
方案详情
文
德国耶拿multiN/C系列TOC分析仪,采用多项世界领先技术,如专利VITA技术,NDIR检测器,EASY CAL等,测定纯水样品,灵敏度,准确度高。
方案详情

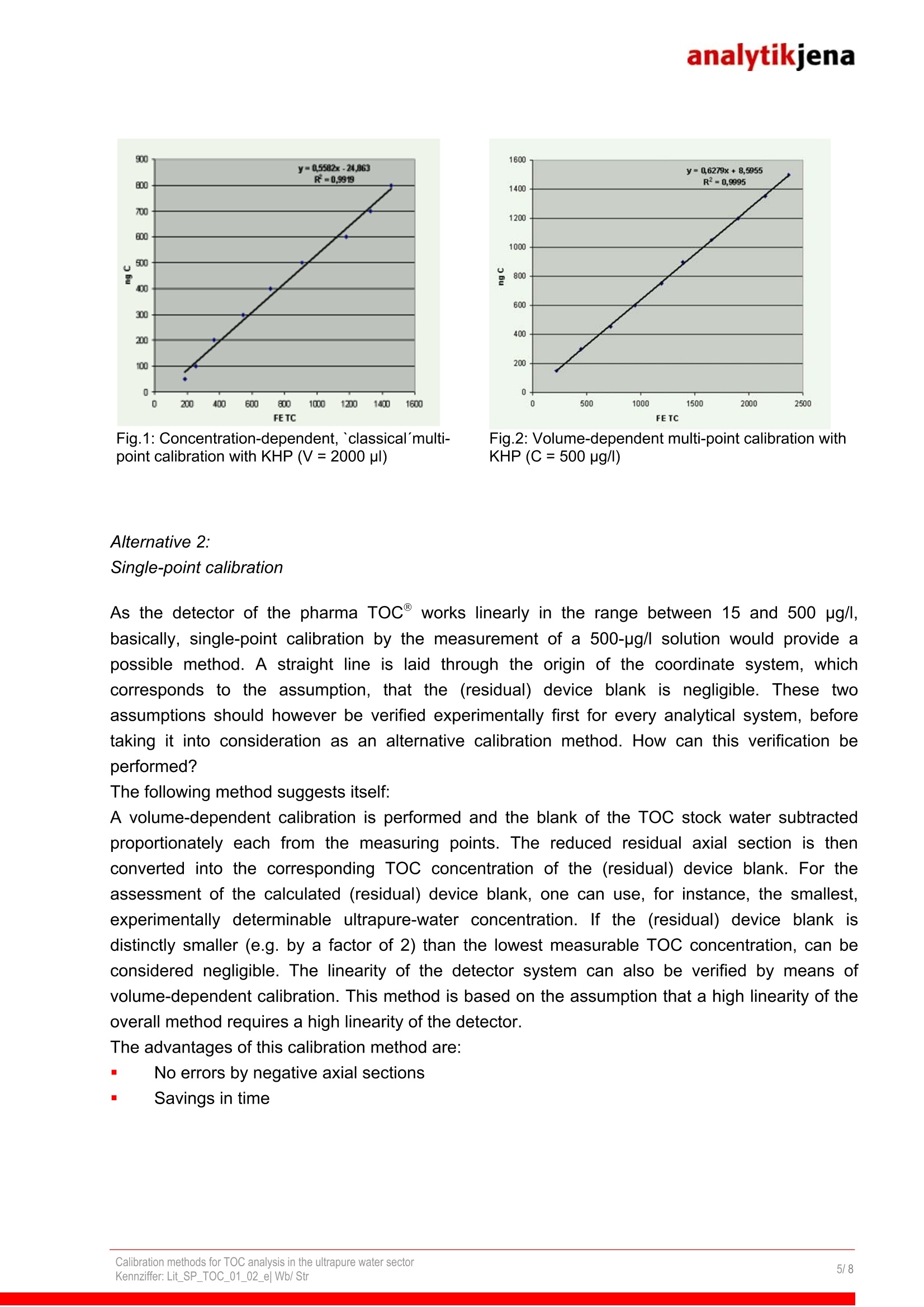
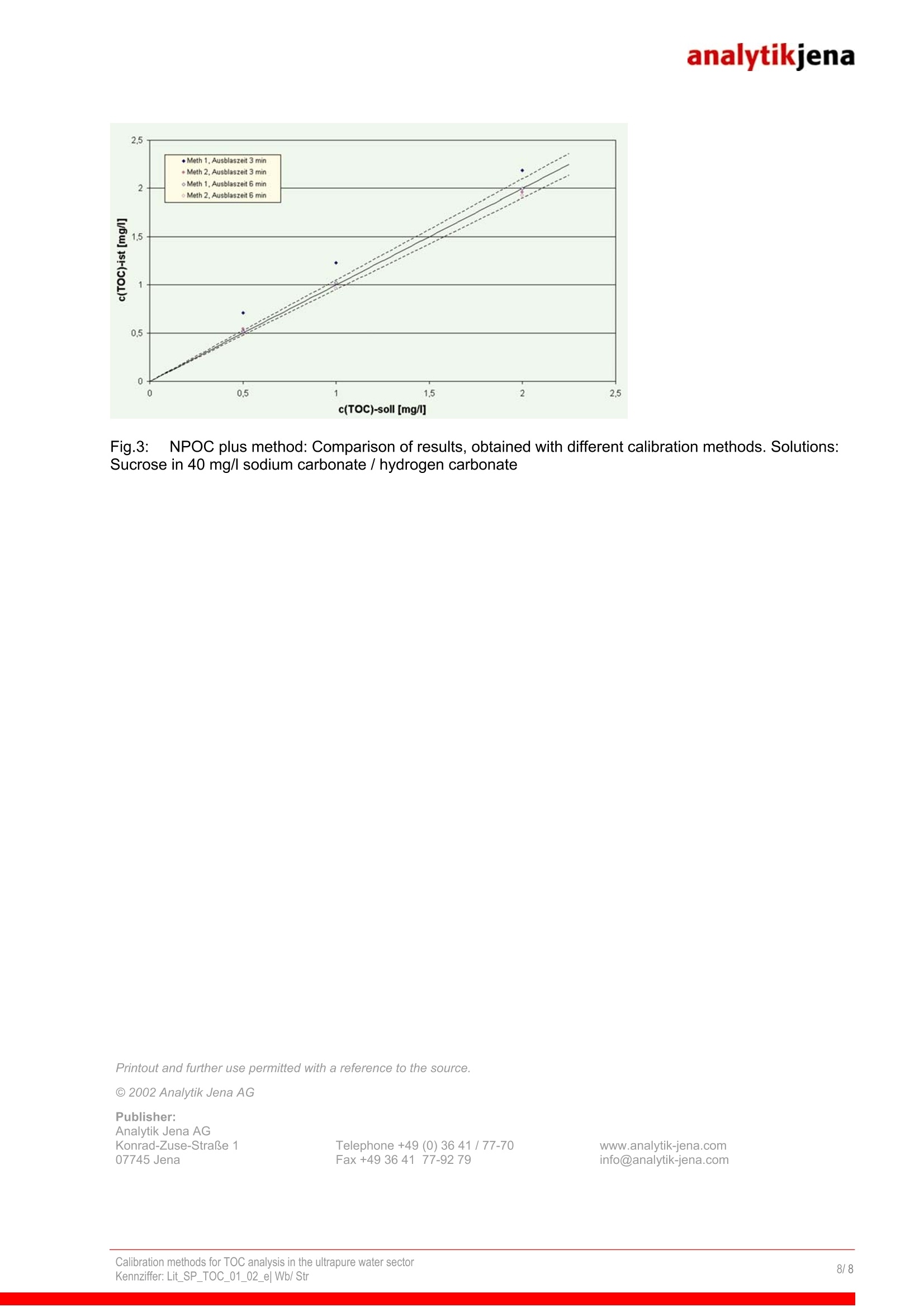
analytikjena Fields of Application / Industry: Chemistry / Polymer Industry Clinical Chemistry / Medicine /Hygiene/ Health Care Electronics Semi-Conductor Technology Energy Environment / Water /Waste Geology /Mining Food / Agriculture Metallurgy / Galvanization Refineries / Petrochemistry Pharmacy Cosmetics Material Analysis Others Calibration methods for TOC analysis in the ultrapure water sector Authors: Thomas Stratesteffen, Birgit Wittenburg, Analytik Jena AG; Konrad-Zuse-Str.1, 07745 Jena Dr. Christiane Ehrling, AJ IDC GmbH Langewiesen Introduction The TOC analysis in the ultrapure water sector makes especially high demands on the methodused to calibrate the analytical instruments. In this connection, blanks and the stability andreliability of standard solutions play a particular part. The fundamental difficulty is that thesensitivity of today's analytical instruments extends as far as into the lower pg/l range, in which it ishardly possible to manually prepare dependable TOC and TIC standard solutions. Here, newcalibration methods are necessary that allow dependable analyses in the ultrapure water sector. Factors affecting TOC calibration in the ultrapure water sector In the ug/l range, the preparation of TOC standards makes particularly high demands on thecleanness of the glass vessels used and requires particular care in preparing and storing thestandard solutions. As a consequence, special requirements are to be met in the calibration of theTOC analyzer. The difficulties of TOC calibration in the ug/l range can be summarized as follows: 1. Compared with higher-concentration TOC standards, the stability of TOC standards of smallconcentrations is limited. 2.The quality of the water used to prepare the standards (stock water) plays an important part1 (water blank). 3.Particular demands are made on the cleanness of the materials of the analytical instrumentused (device blank). 4.CO2 of ambient air accumulates very quickly in the standards. A CO2 concentration that ismuch higher compared to the TOC test quantity results in inaccuracies in the differentialmethod (TOC=TC-TIC). 5.Carbonaceous organic compounds from the laboratory may accumulate inmeasurablequantities in the standards (samples). 6.1.As a rule, when using the differential method, TC and TIC are calibrated separately.Experience has shown, however, that the stability of TIC standard solutions is even moreproblematic than that of TOC standard solutions of the same concentration range, which is dueto the CO2 adsorption from air in the ug/I range. Thus, it is justified to ask, in which way the TOC (TIC) can be calibrated reliably down into thelower ug/l range, if the preparation and the handling of these standards below approx. 