方案详情
文
锂电池鼻祖J.-M. Tarascon的杰作——《Nanomaterials for Rechargeable Lithium Batteries》
方案详情

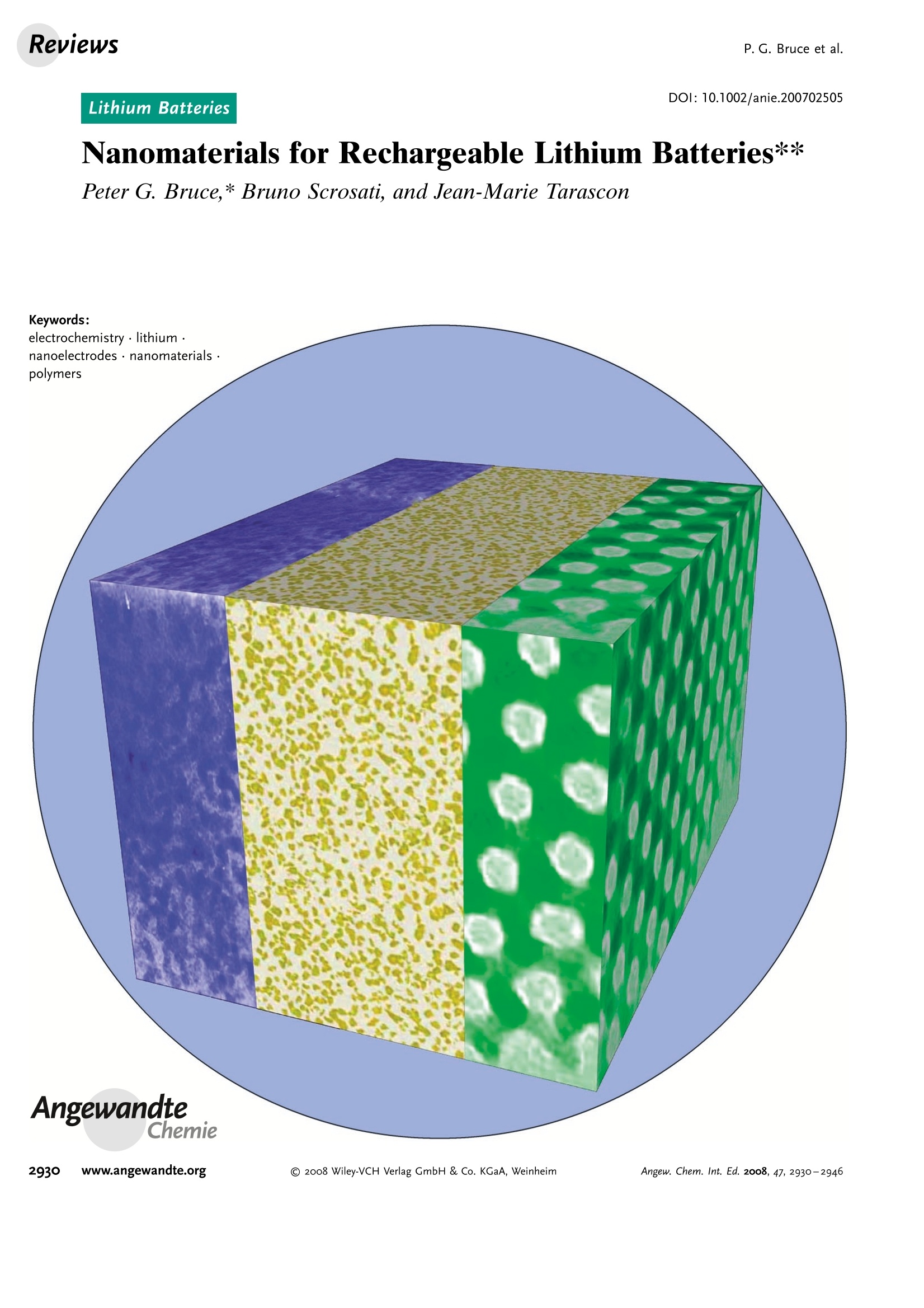
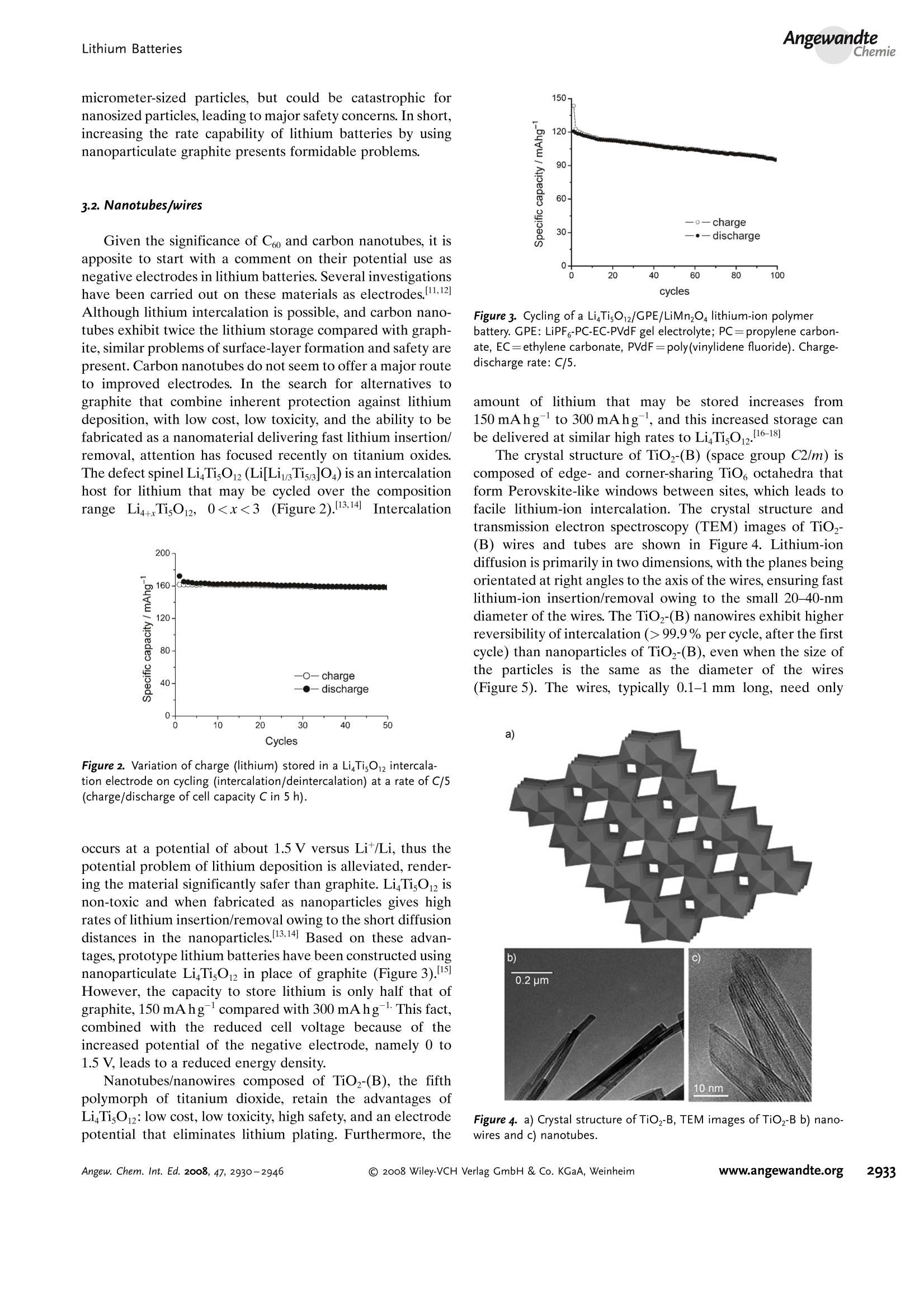
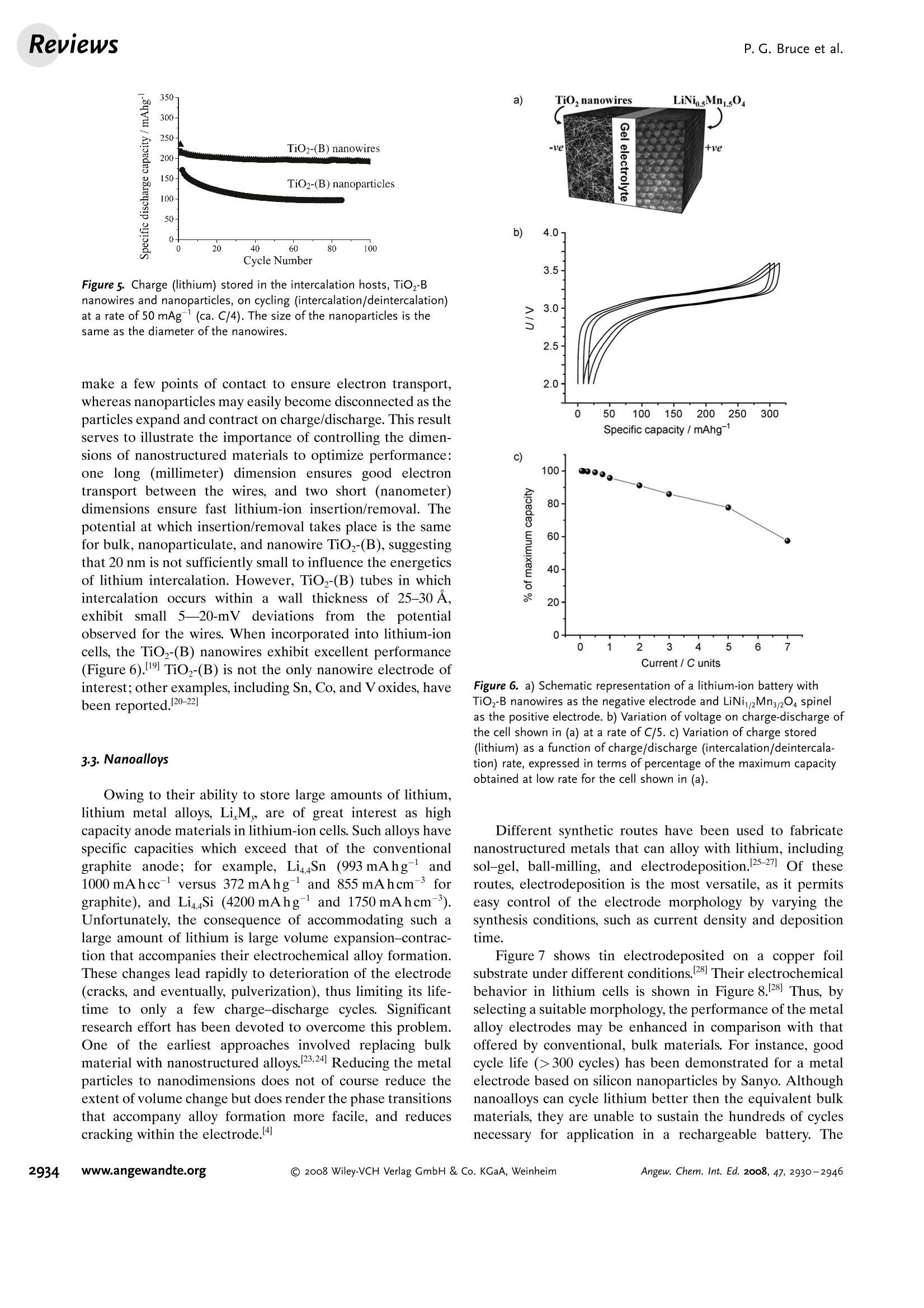
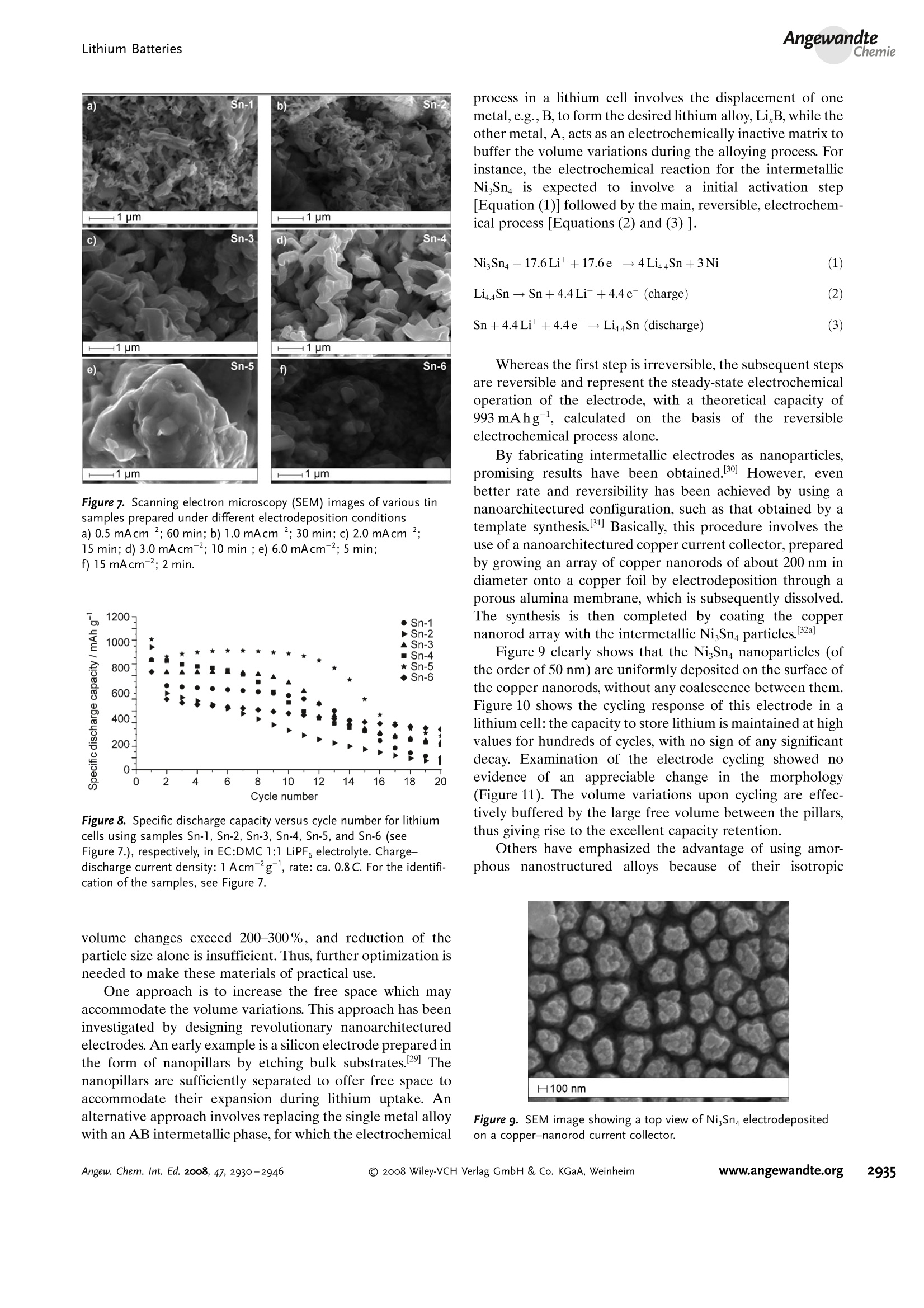
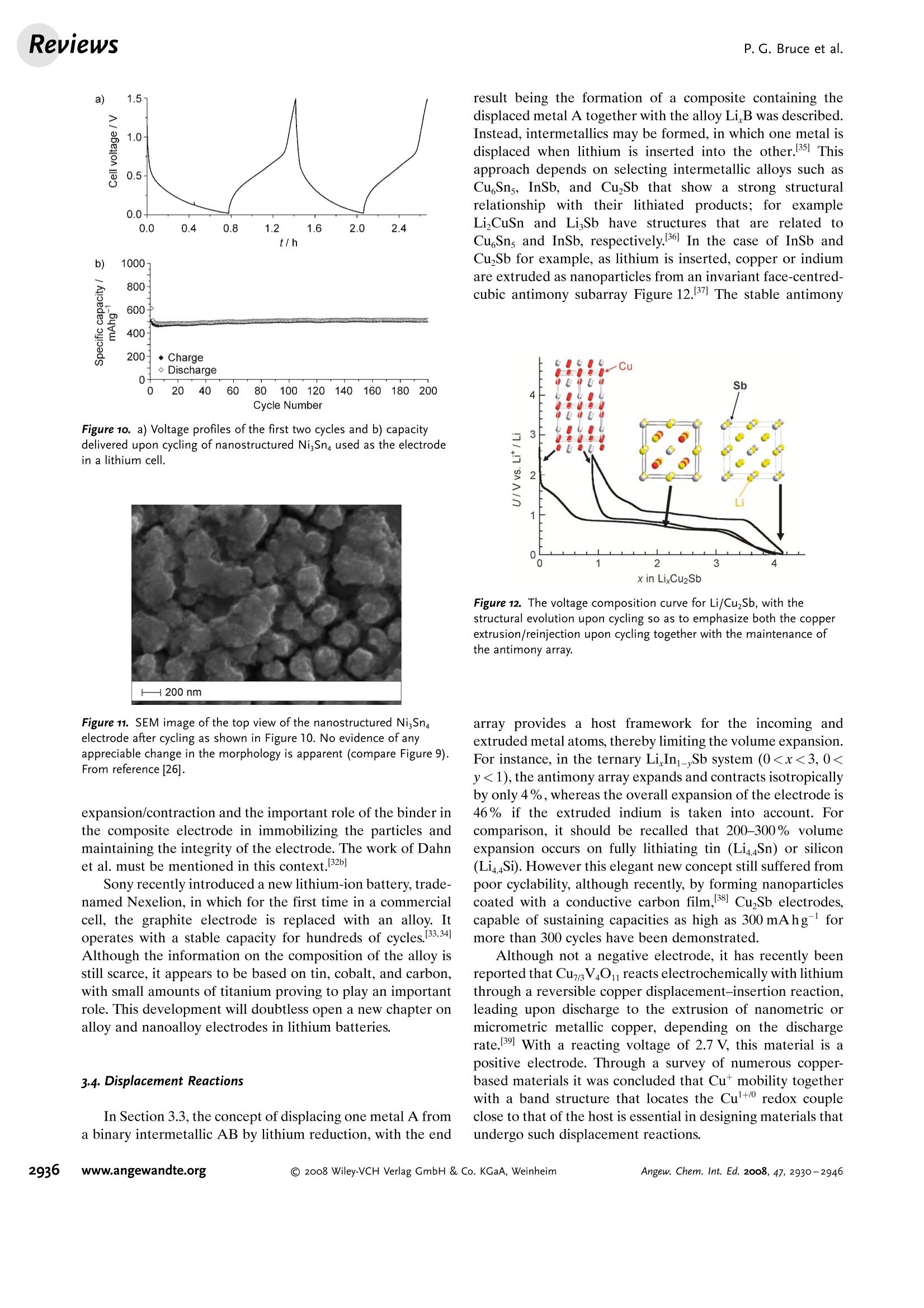
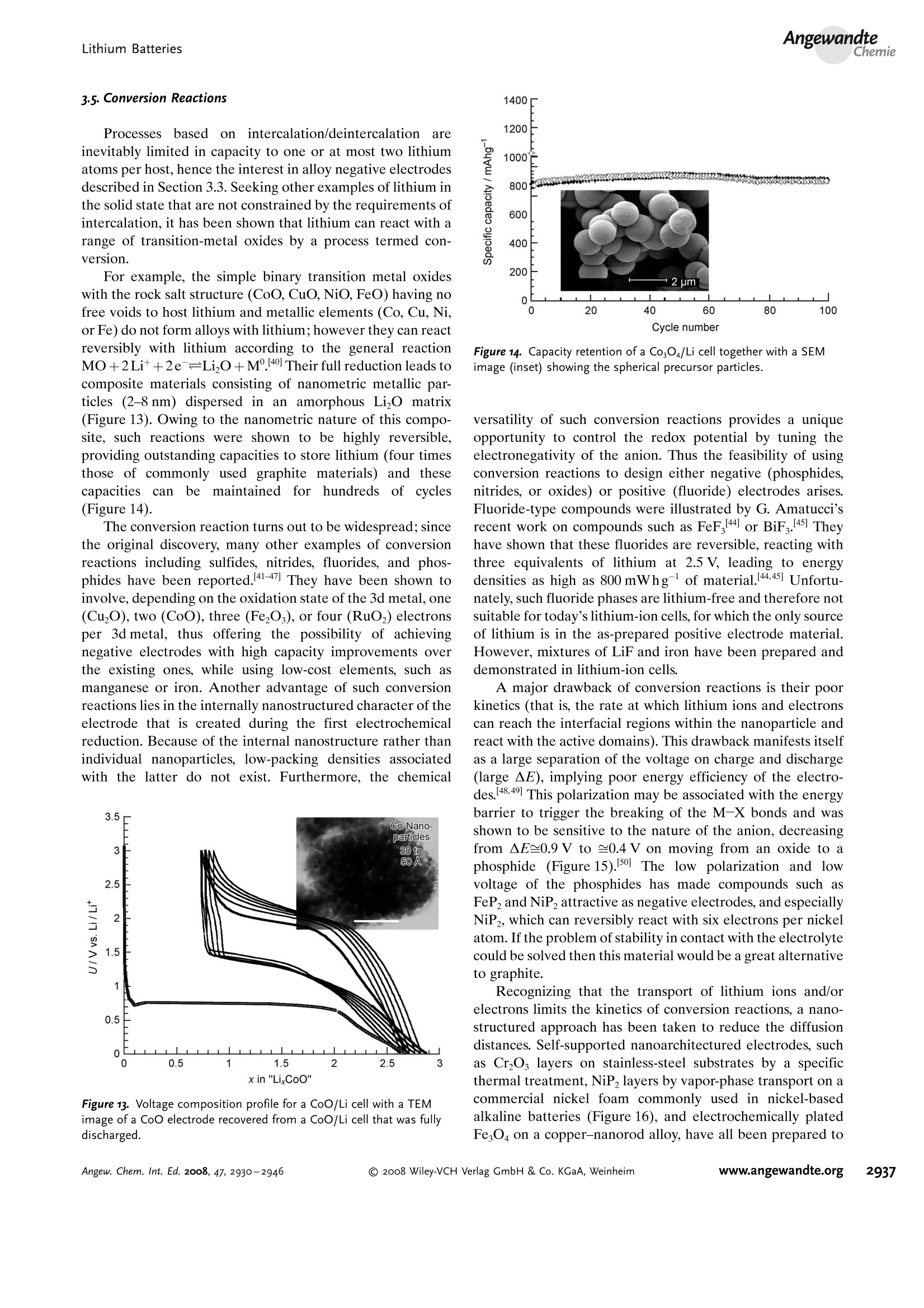
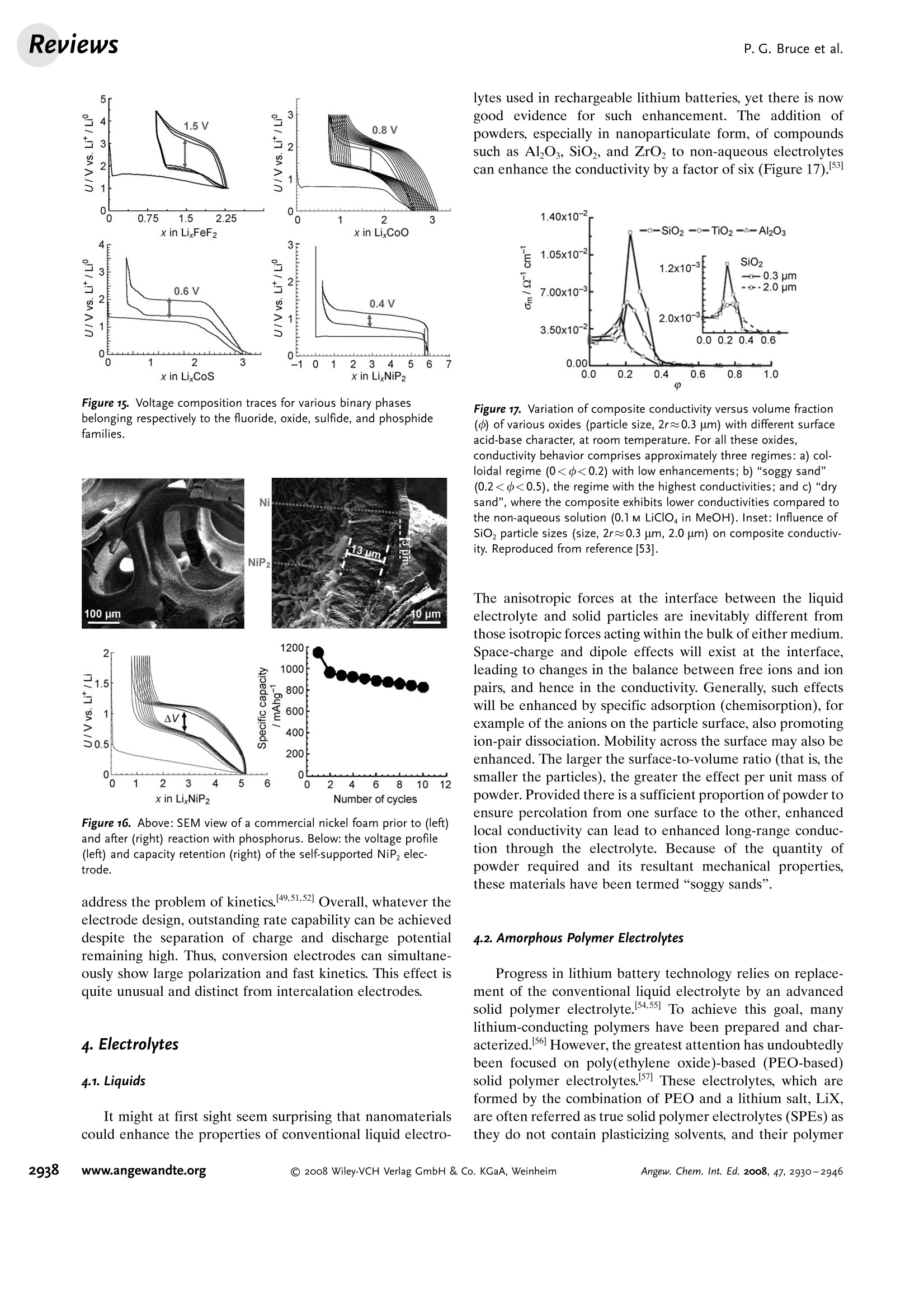
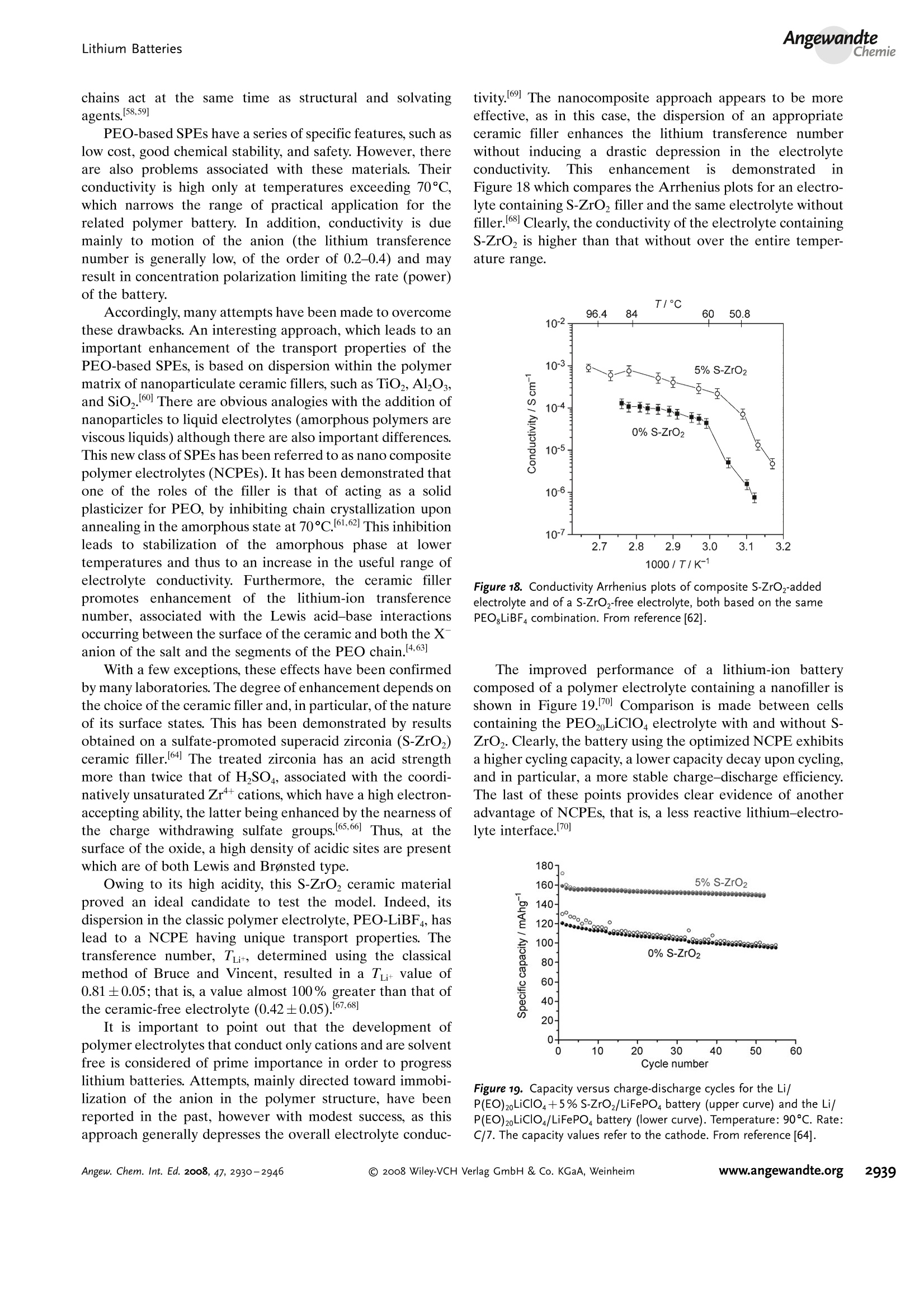
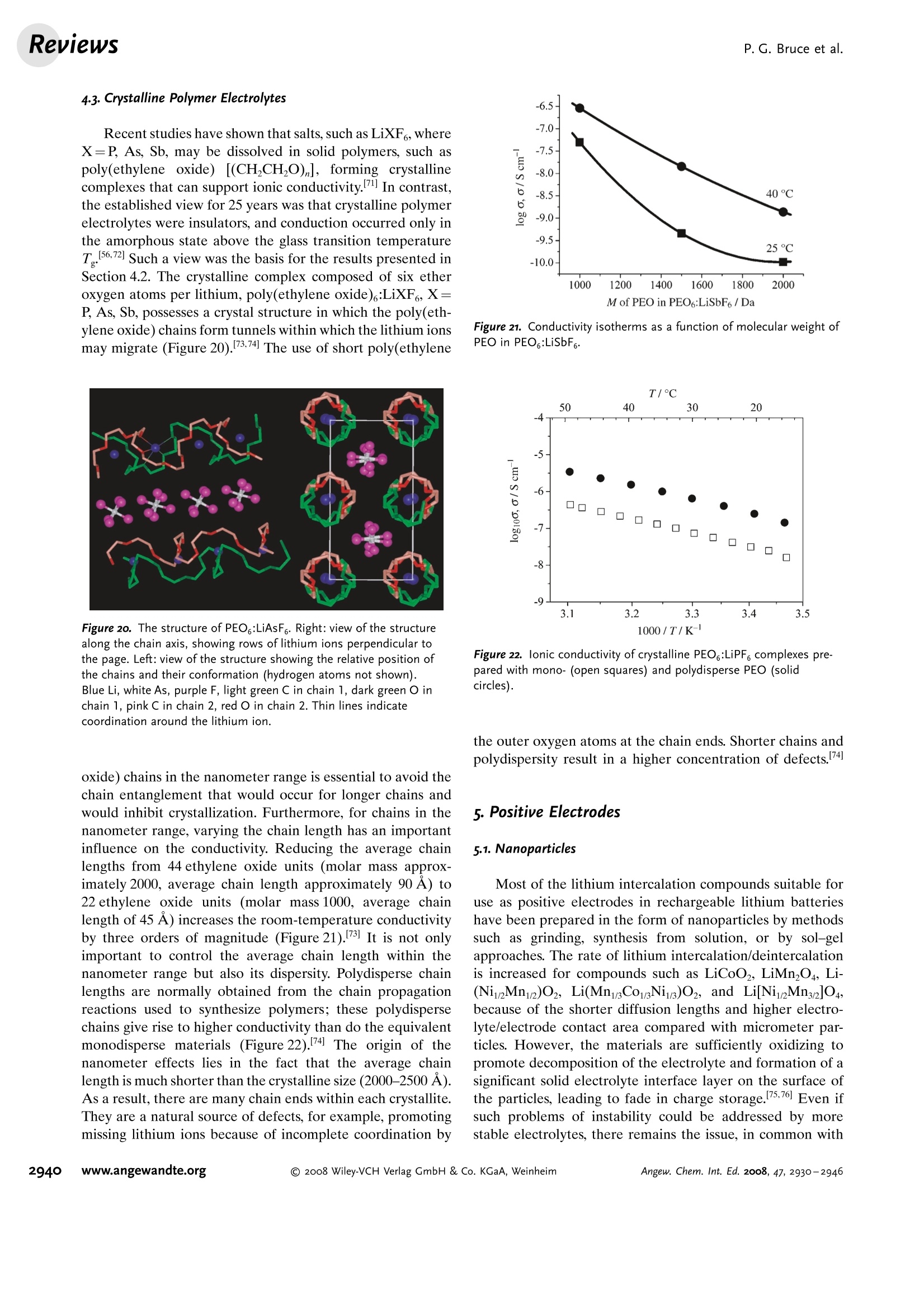
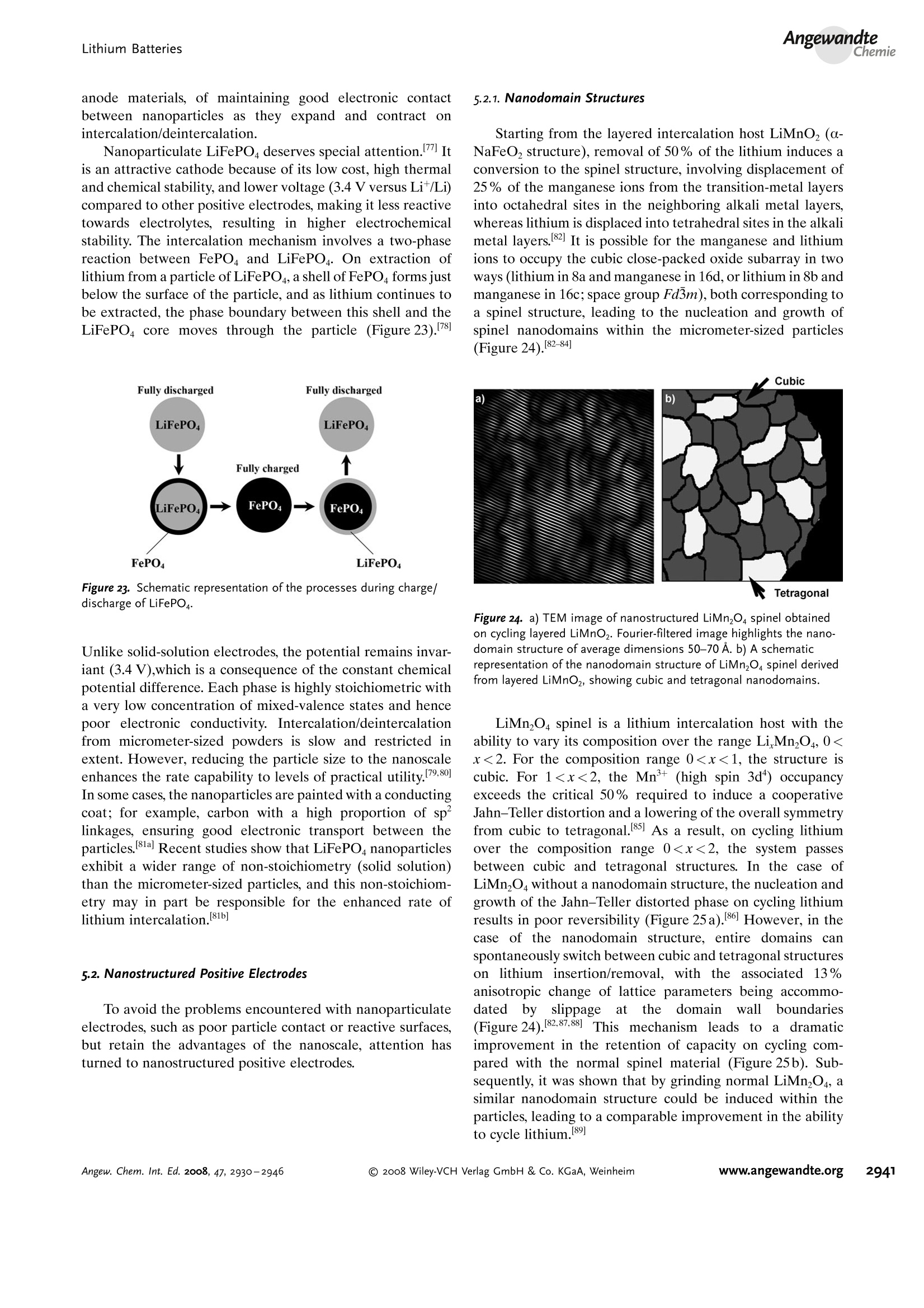
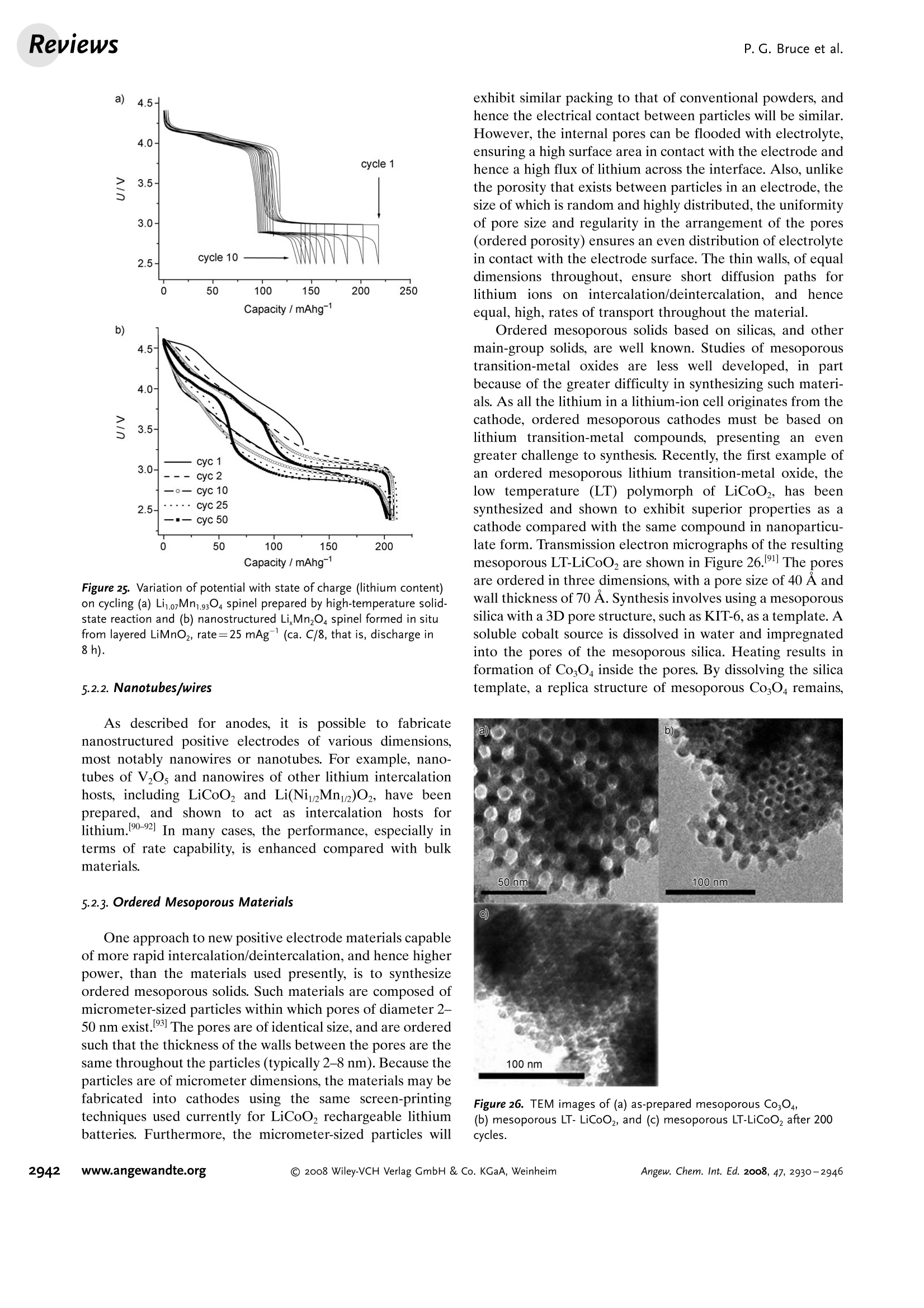
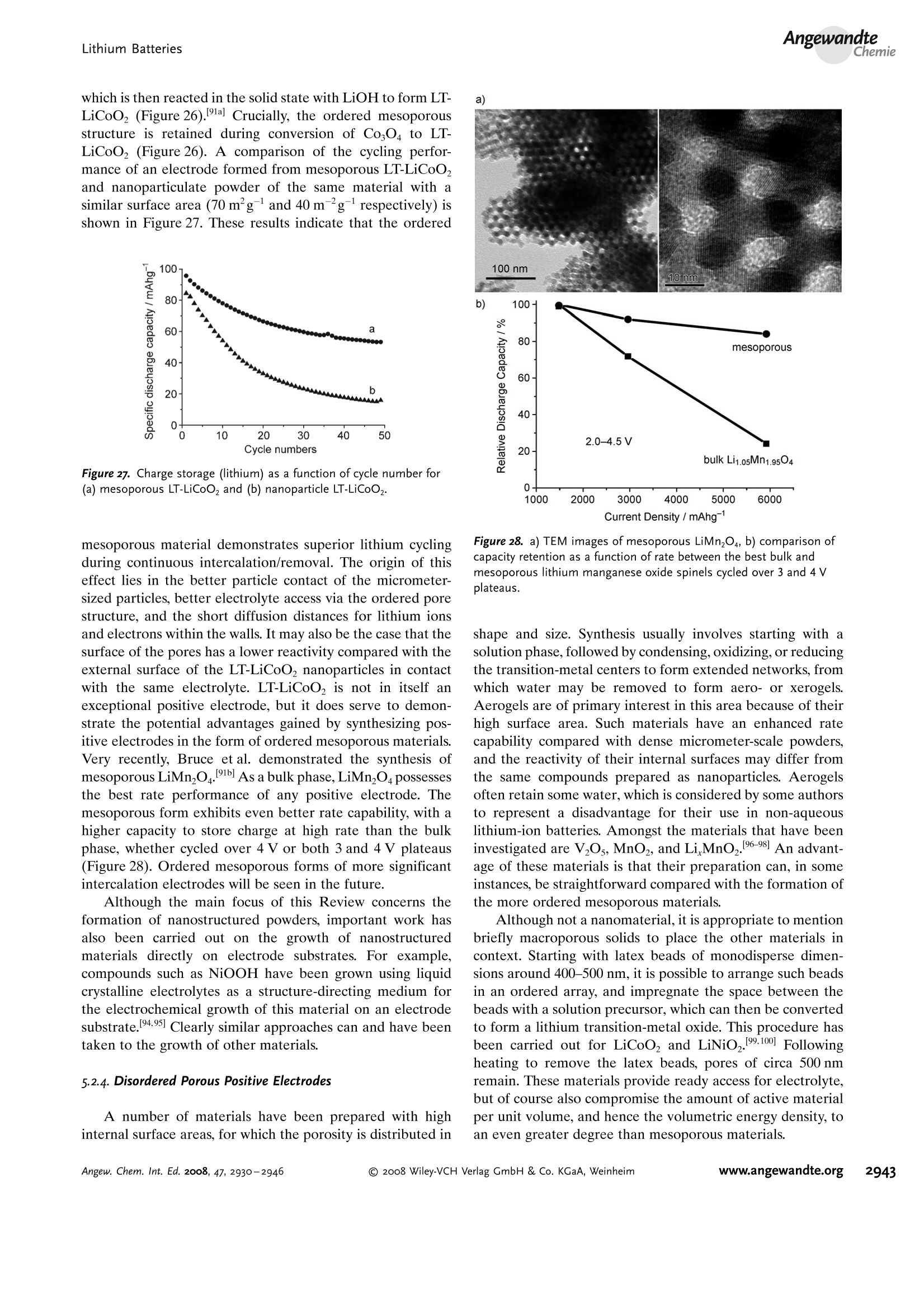
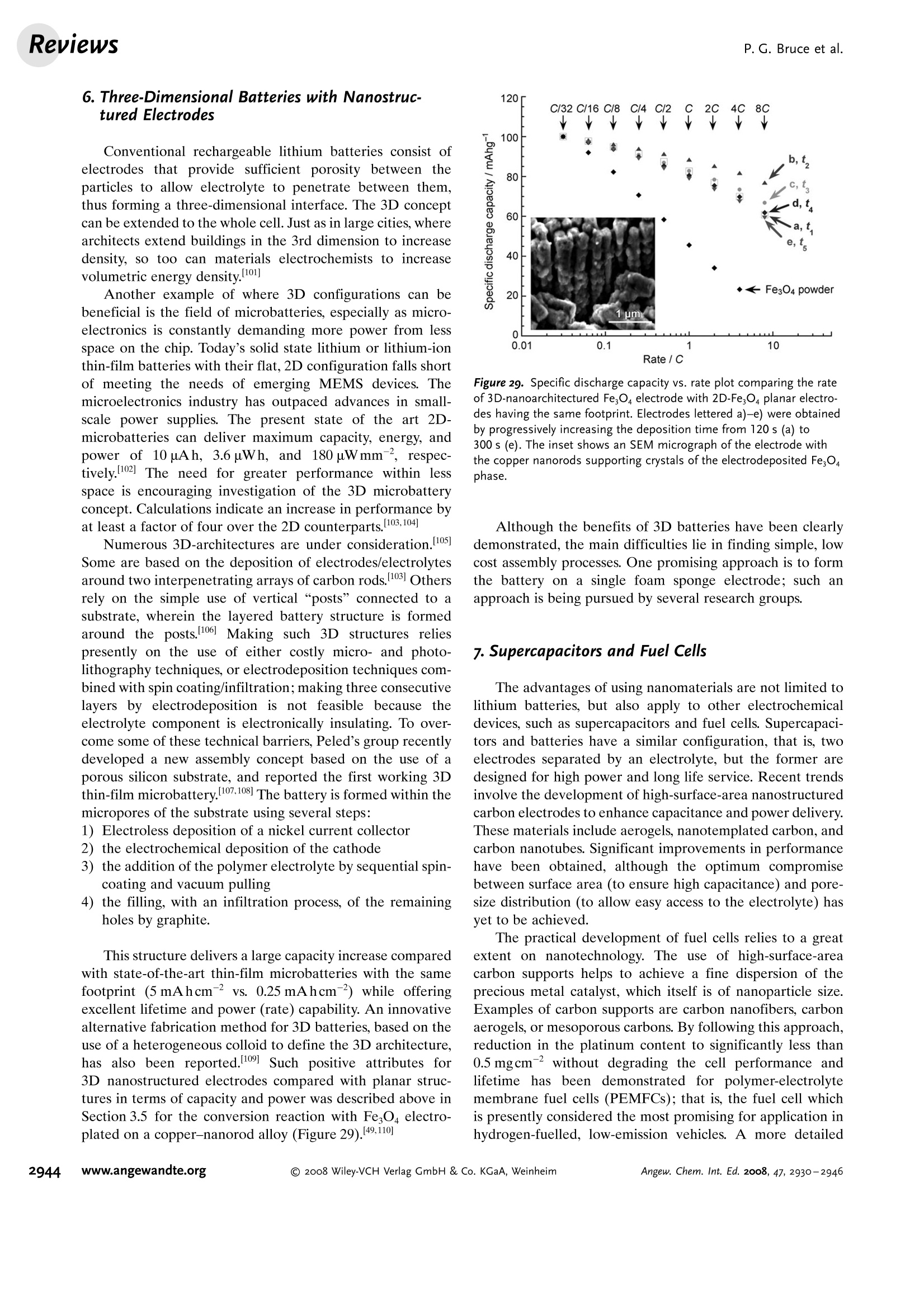
ReviewsP. G. Bruce et al.DOI: 10.1002/anie.200702505 AngewandteLithium BatteriesChemie Lithium Batteries Nanomaterials for Rechargeable Lithium Batteries** Peter G. Bruce,* Bruno Scrosati, and Jean-Marie Tarascon Keywords: electrochemistry. lithium · nanoelectrodes· nanomaterials . Energy storage is more important today than at any time in humanhistory. Future generations ofrechargeable lithium batteries arerequired to power portable electronic devices (cellphones, laptopcomputers etc.), store electricity from renewable sources, and as a vitalcomponent in new hybrid electric vehicles. To achieve the increase inenergy and power density essential to meet the future challenges ofenergy storage, new materials chemistry, and especially new nano-materials chemistry, is essential. We must find ways of synthesizingnew nanomaterials with new properties or combinations ofproperties,for use as electrodes and electrolytes in lithium batteries. Herein wereview some ofthe recent scientific advances in nanomaterials, andespecially in nanostructured materials, for rechargeable lithium-ionbatteries. From the Contents 2. Advantages and Disadvantagesof Nanomaterials for LithiumBatteries 2932 3. Negative Electrodes 2932 6. Three-Dimensional Batterieswith Nanostructured Electrodes 2944 7.Supercapacitors and Fuel Cells 2944 8. Summary and Outlook 2945 The storage ofelectrical energy will be far more importantin this century than it was in the last. Whether to power themyriad portable consumer electronic devices (cell phones,PDAs, laptops, or for implantable medical applications, suchas artificial hearts, or to address global warming (hybridelectric vehicles, storage of wind/solar power), the need forclean and efficient energy storage will be vast. Nanomaterialshave a critical role to play in achieving this change in the waywe store energy. Rechargeable lithium batteries have revolutionized port-able electronic devices. They have become the dominantpower source for cell phones, digital cameras, laptops etc.,because of their superior energy density (capability to store 2-3 