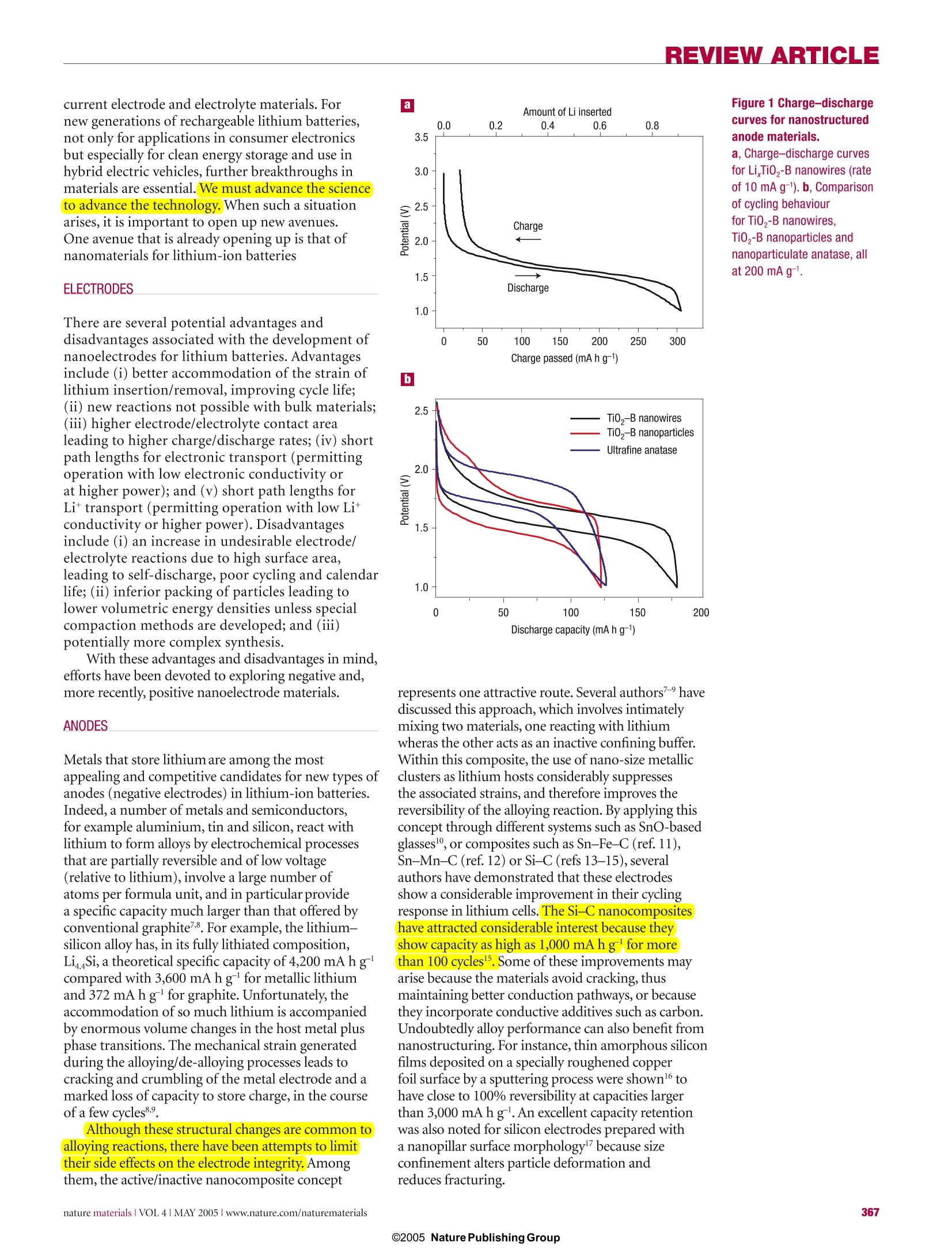
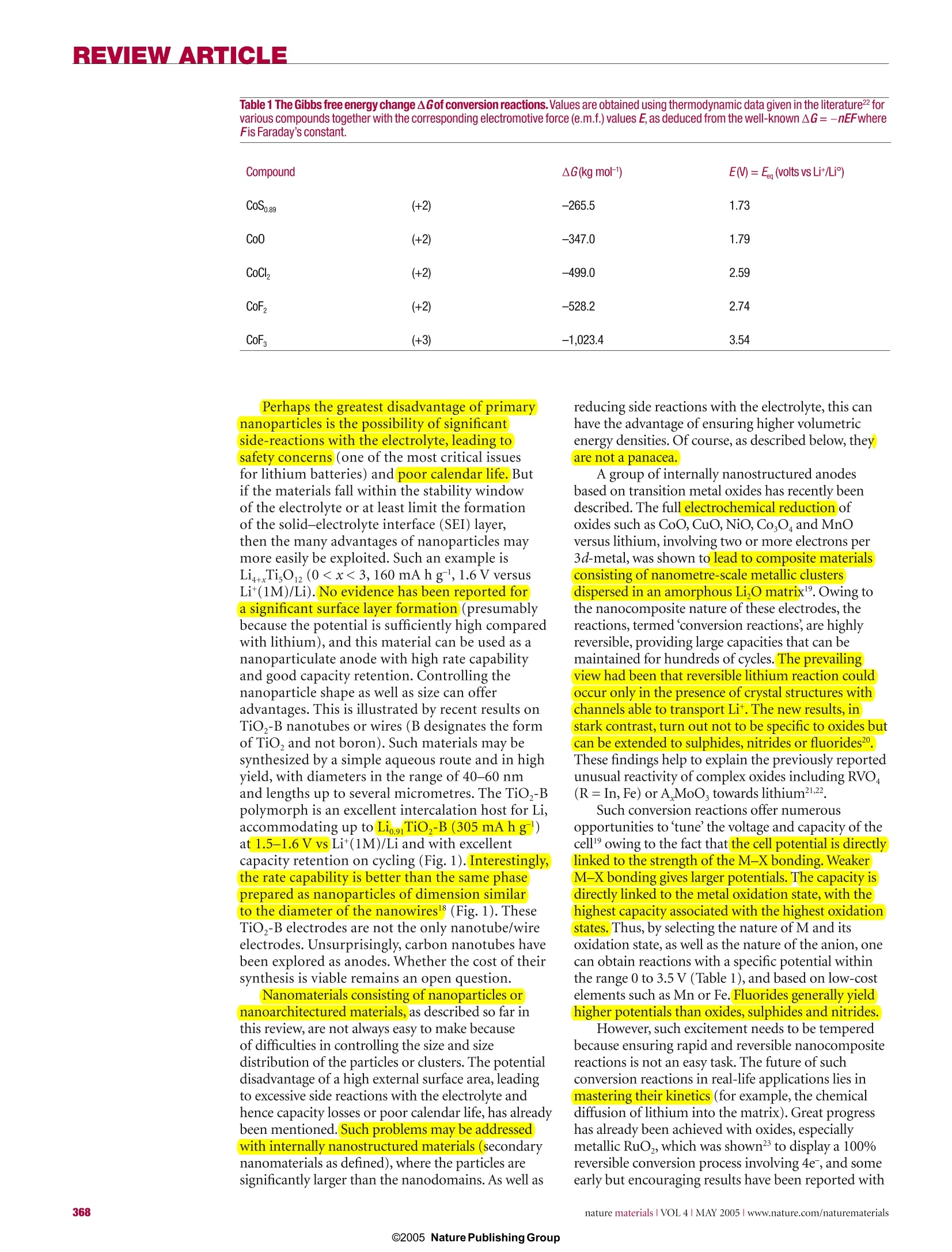
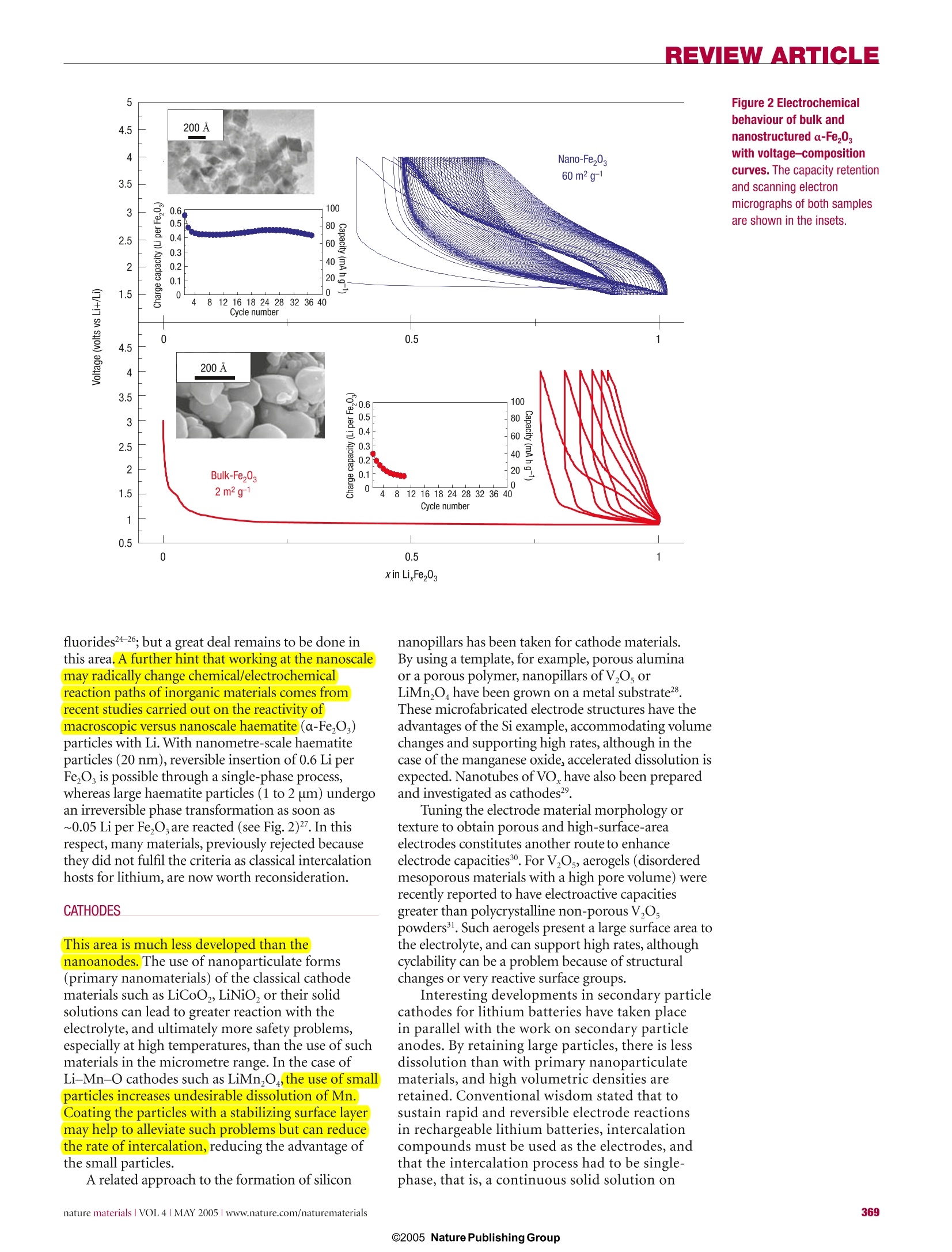
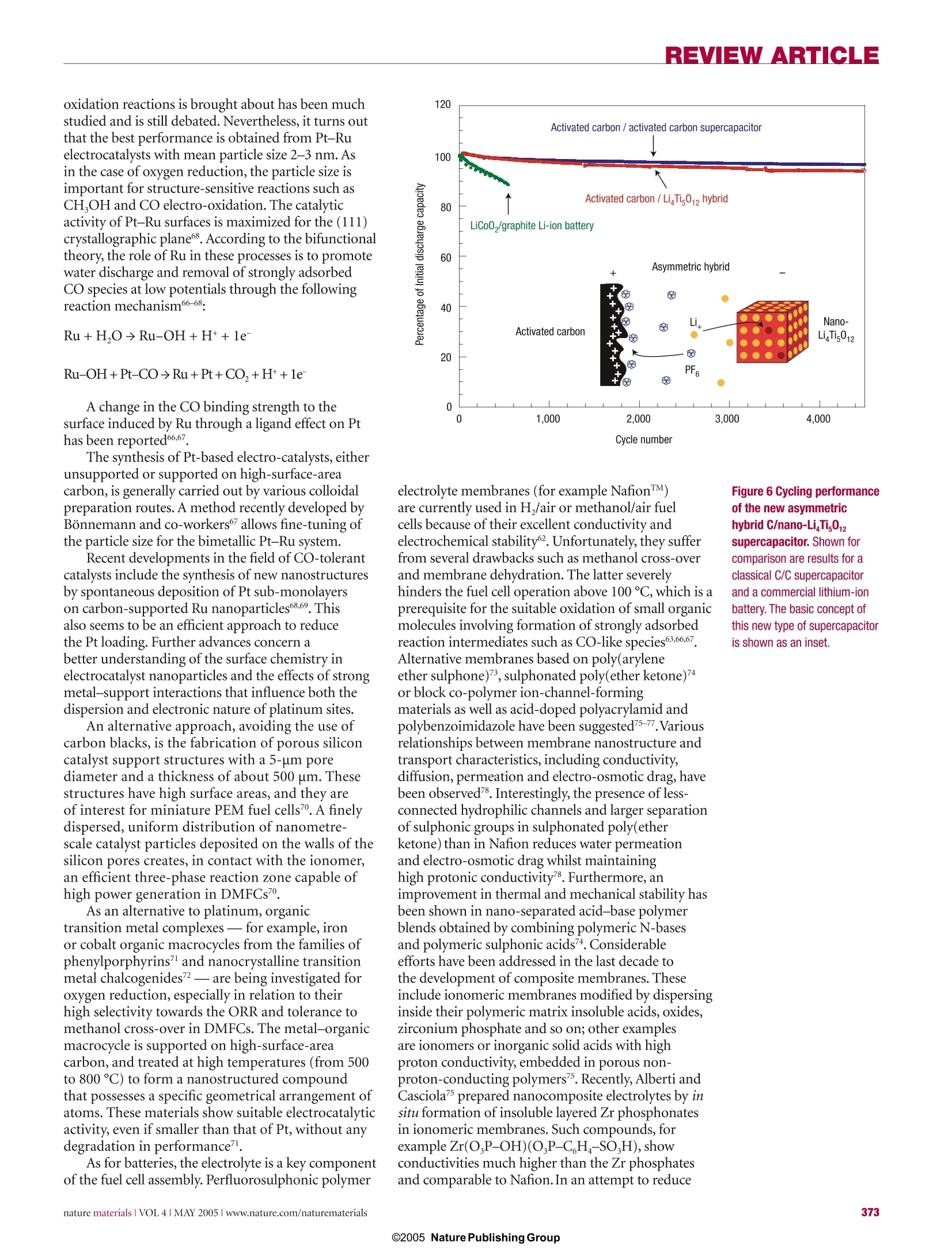
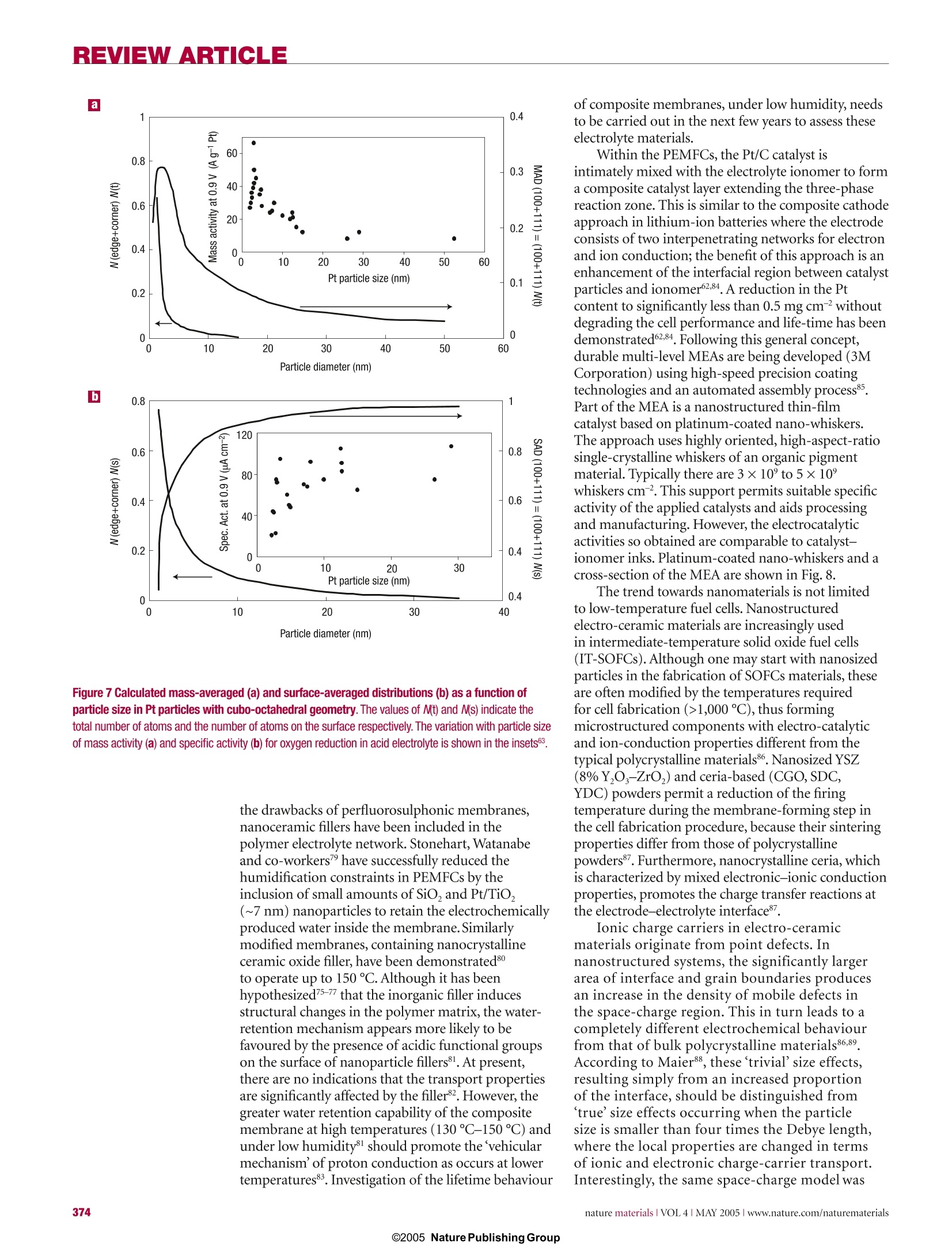
REVIEW ARTICLE REVIEWARTICLE Nanostructured materials for advancedenergy conversion and storage devices New materials hold the key to fundamental advances in energy conversion and storage, both of whichare vital in order to meet the challenge of global warming and the finite nature of fossil fuels. Nanomaterialsin particular offer unique properties or combinations of properties as electrodes and electrolytes ina range of energy devices. This review describes some recent developments in the discovery ofnanoelectrolytes and nanoelectrodes for lithium batteries, fuel cells and supercapacitors. The advantagesand disadvantages of the nanoscale in materials design for such devices are highlighted. ANTONINO SALVATORE ARIC01PETER BRUCE2, BRUNO SCROSATI3*JEAN-MARIE TARASCON4 ANDWALTER VAN SCHALKWIJK5 Istituto CNR-ITAE, 98126 S. Lucia, Messina, Italy2School of Chemistry, University of St Andrews, KY16 9ST,Scotland 3Dipartimento di Chimica, Universita ‘La Sapienza',00186Rome, Italy Universite de Picardie Jules Verne,LRCS; CNRS UMR-6047,80039 Amiens, France 5EnergyPlex Corporation, 1400 SE 112th Avenue, Suite 210, Bellevue, Washington 98004, USA *e-mail: scrosati@uniroma1.it One of the great challenges in the twenty-first centuryis unquestionably energy storage. In responseto the needs of modern society and emergingecological concerns, it is now essential that new,low-cost and environmentally friendly energyconversion and storage systems are found;hencethe rapid development of research in this field. Theperformance of these devices depends intimately onthe properties of their materials. Innovative materialschemistry lies at the heart of the advances that havealready been made in energy conversion and storage,for example the introduction of the rechargeablelithium battery. Further breakthroughs in materials,not incremental changes, hold the key to newgenerations of energy storage and conversion devices. Nanostructured materials have attracted greatinterest in recent years because of the unusualmechanical, electrical and optical propertiesendowed by confining the dimensions of suchmaterials and because of the combination of bulkand surface properties to the overall behaviour. One need only consider the staggering developmentsin microelectronics to appreciate the potential ofmaterials with reduced dimensions. Nanostructuredmaterials are becoming increasingly important forelectrochemical energy storagel2. Here we address thistopic. It is important to appreciate the advantages anddisadvantages of nanomaterials for energy conversionand storage, as well as how to control their synthesisand properties. This is a sizeable challenge facingthose involved in materials research into energyconversion and storage. It is beyond the scope of thisreview to give an exhaustive summary of the energystorage and conversion devices that may now or in thefuture benefit from the use of nanoparticles; rather,we shall limit ourselves to the fields of lithium-basedbatteries, supercapacitors and fuel cells. Furthermore,from now on we shall refer to nanomaterialscomposed of particles that are of nanometredimensions as primary nanomaterials, and thosefor which the particles are typically of micrometredimensions but internally consist of nanometre-sizedregions or domains as secondary nanomaterials. LITHIUM BATTERIES Lithium-ion batteries are one of the great successesof modern materials electrochemistry. Theirscience and technology have been extensivelyreported in previous reviews and dedicated books,to which the reader is referred for more details.A lithium-ion battery consists of a lithium-ionintercalation negative electrode (generally graphite),and a lithium-ion intercalation positive electrode(generally the lithium metal oxide,LiCoO,), thesebeing separated by a lithium-ion conductingelectrolyte, for example a solution of LiPF, inethylene carbonate-diethylcarbonate.Althoughsuch batteries are commercially successful, weare reaching the limits in performance using the current electrode and electrolyte materials. Fornew generations of rechargeable lithium batteries,not only for applications in consumer electronicsbut especially for clean energy storage and use inhybrid electric vehicles, further breakthroughs inmaterials are essential. We must advance the scienceto advance the technology. When such a situationarises, it is important to open up new avenues.One avenue that is already opening up is that ofnanomaterials for lithium-ion batteries ELECTRODES There are several potential advantages anddisadvantages associated with the development ofnanoelectrodes for lithium batteries.Advantagesinclude (i) better accommodation of the strain oflithium insertion/removal, improving cycle life;(ii) new reactions not possible with bulk materials;(iii)higher electrode/electrolyte contact arealeading to higher charge/discharge rates; (iv) shortpath lengths for electronic transport (permittingoperation with low electronic conductivity orat higher power); and (v) short path lengths forLit transport (permitting operation with low Litconductivity or higher power). Disadvantagesinclude (i) an increase in undesirable electrode/electrolyte reactions due to high surface area,leading to self-discharge, poor cycling and calendarlife; (ii) inferior packing of particles leading tolower volumetric energy densities unless specialcompaction methods are developed; and (iii)potentially more complex synthesis. With these advantages and disadvantages in mind,efforts have been devoted to exploring negative and,more recently, positive nanoelectrode materials. ANODES Metals that store lithium are among the mostappealing and competitive candidates for new types ofanodes (negative electrodes) in lithium-ion batteries.Indeed, a number of metals and semiconductors,for example aluminium, tin and silicon, react withlithium to form alloys by electrochemical processesthat are partially reversible and of low voltage(relative to lithium), involve a large number ofatoms per formula unit, and in particular providea specific capacity much larger than that offered byconventional graphite7. For example, the lithium-silicon alloy has, in its fully lithiated composition,LinaSi, a theoretical specific capacity of 4,200 mAhgcompared with 3,600 mA h g for metallic lithiumand 372 mA h g for graphite. Unfortunately, theaccommodation of so much lithium is accompaniedby enormous volume changes in the host metal plusphase transitions. The mechanical strain generatedduring the alloying/de-alloying processes leads tocracking and crumbling of the metal electrode and amarked loss of capacity to store charge, in the courseof a few cycles. Although these structural changes are common toalloying reactions, there have been attempts