100 pg/l aresuch a problem. For the reasons mentioned, a 'classic' calibration by measuring a standard dilutionseries does not appear practicable in the range below 100 pg/l. Within the scope of the presentedconsiderations, the following questions were investigated in detail: 1. How the blank should be taken into account, and what does it consist of? 2. Considering the limited stability of TOC standard solutions in the pg/l range:Which methods lend themselves for the calibration of ultrapure water? 3. lf the TIC/TOC ratio of aqueous samples is very unfavourable: Which TOC measuring andcalibration method (differential or NPOC method) is the method of choice in the ug/I range? The investigations presented here were made with the pharma TOC Analyzer (Analytik Jena AG).Operation of this device is based on the principle of catalytic high-temperature combustion withNDIR detection; the catalyst used is platinum. The lower detection limit of the device is about15 pg/l. Software-assisted normalization of occurring flow variations allows the injection of up to3 ml sample as well as the variation of the injection volume without the need for recalibration of thedevice (residence-time-coupled integration in TOC analysis, VITA) 1. Blank values of TOC analysis In principle, TOC blank values consist of the blank values of water (TOC of used waters) and thedevice blank (blank value of the materials of the device). The used waters include: 1.Water for the preparation of standard solutions, 2'.Dilution water for the dilution of samples and3i. System-rinse waters, if they have an effect on the measurement result. As these waters often are different types of waters, their blank values must always be treatedseparately. In the ultrapure water sector, dilution and rinse waters usually do not play any part, asthe samples need not be diluted and the device can be rinsed with the samples themselves.The water used for the preparation of TOC standard solutions (stock water), however, plays aspecial part in the pg/l range, as the weighed portion and the TOC blank value of the stock wateroften are of the same order of magnitude. If after the calibration any standard solution is measuredas sample (for instance for verification of calibration), in the ideal case, logically the following TOCconcentration will resultTOC measured value = theoretical TOC + water blank. Example: If a 500-pg/l TOC standard solution iss measured as sample, ideally themeasurement result would be e.g. 530 pg/l TOC (500 pg/l