times the energy per unit weight and volume compared withconventional rechargeable batteries). The worldwide marketfor rechargeable lithium batteries is now valued at 10 billiondollars per annum and growing. They are the technology ofchoice for future hybrid electric vehicles, which are central tothe reduction of CO, emissions arising from transportation. The rechargeable lithium battery does not contain lithiummetal. It is a lithium-ion device, comprising a graphitenegative electrode (anode), a non-aqueous liquid electrolyte,and a positive electrode (cathode) formed from layeredLiCoO (Figure 1). On charging, lithium ions are deinterca- GraphiteLit conducting electrolyteLiCoo Figure 1. Schematic representation of a lithium-ion battery. Negativeelectrode (graphite), positive electrode (LiCoO,), separated by a non-aqueous liquid electrolyte. lated from the layered LiCoOintercalation host, pass acrossthe electrolyte, and are intercalated between the graphitelayers in the anode. Discharge reverses this process. Theelectrons, of course, pass around the external circuit. Therechargeable lithium battery is a supreme representation ofsolid-state chemistry in action. A more detailed account oflithium-ion batteries than is appropriate here may beobtained from the literature.1-3] The first-generation lithium-ion battery has electrodesthat are composed of powders containing millimeter-sizedparticles, and the electrolyte is trapped within the millimeter-sized pores of a polypropylene separator. Although thebattery has a high energy density, it is a low-power device(slow charge/discharge). No matter how creative we are indesigning new lithium intercalation hosts with higher rates,limits exist because of the intrinsic diffusivity of the lithiumion in the solid state (ca. 10-8cm’s-), which inevitably limitsthe rate of intercalation/deintercalation,and hence charge/discharge. However, an increase in the charge/discharge rateof lithium-ion batteries of more than one order of magnitudeis required to meet the future demands of hybrid electricvehicles and clean energy storage. Nanomaterials, so often [**] Thanks to Dr. Aurelie Debart for preparation of the frontispiece. hyped or misrepresented by claims of delivering new proper-ties, have the genuine potential to make a significant impacton the performance of lithium-ion batteries, as their reduceddimensions enable far higher intercalation/deintercalationrates and hence high power. This is just one property that maybe enhanced by the use of nanomaterials. However, nano-materials are certainly not a panacea. The advantages anddisadvantages of lithium-ion battery materials are summar-ized in Section 2, and thereafter advances in the use ofnanomaterials, emphasizing in particular nanostructuredmaterials, as negative electrodes, electrolytes, and positiveelectrodes for rechargeable lithium batteries are described.4The illustrative examples that are presented are mainly fromthe work of the authors. 