to limittheir side effects on the electrode integrity. Amongthem, the active/inactive nanocomposite concept b represents one attractive route. Several authors7-havediscussed this approach, which involves intimatelymixing two materials, one reacting with lithiumwheras the other acts as an inactive confining buffer.Within this composite, the use of nano-size metallicclusters as lithium hosts considerably suppressesthe associated strains, and therefore improves thereversibility of the alloying reaction. By applying thisconcept through different systems such as SnO-basedglasses, or composites such as Sn-Fe-C (ref.11),Sn-Mn-C (ref. 12) or Si-C (refs 13-15), severalauthors have demonstrated that these electrodesshow a considerable improvement in their cyclingresponse in lithium cells. The Si-C nanocompositeshave attracted considerable interest because theyshow capacity as high as 1,000 mAhgfor morethan 100 cycles . Some of these improvements mayarise because the materials avoid cracking, thusmaintaining better conduction pathways, or becausethey incorporate conductive additives such as carbon.Undoubtedly alloy performance can also benefit fromnanostructuring. For instance, thin amorphous siliconfilms deposited on a specially roughened copperfoil surface by a sputtering process were showntohave close to 100% reversibility at capacities largerthan 3,000 mA h g. An excellent capacity retentionwas also noted for silicon electrodes prepared witha nanopillar surface morphology because sizeconfinement alters particle deformation andreduces fracturing. Figure 1 Charge-dischargecurves for nanostructuredanode materials. a, Charge-discharge curvesfor Li,Ti0 -B nanowires (rateof 10 mA g-). b, Comparisonof cycling behaviourfor TiO,-B nanowires,TiO -B nanoparticles andnanoparticulate anatase, allat 200 mAg. Compound AG(kg moF1) E()=Eg(volts vs Lit/Li) CoSo.89 (+2) -265.5 1.73 Coo (+2) -347.0 1.79 CoCl, (+2) -499.0 2.59 CoF, (+2) -528.2 2.74 CoF, (+3) -1,023.4 3.54 Perhaps the greatest disadvantage of primarynanoparticles is the possibility of significantside-reactions with the electrolyte, leading tosafety concerns (one of the most critical issuesfor lithium batteries) and poor calendar life. Butif the materials fall within the stability windowof the electrolyte or at least limit the formationof the solid-electrolyte interface (SEI) layer,then the many advantages of nanoparticles maymore easily be exploited. Such an example isLi4+xTi,O12 (099.9% capacity retention, despite undergoingthe same cubic-tetragonal transformation.3.The reason is clearly not suppression of theJahn-Teller driven cubic-tetragonal distortion(something that has been attempted manytimes with limited success). Instead, the systemaccommodates the strain associated with the transformation by developing a nanostructurewithin the micrometre-sized particles (Fig. 3).The nanodomains of spinel switch between cubicand tetragonal structures,with the strain beingaccommodated by slippage at the domain wallboundaries34.35. The nanodomains form during thelayered-to-spinel transformation. Subsequentlyit has been shown that such a nanostructurecan be induced in normal spinel by grinding,with a similar enhancement in cyclability36.Interestingly, the layered-to-spinel transformationis very easy, helping to mitigate any ill effects thetransformation itself may have on cyclability7. A further, somewhat different, example ofthe benefits of nanoelectrodes within the field ofbatteries is the optimization of the environmentallybenign and low-cost phospho-olivine LiFePO4phase8 that displays a theoretical capacity of170 mA h g', as compared with 140 mA h g' forthe LiCoO,electrode used at present (LiFePOoperates at a lower voltage). But the insulatingcharacter of the olivine means that in practice onecould not obtain the full capacity of the materialbecause, as the electrochemical reaction proceeds,electronicallyisolated areas remain inactive inthe bulk electrode. As a result, this material waslargely ignored until it was prepared in the formof carbon-coated nanoparticles (Fig.4) throughvarious chemical and physical means39-41. Thissimultaneously reduces the distance for Littransport, and increases the electronic contactbetween the particles. Procedures of this kindhave led to a greatly improved electrochemicalresponse, and the full capacity of the material isnow accessible even under prolonged cycling(seealso Fig. 4). This example serves to illustrate someof the advantages of nanoelectrode materials listedat the beginning of this section, and to demonstratethat the search for new electroactive materials isnow wider than ever because such materials do notrequire a particularly high electronic conductivity,nor a high diffusion coefficient for lithium, as had been believed for the past 20 years. ELECTROLYTES Progress in lithium batteries relies as much onimprovements in the electrolyte as it does on theelectrodes. Solid polymer electrolytes representthe ultimate in terms of desirable properties forbatteries because they can offer an all-solid-stateconstruction, simplicity of manufacture, a widevariety of shapes and sizes, and a higher energydensity (because the constituents of the cell maybe more tightly wound). No corrosive or explosiveliquids can leak out, and internal short-circuits areless likely, hence greater safety. The most desirablepolymer electrolytes are those formed by solvent-free membranes, for example poly(ethylene oxide),PEO, and a lithium salt, LiX, for example LiPF, orLiCF,SO, (ref. 42). The poor ionic conductivity ofthese materials at room temperature has preventedthem from realizing their otherwise high promise.Dispersing nanoscale inorganic fillers in solvent-free, polyether-based electrolytes increases the conductivity several-fold43-45. The improvement ofthe electrolyte transport properties may be explainedon the basis of the heterogeneous doping modeldeveloped by Maier46. Accordingly, nanocompositescan be defined as the distribution of a second (oreven third) phase,with particles of nanometricdimension in a matrix that can be amorphous orcrystalline. Thus the increase in conductivity may beassociated with Lewis acid-base interactions betweenthe surface states of the ceramic nanoparticle withboth the polymer chains and the anion of thelithium salt4. As in the electrode case, there are ofcourse pros and cons in this particular approach.Indeed, other avenues are being explored to achievehigh-conductivity polymer electrolytes. Relevant inthis respect are the polymer-in-salt nanostructures48and the role of ionic liquids49. For 30 years it has been accepted that ionicconductivity in polymer electrolytes occurredexclusively in the amorphous phase, above the glasstransition temperature T. Crystalline polymerelectrolytes were considered to be insulators. Butrecent studies have shown that this is not the case:the 6:1 crystalline complexes PEO:LiXF ;X=P,As,Sb demonstrate ionic