2. Advantages and Disadvantages of Nanomaterialsfor Lithium BatteriesAdvantages 1.T1hey enable electrode reactions to occur that cannot takeplace for materials composed of micrometer-sized parti- Peter Bruce is Professor of Chemistry at theUniversity of St Andrews, Scotland. Hisresearch interests embrace the synthesis andcharacterization of materials (extendedarrays and polymers) with new properties orcombinations of properties, and in particularmaterials for new generations ofenergyconversion and storage devices. He hasreceived a number ofawards and fellow-ships, and is a fellow of the Royal Society. Bruno Scrosati is Professor of Electrochemis-try at the University of Rome. He has beenpresident ofthe International Society ofSolid State lonics, the Italian ChemicalSociety, and the Electrochemical Society,and is fellow ofthe Electrochemical Society(ECS) and of the International Society ofElectrochemistry (ISE). He has a "honoriscausa" (honorary DSc) from the Universityof St. Andrews in Scotland. He won the XVIEdition ofthe Italgas Prize, Science andEnvironment. He is European editor of thejournal of Power Sources and member ofthe editorial boards of various internationaljournals. jean-Marie Tarascon is Professor at the Uni- versity of Picardie (Amiens). He developstechniques for the synthesis ofelectronicmaterials (superconductors, ferroelectrics,fluoride glasses, and rechargeable batteries)for new solid-state electronic devices. Heplayed a pivotal role in the development ofathin and flexile plastic lithium-ion batterythat is presently being commercially devel-oped. He is investigating new lithium reac-tivity concepts, and electrodes for the nextgeneration of lithium-ion batteries. He is thefounder ofALISTORE. cles; for example, reversible lithium intercalation intomesoporous β-MnO2 without destruction of the rutilestructure.s] 2.The reduced dimensions increases significantly the rate oflithium insertion/removal, because of the short distancesfor lithium-ion transport within the particles. The charac-teristic time constant for diffusion is given by t=LD,where L is the diffusion length and D the diffusionconstant. The time t for intercalation decreases with thesquare of the particle size on replacing micrometer withnanometer particles.4 3. Electron transport within the particles is also enhanced bynanometer-sized particles, as described for lithium ions.4 4.A high surface area permits a high contact area with theelectrolyte and hence a high lithium-ion flux across theinterface. 