conductivity50.51.The Lit ionsreside in tunnels formed by the polymer chains(Fig.5). Significant increases in ionic conductivityin the crystalline 6:1 complexes are observed onreducing the chain length from 3,000 to 1,000:that is, in the nanometre range. It is evident thatin electrolytes, just as in the electrodes describedabove,control of dimensions on the nanoscalehas a profound influence on performance2.Crystalline polymer electrolytes represent aradically different type of ionic conductivity inpolymers, and illustrate the importance of seekingnew scientific directions. The present materials donot support ionic conductivities that are sufficientfor applications, but they do offer a fresh approachwith much scope for further advances. Recentlyit has been shown that the conductivity of thecrystalline polymer electrolytes may be raised bytwo orders of magnitude by partial replacement ofthe XF, ions with other mono or divalent anions(ref. 53,and P. G. Bruce, personal communication).Chemical compatibility with selected electrodematerials has yet to be evaluated. SUPERCAPACITORS Supercapacitors are of key importance in supportingthe voltage of a system during increased loads ineverything from portable equipment to electricvehicles54-56. These devices occupy the area in theRagone plot (that is, the plot of volumetric againstgravimetric energy density) between batteries anddielectric capacitors. Supercapacitors are similar tobatteries in design and manufacture (two electrodes,separator and electrolyte), but are designed for highpower and long cycle life (>100 times battery life),but at the expense of energy density. There are two general categories ofelectrochemical supercapacitors: electric double-layer capacitors (EDLC) and redox supercapacitors.In contrast to batteries, where the cycle life is limited because of the repeated contraction and expansion ofthe electrode on cycling, EDLC lifetime is in principleinfinite, as it operates solely on electrostatic surface-charge accumulation. For redox supercapacitors, somefast faradic charge transfer takes place as in a battery.This gives rise to a large pseudo-capacitance. Progress in supercapacitor technology can benefitby moving from conventional to nanostructuredelectrodes. In the case of supercapacitors, the electroderequirements are less demanding than in batteries,at least in terms of electrode compaction,becausepower prevails over energy density. Thus, the benefitsof nanopowders with their high surface area (primarynanoparticles) are potentially more important, hencethe staggering interest in nanopowders and their rapiduptake for supercapacitor-based storage sources. Recent trends in supercapacitors involve thedevelopment of high-surface-area activated carbonelectrodes to optimize the performance in terms ofcapacitance and overall conductivity. Attention hasbeen focused on nanostructured carbons,such asaerogels7, nanotubes58 and nanotemplates9. Theadvantages of carbon aerogels lie mainly in their lowionic and electronic charging resistance and in theirpotential use as binderless electrodes.Replacing thestandard carbon fibre with carbon aerogel electrodesimproves capacitance and cyclability. Because oftheir unique architecture, carbon nanotubes arenow intensively studied as new electrode materialsfor supercapacitor structures although, as forbatteries, cost may be an issue. A critical aspect innanotechnology for supercapacitors is to reach acompromise between specific surface area (to ensurehigh capacitance) and pore-size distribution (topermit easy access for the electrolyte). Nanostructured materials have led to thedevelopment of new supercapacitor technologies.Capitalizing on the benefits of nanomaterialelectrodes, Telcordia’s researchers have, by meansof the so-called hybrid supercapacitors (HSCs),proposed a new approach to energy storage.HSCsuse one capacitive carbon electrode similar to that of acarbon EDLC; however, the complementary negativeelectrode consists of a nanostructured lithium Figure 4 Capacity against cycling number for a lithiumcoin cell. The experimentsused a 1-cm? disc of Bellcore-type plastic laminate madeout 4.480 mg of pure LiFePOcoated with 5%C in situ. Theoverall plastic composition was69% LiFePO, 11%C total and20% Kynar. The cell was cycledat C/5 (0.160 mA cm-), D/2(-0.400 mA cm-) between 2and 4.5V at room temperature.Here, Dis discharge rate,C is charge rate. Left inset:enlarged transmission electronmicrograph of the LiFePO,particles used, coated withcarbon. The coating used ahomemade recipe103. Thisimage was recorded with aTECNAI F20 scanning TEM. Thequality of the coating - thatis, the interface betweencrystallized LiFePO andamorphous carbon- is nicelyobserved in the high-resolutionelectron micrograph in the rightinset (taken with the samemicroscope).Courtesy ofC. Wurm and C. Masquelier,LRCS Amiens. titanate compound that undergoes a reversiblefaradic intercalation reaction. The key innovationlies in the use of a nanostructured Li Ti,O12 as thenegative electrode, resulting in no degradation inperformance from charge/discharge-induced strain.Here is a nanomaterial that is being explored forcapacitor and battery use because of its excellentcyclability, rate capability and safety. Its use in thehybrid supercapacitor results in a device with cyclelife comparable to that of supercapacitors, freeingdesigners from the lifespan limitations generallyassociated with batteriesgrt. Figure 6 shows somefeatures of these new types of capacitors. BesidesLi Ti,O12, other nanostructured oxide materials canbe engineered so as to obtain hybrid devices operatingover a wide range of voltages. Inorganic nanotubes ornanowires may offer an alternative to nanoparticulateLi Ti,O, for example the LiTiO,-B nanowiresdescribed in the section on lithium batteries. Onecan therefore envisage a supercapacitor composed ofa carbon nanotube as the positive electrode and aninorganic nanotube capable of lithium intercalation(for example Li TiO,-B) as the negative electrode. Fabricating nanopillared electrodes by growingthe materials on a substrate was described in thecontext of lithium batteries. However, a relatedapproach where materials are electro-synthesizedin the presence of an electrolyte, which is also aliquid-crystal template, has been used to formnanoarchitectured electrodes, for example Ni orNiOOH, for possible use in supercapacitors. FUEL CELLS Fuel cell technologies are now approachingcommercialization, especially in the fields of portablepower sources - distributed and remote generationof electrical energy2. Already, nanostructuredmaterials are having an impact on processingmethods in the development of low-temperaturefuel cells (T<200℃), the dispersion of preciousmetal catalysts, the development and dispersionof non-precious catalysts, fuel reformation andhydrogen storage, as well as the fabrication ofmembrane-electrode assemblies (MEA). Polymerelectrolyte membrane fuel cells (PEMFCs) haverecently gained momentum for application intransportation and as small portable power sources;whereas phosphoric acid fuel cells (PAFCS), solidoxide fuel cells (SOFCs) and molten carbonates fuel cells (MCFCs) still offer advantages for stationaryapplications, and especially for co-generation2.Platinum-based catalysts are the most activematerials for low-temperature fuel cells fed withhydrogen, reformate or methanol2. To reduce thecosts, the platinum loading must be decreased (whilemaintaining or improving MEA performance), andcontinuous processes for fabricating MEAs in highvolume must be developed. A few routes are beingactively investigated to improve the electrocatalyticactivity of Pt-based catalysts. They consist mainly ofalloying Pt with transition metals or tailoring the PtDarticle size. The oxygen reduction reaction (ORR) limits theperformance of low-temperature fuel cells. One of thepresent approaches in order to increase the catalystdispersion involves the deposition of Pt nanoparticleson a carbon black support. Kinoshita et al. observedthat the mass activity and specific activity for oxygenelectro-reduction in acid electrolytes varies with thePt particle size according to the relative fraction of Ptsurface atoms on the (111)and (100) faces3 (Fig.7).The mass-averaged distribution of the surface atoms onthe (111) and (100) planes passes through a maximum(~3 nm) whereas the total fraction of surface atomsat the edge and corner sites decreases rapidly with anincrease of the particle size. On the other hand, thesurface-averaged distribution for the (111) and (100)planes shows a rapid increase with the particle size,which accounts for the increase of the experimentallydetermined specific activity with the particle size(Fig.7). A dual-site reaction is assumed as the rate-determining step: Pt-O,+H*+e→Pt-HO,Pt-HO, +Pt→ Pt-OH+Pt-O (r.d.s.) This mechanism accounts for the role of dual sites ofproper orientation63. Alloying Pt with transition metals also enhancesthe electrocatalysis of O,reduction. In low-temperaturefuel cells, Pt-Fe, Pt-Cr and Pt-Cr-Co alloyelectrocatalysts were observed62.64.65 to have high specificactivities for oxygen reduction as compared with thaton platinum. This enhancement in electrocatalyticactivity has been ascribed to several factors suchas interatomic spacing, preferred orientation orelectronic interactions. The state of the art Pt-Co-Crelectrocatalysts have a particle size of 6 nm (ref.65). Both CO,and CO are present in hydrogenstreams obtained from reforming. These moleculesare known to adsorb on the Pt surface under reducingpotentials. Adsorbed CO-like species are also formedon Pt-based anode catalysts in direct methanol fuelcells (DMFCs). Such a poisoning of the Pt surfacereduces the electrical efficiency and the power densityof the fuel cell66.The electrocatalytic activity of Ptagainst, for example, CO,/CO poisoning is known tobe promoted by the presence of a second metal64,65,67,such as Ru, Sn or Mo. The mechanism by which suchsynergistic promotion of the H,/COand methanol oxidation reactions is brought about has been muchstudied and is still debated.Nevertheless,it turns outthat the best performance is obtained from Pt-Ruelectrocatalysts with mean particle size 2-3 nm. Asin the case of oxygen reduction, the particle size isimportant for structure-sensitive reactions such asCH,OH and CO electro-oxidation. The catalyticactivity of Pt-Ru surfaces is maximized for the (111)crystallographic plane. According to the bifunctionaltheory, the role of Ru in these processes is to promotewater discharge and removal of strongly adsorbedCO species at low potentials through the followingreaction mechanism66-68: A change in the CO binding strength to thesurface induced by Ru through a ligand effect on Pthas been reported66,67. The synthesis of Pt-based electro-catalysts, eitherunsupported or supported on high-surface-areacarbon, is generally carried out by various colloidalpreparation routes. A method recently developed byBonnemann and co-workers7allows fine-tuning ofthe particle size for the bimetallic Pt-Ru system. Recent developments in the field of CO-tolerantcatalysts include the synthesis of new nanostructuresby spontaneous deposition of Pt sub-monolayerson carbon-supported Ru nanoparticles68,69. Thisalso seems to be an efficient approach to reducethe Pt loading. Further advances concern abetter understanding of the surface chemistry inelectrocatalyst nanoparticles and the effects of strongmetal-support interactions that influence both thedispersion and electronic nature of platinum sites. An alternative approach, avoiding the use ofcarbon blacks, is the fabrication of porous siliconcatalyst support structures with a 5-um porediameter and a thickness of about 500 um. Thesestructures have high surface areas, and they areof interest for miniature PEM fuel cells7. A finelydispersed, uniform distribution of nanometre-scale catalyst particles deposited on the walls of thesilicon pores creates, in contact with the ionomer,an efficient three-phase reaction zone capable ofhigh power generation in DMFCs. As an alternative to platinum, organictransition metal complexes - for example, ironor cobalt organic macrocycles from the families ofphenylporphyrins’ and nanocrystalline transitionmetal chalcogenides72 - are being investigated foroxygen reduction, especially in relation to theirhigh selectivity towards the ORR and tolerance tomethanol cross-over in DMFCs. The metal-organicmacrocycle is supported on high-surface-areacarbon, and treated at high temperatures (from 500to 800℃) to form a nanostructured compoundthat possesses a specific geometrical arrangement ofatoms. These materials show suitable electrocatalyticactivity, even if smaller than that of Pt, without anydegradation in performance. As for batteries, the electrolyte is a key componentof the fuel cell assembly. Perfluorosulphonic polymer electrolyte membranes (for example Nafion)are currently used in H/air or methanol/air fuelcells because of their excellent conductivity andelectrochemical stability62. Unfortunately, they sufferfrom several drawbacks such as methanol cross-overand membrane dehydration. The latter severelyhinders the fuel cell operation above 100℃, which is aprerequisite for the suitable oxidation of small organicmolecules involving formation of strongly adsorbedreaction intermediates such as CO-like species63,66,67.Alternative membranes based on poly(aryleneether sulphone)73, sulphonated poly(ether ketone)74or block co-polymer ion-channel-formingmaterials as well as acid-doped polyacrylamid andpolybenzoimidazole have been suggested75-77.Variousrelationships between membrane nanostructure andtransport characteristics, including conductivity,diffusion, permeation and electro-osmotic drag, havebeen observed7. Interestingly, the presence of less-connected hydrophilic channels and larger separationof sulphonic groups in sulphonated