5. For very small particles, the chemical potentials for lithiumions and electrons may be modified, resulting in a changeof electrode potential (thermodynamics of the reaction). 6. The range of composition over which solid solutions existis often more extensive for nanoparticles,and the strainassociated with intercalation is often better accommo-dated. Disadvantages 1. Nanoparticles may be more difficult to synthesize andtheir dimensions may be difficult to control. 2. High electrolyte/electrode surface area may lead to moresignificant side reactions with the electrolyte, and moredifficulty maintaining interparticle contact. 3. The density of a nanopowder is generally less than thesame material formed from micrometer-sized particles.The volume of the electrode increases for the same mass ofmaterial thus reducing the volumetric energy density. 3. Negative Electrodes 3.1. Nanoparticles Graphite powder, composed of micrometer-sized parti-cles, has been the stalwart of negative electrodes forrechargeable lithium batteries for many years. 1.2 Replace-ment by nanoparticulate graphite would increase the rate oflithium insertion/removal and thus the rate (power) of thebattery. Lithium is inserted into graphite at a potential of lessthan 1 V versus Lit/Li. At such low potentials, reduction ofthe electrolyte occurs, accompanied by the formation of apassivating (solid electrolyte interface) layer on the graphitesurface.[8-10 The formation of such a layer is essential for theoperation of graphite electrodes, as it inhibits exfoliation. Theseverity of layer formation would, in the case of high-surface-area nanoparticulate graphite, result in the consumption ofexcessive charge, which would then be lost to the cell. Of evengreater importance is the fact that most of the lithium isintercalated into graphite at potentials of less than 100 mVversus Lit/Li; were it not for careful electronic control ofcharging, lithium could deposit on the graphite surface. Thedeposition of highly reactive lithium would be serious for micrometer-sized particles, but could be catastrophic fornanosized particles, leading to major safety concerns. In short,increasing the rate capability of lithium batteries by usingnanoparticulate graphite presents formidable problems. 