poly(etherketone) than in Nafion reduces water permeationand electro-osmotic drag whilst maintaininghigh protonic conductivity78. Furthermore, animprovement in thermal and mechanical stability hasbeen shown in nano-separated acid-base polymerblends obtained by combining polymeric N-basesand polymeric sulphonic acids74.Considerableefforts have been addressed in the last decade tothe development of composite membranes. Theseinclude ionomeric membranes modified by dispersinginside their polymeric matrix insoluble acids, oxides,zirconium phosphate and so on; other examplesare ionomers or inorganic solid acids with highproton conductivity, embedded in porous non-proton-conducting polymers7. Recently, Alberti andCasciola prepared nanocomposite electrolytes by insitu formation of insoluble layered Zr phosphonatesin ionomeric membranes. Such compounds, forexample Zr(O,P-OH)(O,P-C,H-SO,H),showconductivities much higher than the Zr phosphatesand comparable to Nafion. In an attempt to reduce Figure 6 Cycling performanceof the new asymmetrichybrid C/nano-Li,Ti,0supercapacitor.Shown forcomparison are results for aclassical C/C supercapacitorand a commercial lithium-ionbattery. The basic concept ofthis new type of supercapacitoris shown as an inset. a 一+二一 Figure 7 Calculated mass-averaged (a) and surface-averaged distributions (b) as a function ofparticle size in Pt particles with cubo-octahedral geometry. The values of /(t) and N(s) indicate thetotal number of atoms and the number of atoms on the surface respectively. The variation with particle sizeof mass activity (a) and specific activity (b) for oxygen reduction in acid electrolyte is shown in the insets. the drawbacks of perfluorosulphonic membranes,nanoceramic fillers have been included in thepolymer electrolyte network. Stonehart, Watanabeand co-workers? have successfully reduced thehumidification constraints in PEMFCs by theinclusion of small amounts of SiO,and Pt/TiO,(~7 nm) nanoparticles to retain the electrochemicallyproduced water inside the membrane. Similarlymodified membranes, containing nanocrystallineceramic oxide filler, have been demonstrated80to operate up to 150℃. Although it has beenhypothesized75-77 that the inorganic filler inducesstructural changes in the polymer matrix, the water-retention mechanism appears more likely to befavoured by the presence of acidic functional groupson the surface of nanoparticle fillers°1. At present,there are no indications that the transport propertiesare significantly affected by the filler2. However, thegreater water retention capability of the compositemembrane at high temperatures (130C-150℃) andunder low humidity8l should promote the vehicularmechanism'of proton conduction as occurs at lowertemperatures83. Investigation of the lifetime behaviour of composite membranes, under low humidity, needsto be carried out in the next few years to assess theseelectrolyte materials. Within the PEMFCs, the Pt/C catalyst isintimately mixed with the electrolyte ionomer to forma composite catalyst layer extending the three-phasereaction zone. This is similar to the composite cathodeapproach in lithium-ion batteries where the electrodeconsists of two interpenetrating networks for electronand ion conduction; the benefit of this approach is anenhancement of the interfacial region between catalystparticles and ionomer62,84. A reduction in the Ptcontent to significantly less than 0.5 mg cmwithoutdegrading the cell performance and life-time has beendemonstrated62.84. Following this general concept,durable multi-level MEAs are being developed (3MCorporation) using high-speed precision coatingtechnologies and an automated assembly process85.Part of the MEA is a nanostructured thin-filmcatalyst based on platinum-coated nano-whiskers.The approach uses highly oriented, high-aspect-ratiosingle-crystalline whiskers of an organic pigmentmaterial. Typically there are 3 × 10° to 5 ×10°whiskers cm. This support permits suitable specificactivity of the applied catalysts and aids processingand manufacturing. However, the electrocatalyticactivities so obtained are comparable to catalyst-ionomer inks. Platinum-coated nano-whiskers and across-section of the MEA are shown in Fig. 8. The trend towards nanomaterials is not limitedto low-temperature fuel cells. Nanostructuredelectro-ceramic materials are increasingly usedin intermediate-temperature solid oxide fuel cells(IT-SOFCs). Although one may start with nanosizedparticles in the fabrication of SOFCs materials, theseare often modified by the temperatures requiredfor cell fabrication (>1,000 ℃), thus formingmicrostructured components with electro-catalyticand ion-conduction properties different from thetypical polycrystalline materials86. Nanosized YSZ(8%Y,O-ZrO,) and ceria-based (CGO,SDC,YDC) powders permit a reduction of the firingtemperature during the membrane-forming step inthe cell fabrication procedure, because their sinteringproperties differ from those of polycrystallinepowders7. Furthermore, nanocrystalline ceria, whichis characterized by mixed electronic-ionic conductionproperties, promotes the charge transfer reactions atthe electrode-electrolyte interface87. Ionic charge carriers in electro-ceramicmaterials originate from point defects. Innanostructured systems, the significantly largerarea of interface and grain boundaries producesan increase in the density of mobile defects inthe space-charge region. This in turn leads to acompletely different electrochemical behaviourfrom that of bulk polycrystalline materials86,89.According to Maier8, these trivial' size effects,resulting simply from an increased proportionof the interface, should be distinguished from‘true’size effects occurring when the particlesize is smaller than four times the Debye length,where the local properties are changed in termsof ionic and electronic charge-carrier transport.Interestingly, the same space-charge model was used by Maier to explain the observed increasein ionic conductivity of dry or hybrid Li-basedpolymer systems loaded with nano-inorganic fillers (SiO,,Al,O,TiO,) as discussed previously.Notwithstanding the importance ofnanostructured materials, the performance ofpractical fuel cells remains limited by scale-up,stack housing design, gas manifold and sealing.Although progress has been achieved in thedirect electrochemical oxidation of alcohol andhydrocarbon fuels62,90,91, fuel cells are still mostlyfed by hydrogen. Much research is now focusedon nanostructured hydrides including carbonnanotubes, nanomagnesium-based hydrides,metal-hydride/carbon nanocomposites, and alanatesfor hydrogen storage2.93. Early reports suggestedhydrogen adsorption capacities of 14-20 wt% forK-and Li-doped carbon nanotubes94.95, but today’sreliable hydrogen-storage capacities in single-walledies 5carbon nanotubes appear comparable to or even lessthan those of metal hydrides96-98, and probably notsufficient to store the amount of hydrogen requiredfor automotive applications, which has been set by theUS Department of Energy as 6.5% (ref. 98). Recentdevelopments in this field include modification ofMg-hydrides with transition metals99,100, and theinvestigation of boron-nitride nanostructures01.Magnesium hydride, MgH, is often modified byhigh-energy ball-milling with alloying elementsincludingNi, Cu, Ti,Nb and Al so as to obtain,after 20 h of milling, nanoparticles in the range of20-30 nm providing hydrogen-storage capacities ofabout 6-11 wt% (refs 99,100). Besides improvingthe hydrogen sorption kinetics of MgH,partialsubstitution of Al for Mg in Mg,Ni hydrogen storagesystems seems to increase the life-cycle characteristics,as a consequence of the existence of an Al,O, film onthe alloy surface.Further advances resulted from theinvestigation of MgH,-V nanocomposites100 and theaddition of very small amounts of nanoparticulatetransition metal oxides (Nb,O,, WO, Cr,O;) toMg-based hydrides 02.The presence of vanadiumas well as the particular nanostructure of thenanocomposite aids the hydrogen penetration intothe material.Also, in the case of transition metaloxide promoters, the catalytic effect of compoundssuch as Cr,O, the reactive mechanical grinding andthe nanosized particles seem to produce a substantialimprovement in the adsorption and desorptionproperties of magnesium102. CONCLUSION It is a regrettable feature of important scientific. discoveries that they often suffer from hyperbole;perhaps it has always been so, but the speed oftoday’s communications exacerbates the situation.Nanoscience suffers from this disease. Yet there aremany genuine scientific advances that fall underthe umbrella of nanoscience.This short reviewdemonstrates how moving from bulk materials tothe nanoscale can significantly change electrodeand electrolyte properties, and consequently theirperformance in devices for energy storage andconversion. In some cases the effects may be simple a consequences of a reduction in size, for examplewhen nanoparticulate electrodes or electrocatalystslead to higher electrode/electrolyte contact areas andhence higher rates of electrode reaction. In othersthe effects may be more subtle, involving internallynanostructured materials or nanostructures withparticular morphologies, for example the nanotubes.Space-charge effects at the interface between smallparticles can result in substantial improvementsof properties. There is a profound effect of spatialconfinement and contribution of surfaces, due tosmall particle size, on many of the properties ofmaterials; this challenges us to develop new theoryor at least adapt and develop theories that have beenestablished for bulk materials. We also foresee thatthis subject will bring together the disciplines ofmaterials chemistry and surface science, as both arenecessary to understand nanomaterials. doi:10.1038/nmat1368 ( References ) ( 1 . N azar, L. E. et al. Nanostructured m a terials for energy st o rage. In t . J . I n org M ater. 3 , 1 91- 2 00(2001). ) ( 2. Hirshes, M. N a noscale materials f o r e nerg y storage. Mate r. Sci. Eng. B 108, 1 (2004) ) ( 3 Scrosati, B. Challenge of portable p o w e r. Nature 373, 557 -5 58( 199 5) 4 ) ( T arascon, J.-M. & A rmand, M. Is s ues and challenges facing rec h argeablebatteries . Nature 414,3 5 9-367(2 0 01). ) ( 5. W V akihara, W. & Yamamoto,O. (e d s) Lithium Ion B a tteries - F u ndamentals an d P erformance ( Kodansha-Wi l ey-VCH, Weinheim, 1 998). ) ( 6. v an Schalkwijk, W. & Scrosati, B. (e d s) Ad v ances in Lithium-Ion Batter i es . (Kluwer Academic/Plenum, New York,2002) . ) ( 7 . H uggins,R. A. i n Handbook of Battery M aterials (ed. Be s enhard, J. O .) Part III, C hapter 4 ( Wi l ey -V CH, Weinheim, 1 9 99). ) ( 8 W inter,M . & Besenhard,J. O. E le c trochemical l it hiation of t in and t in-based i ntermeta l lic and composites. Electrochim. Acta 45,31 - 50( 1 9 99). ) ( 9 N azar, L. F. & C rosnier, O. In Lithium Batteries Scien c e a n d Te c hnology (e ds Nazri,G.- A . & Pistoia, G . ) 112 - 143 (Kluw e r Academic/Plenum,Boston,2004). ) ( 10 . Idota, Y., Kabuto, T ., Matsufuji, A ., Maekawa, Y. & Miyasaki, T. T i n-based a mo r ph o us oxi d es: a h i gh-cap a city li t hium-ion s torage material. Sci e n c e 276 , 1395-1397( 1 997). ) ( 1 1 . Mao, O. & Dahn,J. R. Mechanically alloyed Sn-F e ( - C) powders as anode m aterials for Li ion b atteries. III . Sn,Fe:SnFe,C active/inactive composites. J . Electrochem. Soc . 146 , 423 -4 27( 1 99 9). ) ( 1 2. Beaulieu, L. Y. & Dahn, J . R. The reaction of lithium with Sn-Mn-C i ntermetallics prepared by m echanical alloying.J. E lectrochem. Soc. 147, 3237 -3 241(2000). ) ( 1 3. Graetz,J., Ahn, C. C., Yazami, R. & Fuetz, B. Highly reversible lithium storage i n n anostructured silicon. El ec tr ochem. Solid-State L ett. 6, A1 94-197 (20 03 ). ) ( 1 4 . Yang,J. et al . Si/C composites f or h igh capacity lithium st o rage materials. Electrochem. Solid - State Le t t . 6,A154 - 156 (2003)). ) ( 1 5. N ovak, P . e t al. in Int . Meeting L i Batteries IMLB12 N ara, Japan Abs t ract 9 (200 4 ). 1 6. Ikeda, H. e t al. i n Proc . 42nd Batt e ry S y mposium, Yokohama, Japan 282 ( 200 1 ). ) ( 1 7. G r een, M., Fielder,E.,S c rosati, B., Wachtler , M. & Se r ra Moreno, J. Structured silicon anodes for lithium battery a pplications. El e ctrochem. S o l i d-S t a t e Lett. 6, A 75-79(2003). ) ( 18. Ar m strong, A. R ., Armstrong,G., Ca n ales, Garcia, J. R . &Bruce, P. G. Lithium i ntercalation intoTiO, - B nanowires . Ad v. Mater. (in the pre s s). ) ( 1 9. Poizo t , P., Laru e lle, S., Grugeon, S.,Dupont, L . & Tarascon,J.-M . Nano-siz e d ) Figure 8 Platinum-coatednanostructured whiskersupports (0.25 mg cm2).a, Plane view; b, 45° view(higher magnification). Thenanostructured film of theMEA (C) shows the Pt-coatednanowhiskers sandwichedbetween the PEM and thegas-diffusion layer. Courtesyof R. Atanasoski, 3M,St. Paul,Minnesota,USA. ( t ransition m etal oxides as negative elec t rode material for lithium-ion batteries. Nature 407,496- 4 99 ( 2000). ) ( 2 0. Tarascon, J . -M .,Grugeon, S., Laruelle, S.,L a rcher, D. & Poizot, P . In L ithium B atteries Scienc e and Technology (eds Na z ri, G.-A. & Pistoia, G.) C h . 7, 220-246( K luwer Academic/Plenum, Boston,2004). ) ( 21. Denis, S . , Baudrin,E . , Touboul, M.& Tarascon, J . - M. Synthesis andelectrochemical p roperties vs. Li of amorphous vanadat e s of general formula R VO ,( R = I n , C r, F e , A l, Y) J. Electrochem . S o c. 1 44, 4 099-4109 (1997). ) ( 22. Leroux,E,Coward, G. R. , Power, W. P. & Nazar, L. E. U n derstanding the n a tureof low- p otential L i uptake into high volumetric capacity molybedenum oxides. E lectrochem S olid-State Lett . 