3.2. Nanotubes/wires Given the significance of C60 and carbon nanotubes, it isapposite to start with a comment on their potential use asnegative electrodes in lithium batteries. Several investigationshave been carried out on these materials as electrodes.l11,12]Although lithium intercalation is possible, and carbon nano-tubes exhibit twice the lithium storage compared with graph-ite, similar problems of surface-layer formation and safety arepresent. Carbon nanotubes do not seem to offer a major routeto improved electrodes. In the search for alternatives tographite that combine inherent protection against lithiumdeposition, with low cost, low toxicity, and the ability to befabricated as a nanomaterial delivering fast lithium insertion/removal, attention has focused recently on titanium oxides.The defect spinel Li Ti,O12(Li[Li13Ti5/3]O4) is an intercalationhost for lithium that may be cycled over the compositionrange Li4+xTisO12, 099.9% per cycle, after the firstcycle) than nanoparticles of TiO-(B), even when the size ofthe particles is the same as the diameter of the wires(Figure 5). The wires, typically 0.1-1 mm long, need only Figure 4.a) Crystal structure of TiOz-B,TEM images ofTiOz-B b) nano-wires and c) nanotubes. Figure 5. Charge (lithium) stored in the intercalation hosts, TiOz-Bnanowires and nanoparticles, on cycling (intercalation/deintercalation)at a rate of 50mAg(ca. C/4). The size of the nanoparticles is thesame as the diameter of the nanowires. make a few points of contact to ensure electron transport,whereas nanoparticles may easily become disconnected as theparticles expand and contract on charge/discharge. This resultserves to illustrate the importance of controlling the dimen-sions of nanostructured materials to optimize performance:one long (millimeter) dimension ensures good electrontransport between the wires, and two short (nanometer)dimensions ensure fast lithium-ion insertion/removal. Thepotential at which insertion/removal takes place is the samefor bulk, nanoparticulate, and nanowire TiOz-(B), suggestingthat 20 nm is not sufficiently small to influence the energeticsof lithium intercalation. However, TiO2-(B) tubes in whichintercalation occurs within a wall thickness of 25-30 A.exhibit small 5—20-mV deviations from the potentialobserved for the wires. When incorporated into lithium-ioncells, the TiO-(B) nanowires exhibit excellent performance(Figure 6).19 TiO-(B) is not the only nanowire electrode ofinterest; other examples, including Sn, Co, and V oxides,havebeen reported.20-22 3.3. Nanoalloys Owing to their ability to store large amounts of lithium,lithium metal alloys, LiM,, are of great interest as highcapacity anode materials in lithium-ion cells. Such alloys havespecific capacities which exceed that of the conventionalgraphite anode; for example, Lin4Sn (993 mAhgand1000 mAhcc- versus 372 mAhg-and 855 mAhcm- forgraphite), and Li44Si (4200 mAhg-and 1750 mAhcm).Unfortunately, the consequence of accommodating such alarge amount of lithium is large volume expansion-contrac-tion that accompanies their electrochemical alloy formation.These changes lead rapidly to deterioration of the electrode(cracks, and eventually, pulverization), thus limiting its life-time to only a few charge-discharge cycles. Significantresearch effort has been devoted to overcome this problem.One of the earliest approaches involved replacing bulkmaterial with nanostructured alloys.23.24 Reducing the metalparticles to nanodimensions does not of course reduce theextent of volume change but does render the phase transitionsthat accompany alloy formation more facile, and reducescracking within the electrode.4 a) Figure 6. a) Schematic representation of a lithium-ion battery withTiOz-B nanowires as the negative electrode and LiNi2Mn3204 spinelas the positive electrode. b) Variation of voltage on charge-discharge ofthe cell shown in (a) at a rate of C/5.c) Variation of charge stored(lithium) as a function of charge/discharge (intercalation/deintercala-tion) rate, expressed in terms of percentage of the maximum capacityobtained at low rate for the cell shown in (a). Different synthetic routes have been used to fabricatenanostructured metals that can alloy with lithium, includingsol-gel, ball-milling, and electrodeposition.25-27 Of theseroutes, electrodeposition is the most versatile, as it permitseasy control of the electrode morphology by varying thesynthesis conditions, such as current density and depositiontime. Figure 7 shows tin electrodeposited on a copper foilsubstrate under different conditions.[28] Their electrochemicalbehavior in lithium cells is shown in Figure 8.128 Thus, byselecting a suitable morphology, the performance of the metalalloy electrodes may be enhanced in comparison with thatoffered by conventional, bulk materials. For instance, goodcycle life (>300 cycles) has been demonstrated for a metalelectrode based on silicon nanoparticles by Sanyo. Althoughnanoalloys can cycle lithium better then the equivalent bulkmaterials, they are unable to sustain the hundreds of cyclesnecessary for application in a rechargeable battery. The Figure 7. Scanning electron microscopy (SEM) images of various tinsamples prepared under different electrodeposition conditionsa) 0.5 mAcm-2; 60 min; b) 1.0 mAcm-2; 30 min; c) 2.0 mAcm-;15 min; d) 3.0 mAcm-2;10 min; e) 6.0 mAcm-;5 min; f) 15 mAcm-; 2 min. Figure 8. Specific discharge capacity versus cycle number for lithiumcells using samples Sn-1, Sn-2, Sn-3,Sn-4, Sn-5, and Sn-6 (seeFigure 7.), respectively, in EC:DMC 1:1 LiPF,electrolyte. Charge-discharge current density:1Acm’g, rate:ca. 0.8C. For the identifi-cation of the samples, see Figure 7. volume changes exceed 200-300%, and reduction of theparticle size alone is