1 ,2 55-258 (1998). ) ( 2 3. Ba l aya, P , L i, H. , Kienle, L. & Maier, J . Fully reversible homogeneous Li s toragein RuO,w i th high capacity. Adv. F unct. M ater. 13, 6 21- 62 5(20 0 3). ) ( 2 4. Badway, F ., Cosandey, E, Pereira , N . & Amatucci , G. G. Carbon m etal f luoridenanocomposites: high capacity reversible metal fluoride conversion materials as rechargeable positive elec tr odes for L i b a tteries. J . Elec t rochem. S oc. 1 50, A1318- 1 327( 2 003) . ) 25.Li, H.,Ritcher,G. & Maier, J. Reversibile formation and decomposition of LiFclusters using transition metal fluorides as precursors and their application inrechargeable Li batteries. Adv. Mater. 15, 736-739 (2003). ( 2 6. J amnik,J, & M ai er, J. N a nocrystallinity ef f ects in lithium battery materials. Aspects of nano-ionics. Part IV . Phys. Chem. Chem. Phys. 5, 5 2 15-5 2 2 0 (2 00 3). ) ( 2 7. L arche r ,D. e t a l. E f fect o f particle s i ze on l i thium i n t e r calat ion int o a- F e ,O, J .E l ectrochem. S oc. 150,A133- 13 9(2003). ) ( 2 8. S ides,C. R ., L i ,N. C . , P atrissi, C. J . , Scrosati, B . & Martin, C. R. Na n oscale materials for l ithium-ion batter i es . M ater. R es . B ull. 27, 6 04-607(2 0 0 2 ). ) ( 2 9.Nordliner,S., Edstrom, K. & Gustafsson, T. E lect r ochemistry o f v a nadium o xide nanotubes. El ec tr o chem. So l id St a te Lett . 4 ,A129 (2001). ) ( 3 0. L e , D. B. et al . High surface area V,O, a erogel intercalation e l ectrodes.J. Electrochem. Soc . 143 , 2099-2104(1996). ) ( 31. Dong, W., Rolison, D . R. & Dunn, B. E lectrochemical properties of h igh surface area vanadium oxides aerogels. Elect r ochem. S o lid State Lett . 3, 457 - 459( 2 000). ) ( 32. T hackeray, M . M . , David, W. I . E, B ruce,P . G . & Goodenough, J. B . L i thium i nsertion into manganese spinels. Mater. Re s . Bull. 18, 461-472 (19 8 3 ). ) 33. Robertson,A. D., Armstrong, A. R. & Bruce, P. G. Layered Li Mn,Co,O,intercalation electrodes: influence of ion exchange on capacity and structureupon cycling. Chem. Mater. 13, 2380-2386 (2001). 34. Shao-Horn,Y. et al. Structural characterisation of layered LiMnO, electrodesby electron diffraction and lattice imaging. J. Electrochem. Soc. 146, 2404-2412(1999). 35. Wang, H. E, Jang, Y. I. & Chiang, Y.-M. Origin of cycling stability inmonoclinic and orthorhombic-phase lithium manganese oxide cathodesElectrochem. Solid-State Lett. 2, 490-493(1999). 36. Kang,S. H., Goodenough, J. B. & Rabenberg, L. K. Effect of ball-milling on3 V capacity of lithium manganese oxospinel cathodes. Chem.Mater. 13,1758-1764(2001). 37. Reed,J, Ceder, G. & van der Ven, A. Layered-to-spinel phase transformation inLiMnO,. Electrochem. Solid-State Lett. 4,A78-81(2001). 38. Padhi,A. K., Nanjundaswamy, K. S., Masquelier, C., Okada,S. & Goodenough,J. B. Effect of structure on the Fet/Fe"tredox couple in iron phosphates.J. Electrochem. Soc. 144,1609-1613(1997). 39. Ravet, A. et al. Electroactivity of natural and synthetic triphylite. J. PowerSources 97-98,503-507(2001). 40. Huang, H., Yin, S.-C. & Nazar, L. F. Approaching theoretical capacity ofLiFePO,at room temperature and high rates. Electrochem. Solid-State Lett. 4,A170-172(2001). 41. Croce,E, Scrosati, B.,Sides, B. R. & Martin, C. R. in 206th MeetingElectrochemical Society, Honolulu, Hawaii Abstract 241(2004). 42. Scrosati. B. & Vincent, C. A. Polymer electrolytes: the key to lithium polymerbatteries. Mater. Res. Soc. Bull.25,28-30(2000). 43. Croce,E,Appetecchi,G. B., Persi, L. & Scrosati, B. Nanocomposite polymerelectrolytes for lithium batteries. Nature 394, 456-458(1998). 44. MacFarlane, D. R., Newman, P. J.,Nairn, K. M. & Forsyth,M.Lithium-ionconducting ceramic/polyether composites. Electrochim. Acta 43, 1333-1337(1998). ( 4 5. Kumar,B . , R o drig u es, S. J . & Scanlon, L . Ionic c onductivity of polymer- ) ceramic composites. J. Electrochem.Soc. 148,A1191-1195(2001) ( 46. Maier,J. Ionic conduction in space charge regions. P rog. S olid Stat e Chem. 2 3, 1 71- 2 63 (1995). ) ( 4 7.A p petecchi, G. B . , Croce,E, Pe r si, L., Ro n ci, F. & Sc r osati, B. Transport and inte rf acial properties of composite polymer electrolytes. Electrochim. A cta 4 5 ,1481- 1 490 ( 2 000). ) ( 4 8. A ngell, C. A. , Liu, C . & Sanchez,S . Rubbery solid electrolytes with dominant cationic transport and h igh a mbient conductivit y . Nature 362,13 7- 139(199 3 ). ) ( 4 9. Hawett, P . C . , MacFarlane,D. R. & Hollenkamp, A. F. High lithium metalc y cling efficiency in a room-temperature i o nic liquid. E le ct rochem. S olid- S tate L ett . 7, A 97-101 ( 2004) . ) ( 5 0 . MacGl a shan, G., Andreev, Y . G . &. B r uce, P . G. T he str u cture of poly(ethylene oxide):L i AsF . Nature 398, 792-794 ( 1999) . ) ( 5 1 . G adjourova, Z ., Andreev, Y. G. , T unstall D. P . & Bru c e, P. G . Ionic conductivity i n crystalline p olymer electrolytes. N a ture 412, 520-523(2001). ) ( 5 2. Stoeva,Z . ,Martin-Lita s , L. , Staunton,E.,And r eev Y. G . & B r uce, P. G . Ionicconductivity i n the crystalline pol y mer electrolytes PEO: L iXF,X = P,A s , Sb. J . A m. Chem. Soc . 125, 4 619-2626( 2 0 03). ) ( 5 3. Christie, A. M. , Lilley , S .J., Staunton , E,Andreev, Y . G. & Bruce, P. G . Increasing the conducti v ity of c r ystalline polymer electrolytes . Na t ure 433, 50-53 (2005). 5 4. Conway, B. E. Electrochemical Supercapacitors . (Kluwer Acade m ic/ P lenum, N e w York, 1999). ) ( 5 5. Mastragostino, M .,A r bizzani, C. & Soavi, E. In A dv a nces in Lithium-Ion Ba t teries . ( V an Schal k w i jk, W. & Scrosat i , B., eds ) Ch. 16,48 1 -50 5 ( Kluwe r Academic/Pl e num, New York, 2002). ) ( 56.Arbizzani, C. , Ma s tragostino,M . & Soavi, S . New trends in electrochemicalsupercapacitors. J. P o wer Sources 100, 164-170(2001) . ) ( 5 7. Wang,J. e t al. M orphological ef f ect s on the electrical and electrochemical p roperties of carbon aerogels. J . El e ctrochem . Soc. 1 4 8 ,D7 5- 77 (2001). ) ( 58 . N iu, C., Sichje l , E. K., Hoch, R., Hoi, D. & Tennent, H . High po w er ) ( electrochemica l c apa c itors b ased o n carbon n a notube electr o des. A p pl. P h ys. Lett . 70 , 148 0 - 1 482(1997). ) 59. Nelson, P. A. & Owen, J. R. A high-performance supercapacitor/battery hybridincorporating templated mesoporous electrodes. J. Electrochem. Soc. 150,A1313-1317(2003). ( 60. Amatucci,G. G., Badway, E , Du Pasquier, A. & Zheng , T . An asymmetric h ybrid nonaqueou s energy storage cell. J.Electrochem. Soc. 1 48,A 9 30-939 ( 2 001). ) ( 61. Brodd, R. J . et al. Batter i es 1977 to 2002 . J . Electrochem. So c . 151, K 1-1 1 ( 2004) . ) 62. Srinivasan, S.,Mosdale, R., Stevens, P. & Yang, C. Fuel cells: reaching the era ( o f clean a nd efficient p ower generation in the twenty-fir s t century. Annu. Rev. E nergy Env i ron. 2 4, 281 -2 38(1999). ) ( 63. Giordano, N. e t a l. Ana l ysis of p l atinum particle size and oxygen reduction i n p hosphoric acid. E le c trochim. A cta 36, 1979-1984 (1 991 ). ) ( 64. Freund, A. , Lang, J .,Lehman,T.& S tarz, K. A. Improved Pt alloy catalysts for f uel cells . Ca t al. Today 27,279 - 283(1996). ) ( 65. M ukerjee,S., Srinivasan, S., Soriaga, M. P. & Mc B ree n ,J. Role of structural a nd electronic properties of Pt and Pt al l oys on electrocatalysis o f oxygen r eduction. An in- s itu XANES and EXAFS investigation. J . Electro c hem. S oc. 1 42, 1 409- 1 422 (1995). ) ( 66. Arico,A. S., Srinivasan,S . & Antonucci, V . D MFC s : From fundame n tal asp e cts to technology development. F u el C ells 1, 1 33-1 6 1 (2 0 01) ) . ) ( 67. Schmidt,T.J. et al. E lectrocatalytic activity of Pt - Ru alloy colloids f or C O and C O/H, e l ectrooxidation: s t ripping voltammetry an d rotating-diskmeasurements. Langmuir 1 4 , 25 9 1-2595 ( 1 997)). ) ( 68. Chrzanowski, W. & Wieckowski, A. Enhancement in methanol oxida t ion by s pontaneously d e posited rutheni u m on low - ind e x platinum-electrodes. Cata l .Lett,50,69-75( 1 998) ) 69. Brankovic, S. R., Wang,J. X. & Adzic, R. R. Pt submonolayers on Ru nanoparticles-a novel low Pt loading, high CO tolerance fuel-cell . electrocatalyst. Electrochem. Solid-State Lett. 4,A217-220 (2001). 70.Hockaday, R. G. et al. in Proc. Fuel Cell Seminar, Portland, Oregon, USA 791-794 (Courtesy Associates, Washington DC, 2000). 71. Sun, G. R., Wang,J. T.& Savinell, R. F. Iron(III) tetramethoxyphenylporphyrin (Fetmpp) as methanol tolerant electrocatalyst for oxygen reduction in direct methanol fuel-cells. J. Appl. Electrochem.28, 1087-1093 (1998). 72. Reeve,R. W., Christensen, P. A., Hamnett, A., Haydock, S. A. & Roy, S. C. Methanol tolerant oxygen reduction catalysts based on transition metal sulfides.J. Electrochem. Soc. 145, 3463-3471(1998). 73. Nolte, R., Ledjeff, K., Bauer,M. & Mulhaupt, R. Partially sulfonated poly(arylene ether sulfone)-a versatile proton conducting membrane material for modern energy conversion technologies. J. Membrane Sci. 83, 211-220(1993) 74. Kerres, J., Ullrich, A., Meier, F.& Haring, T. Synthesis and characterization of novel acid-base polymer blends for application in membrane fuel cells. Solid State Ionics 125, 243-249 (1999). 75. Alberti, G. & Casciola, M. Composite membranes for medium-temperature PEM fuel cells. Annu. Rev. Mater.Res. 33,129-154(2003). 76. Savadogo,O. Emerging membranes for electrochemical systems:(I) solid polymer electrolyte membranes for fuel cell systems. J. New Mater. Electrochem. Syst. 1, 47-66 (1998). 77. Li, Q.,He, R., Jensen, J.O. & Bjerrum, N. J. Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100°C. Chem. Mater. 15,4896-4815(2003). 78. Kreuer,K. D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membrane Sci. 185, 29-39 (2001). 79. Watanabe, M., Uchida, H., Seki, Y., Emori, M. & Stonehart, P. Self-humidifying polymer electrolyte membranes for fuel cells. J. Electrochem. Soc. 143, 3847-3852(1996)). 80. Arico, A. S., Creti, P,Antonucci, P. L. & Antonucci, V. Comparison of ethanol and methanol oxidation in a liquid feed solid polymer electrolyte fuel cells at high temperature. Electrochem. Solid-State Lett. 1, 66-68(1998). 81. Arico, A. S., Baglio,V., Di Blasi, A. & Antonucci, V. FTIR spectroscopic investigation of inorganic fillers for composite DMFC membranes. Electrochem. Commun.5,862-866(2003). 82. Kreuer, K. D., Paddison,S. J, Spohr, E. &Schuster, M. Transport in proton conductors for fuel cell applications: simulation, elementary reactions and phenomenology. 104, 4637-4678 (2004). ( 8 3 . Kreuer, K. D. On the development of p roton conducting materials for t echnological applications. Solid State I onics 97, 1 -15 ( 1997). ) ( 84.Debe, M. K . Handbook of Fue l Cell s- Fundamen t als, TechnologyandApplica t ions V o l. 3 ( e ds Vi e l stich, W . , G asteiger, H. A . & L am m , A.) C h . 4 5 , 5 76-589 ( W iley, C h i ches t er, UK,2 0 03). ) 85. Atanasoski, R. in 4th Int. Conf. Applications of Conducting Polymers, ICCP-4, Como, Italy Abstract 22 (2004). 86.Schoonman, J. Nanoionics. Solid State Ionics 157,319-326(2003) ( 8 7. S teele, B. C . H. C u r rent status of interm e diate temperature fuel cells (It-SO F C s ). European F u el Ce l l News 7, 16- 1 9 ( 2 000) . ) ( 8 8. M aier , J . De f ect che m is t ry a nd ion transport i n n a nostructured materials. P art II Aspects of nanoionics. S olid State Ionics 1 57, 32 7 -334 ( 2 003 ) . ) ( 8 9.K n auth, P & Tuller, H. L . S o l id-state ion i c s : r o o t s , status, and future- prospects. J . Am . C eram. Soc.85, 1 654- 16 80(2 0 0 2). ) ( 9 0. P erry M urray, E., Tsai, T.& B arnett, S . A . A direct-methane fuel ce l l with aceria-based anode. Nature 400, 649-65 1 (1999). ) ( 9 1. Park, S., Vohs , J . M . & Gorte, R . J. Direct oxidatio n o f hydrocarbons in a s olid- o xide f uel cell. Nature 404, 265- 2 67(2000). ) ( 9 2 . Z hou, Y. P, Fen g , K . , Sun, Y.& Zhou, L. A b r ief review on t he study of hydrogen storage in terms of c arbon nanotubes. P r og. C h em. 15 , 345- 3 50( 2 003). ) ( 9 3 . Schlappac h , L . & Z u ttel, A. H ydrogen-storage m a terials f o r m obileapplications. Nature 414,3 5 3-35 8 (2001). ) 94. Dillon A. C. et al. Storage of hydrogen in single-walled carbon nanotubes.Nature 386,377-379(1997). ( 95 . Chambers, A. , P ark, C., Baker, R. T. K . & Rodriguez, N. M. H yd r ogenstorage i n g raphite n anofibers. J. P hys. Chem.102, 4253-4256 (199 8 ) . ) 96. Yang, R. T. Hydrogen storage by alkali-doped carbon nanotubes-revisited.Carbon 38, 623-626(2000). ( 97. Dil l on , A. C.& H eben, M. J. Hydrogen Storage us i ng carbon adsorbents p as t , present and future. Appl . Phys . A 72 , 133- 1 42 ( 2001). ) ( 9 8. Hirsc h er, M. & B echer, M. H ydrogen storage in carbon nanotubes. J. Nanosci. Nanotechnol.3,3-17( 20 03). ) ( 9 9. Sha n g, C. X., B ououdina, M. , Song, Y. & Gu o , Z. X. Mechanical alloyingand e lec t ronic simulation of MgH,- M systems (M-Al , Ti, Fe, Ni, Cu, and Nb) for h ydrogen storage. Int. J.Hydrogen Ene r gy 29, 73 - 8 0 (2004) . ) ( 1 00. B obet, J. L . , Grigorova,E. , K hrussanova, M. , Khristov, M. & Peshev, P. H ydrogen sorption p roper t ies o f the nanocomposite 90wt . % MgNi-10 w t.% V. J . A llo ys Comp o unds 356, 593 - 597 ( 2003). ) ( 1 01. Ma, R. Z . e t al. Synthesis of boron- n itride nanofibers and m e asurement o f t heir hydrogen uptake ca p acity. Ap p l. Phys. Let t . 81,52 2 5-5227 ( 2 002 ) . ) ( 102 . Bobet, J . L . , Desmoulinskrawiec,S., Grigorova, E. , C a ns e ll, F. & ) ( Chevalier, B. Addition o f nanosiz e d Cr, O, to magnesium f or improvement o f the hydrogen s orption p roperties. J . Alloy s Compounds 351, 217-221(2003). ) ( 1 03. Audemer, A., Wurm,C. , Mo rcrette, M., Gwizdala, S.& Masquellier,C.Carbon-c o ated m e tal b e aring powders and pr o cess for production thereof. World Patent WO 04/001881 A 2 ( 2 004). ) ature materials| VOL AY www.nature.com/naturematerialsCNature Publishing Group
确定







还剩10页未读,是否继续阅读?
武汉科思特仪器股份有限公司为您提供《Nanostructured materials for advanced energy conversion and storage devices》,该方案主要用于其他中--检测,参考标准--,《Nanostructured materials for advanced energy conversion and storage devices》用到的仪器有CS2350H双单元电化学工作站(双恒电位仪)、CS350M电化学工作站/电化学测试系统
相关方案
更多
该厂商其他方案
更多