insufficient. Thus, further optimization isneeded to make these materials of practical use. One approach is to increase the free space which mayaccommodate the volume variations. This approach has beeninvestigated by designing revolutionary nanoarchitecturedelectrodes. An early example is a silicon electrode prepared inthe form of nanopillars by etching bulk substrates.29 Thenanopillars are sufficiently separated to offer free space toaccommodate their expansion during lithium uptake. Analternative approach involves replacing the single metal alloywith an AB intermetallic phase, for which the electrochemical process in a lithium cell involves the displacement of onemetal,e.g., B, to form the desired lithium alloy, LiB, while theother metal, A, acts as an electrochemically inactive matrix tobuffer the volume variations during the alloying process. Forinstance, the electrochemical reaction for the intermetallicNigSn is expected to involve a initial activation step[Equation (1)] followed by the main, reversible, electrochem-ical process [Equations (2) and (3)]. Whereas the first step is irreversible, the subsequent stepsare reversible and represent the steady-state electrochemicaloperation of the electrode, with a theoretical capacity of993 mAhg, calculated on the basis of the reversibleelectrochemical process alone. By fabricating intermetallic electrodes as nanoparticles,promising results have been obtained.30] However, evenbetter rate and reversibility has been achieved by using ananoarchitectured configuration, such as that obtained by atemplate synthesis. 31 Basically, this procedure involves theuse of a nanoarchitectured copper current collector, preparedby growing an array of copper nanorods of about 200 nm indiameter onto a copper foil by electrodeposition through aporous alumina membrane, which is subsequently dissolved.The synthesis is then completed by coating the coppernanorod array with the intermetallic Ni,Sna particles.[32a] Figure 9 clearly shows that the NigSn nanoparticles (ofthe order of 50 nm) are uniformly deposited on the surface ofthe copper nanorods, without any coalescence between them.Figure 10 shows the cycling response of this electrode in alithium cell: the capacity to store lithium is maintained at highvalues for hundreds of cycles, with no sign of any significantdecay. Examination of the electrode cycling showed noevidence of an appreciable change in the morphology(Figure 11). The volume variations upon cycling are effec-tively buffered by the large free volume between the pillars,thus giving rise to the excellent capacity retention. Others have emphasized the advantage of using amor-phous nanostructured alloys becausee(of their isotropic Figure g. SEM image showing a top view of Ni,Sng electrodepositedon a copper-nanorod current collector. Figure 10. a) Voltage profiles of the first two cycles and b) capacitydelivered upon cycling of nanostructured Ni,Sna used as the electrodein a lithium cell. Figure 11. SEM image of the top view of the nanostructured Ni,Snaelectrode after cycling as shown in Figure 10. No evidence of anyappreciable change in the morphology is apparent (compare Figure 9).From reference [26]. expansion/contraction and the important role of the binder inthe composite electrode in immobilizing the particles andmaintaining the integrity of the electrode. The work of Dahnet al. must be mentioned in this context.32b] Sony recently introduced a new lithium-ion battery, trade-named Nexelion, in which for the first time in a commercialcell, the graphite electrode is replaced with an alloy. Itoperates with a stable capacity for hundreds of cycles.33,34]Although the information on the composition of the alloy isstill scarce, it appears to be based on tin, cobalt, and carbon,with small amounts of titanium proving to play an importantrole. This development will doubtless open a new chapter onalloy and nanoalloy electrodes in lithium batteries. 3·4.Displacement Reactions In Section 3.3, the concept of displacing one metal A froma binary intermetallic AB by lithium reduction, with the end result being the formation of a composite containing thedisplaced metal A together with the alloy Li,B was described.Instead, intermetallics may be formed, in which one metal isdisplaced when lithium is inserted into the other.[35] Thisapproach depends on selecting intermetallic alloys such asCugSns, InSb, and CuSb that show a strong structuralrelationship with their lithiated products; for exampleLi,CuSn and Li,Sb have structures that are related toCuSns and InSb, respectively.36] In the case of InSb andCuSb for example, as lithium is inserted, copper or indiumare extruded as nanoparticles from an invariant face-centred-cubic antimony subarray Figure 12.37 The stable antimony Figure 12. The voltage composition curve for Li/CuzSb, with thestructural evolution upon cycling so as to emphasize both the copperextrusion/reinjection upon cycling together with the maintenance ofthe antimony array. array provides a host framework for the incoming andextruded metal atoms, thereby limiting the volume expansion.For instance, in the ternary Li,In Sb system (0
确定




还剩15页未读,是否继续阅读?
武汉科思特仪器股份有限公司为您提供《Nanomaterials for Rechargeable Lithium Batteries》,该方案主要用于其他中--检测,参考标准--,《Nanomaterials for Rechargeable Lithium Batteries》用到的仪器有CS2350H双单元电化学工作站(双恒电位仪)、CS350M电化学工作站/电化学测试系统、CS310M电化学工作站/电化学测试系统
相关方案
更多
该厂商其他方案
更多