TOC weighed substance +30pg/l TOC of the stock water) It is sensible to take this addition of blanks into account already in the calibration by measuring theTOC blank of the stock water separately and subtracting it in the calibration. Mathematically, thiscorresponds to a parallel displacement of the calibration curve relative to the y-axis. By thisoperation, the axial section of the equation of a straight line is reduced by an amount, whichcorresponds to the level of the TOC blank of the stock water. Where, however, the device blank isincluded mathematically? One part of the device blank is already included in the subtracted blank.Another part is not determined when measuring the stock water, as it is liberated only during calibration (e.g. carry-over effects). Now, the remaining residual axial section represents this(residual) device blank, which ideally is negligible. 2. Which method is the method of choice for the calibration of ultrapure water? When calibrating a TOC analyzer with TOC standard solutions below 500 pg/l, usually it can beobserved first, that the relative standard deviation is unacceptably high. Besides, these variations raise the determination limit of the analytical system, although theircause is not to be attributed to the system but to the deficient stability and manageability of theused standard solutions. Special rinsing techniques for the used vials as well as immediaterealization of the calibration with freshly prepared standard solutions and the use of a CO2-freeinert gas are helpful measures, but do not eliminate this fundamental problem: Meanwhile, manyTOC analyzers are more accurate and reliable in the lower pg/l range compared to the reliability ofmanually prepared standard solutions. Alternative 1: Volume-dependent calibration with a single standard solution Instead of calibration by means of a series of separately prepared standard solutions, calibration isbasically possible also by the injection of various volumes of a single standard solution. In this way,different CO2 concentrations are generated in the sample gas, i.e. different signals are obtained,which permit an equivalent linear regression to be performed. With this calibration method, thefollowing items must be considered: 1..Due to the varying sample gas flows occurring when injecting the sample into a high-temperature combustion system, the analytical instrument should incorporate the principle offlow normalization (VITA) described above, in order to make the measurement independent ofthe fluctuations caused by flow variations. 2.The precision of the sample metering pump must meet especially high requirements, as thisrepresents the limiting factor of the 99:method. 3.The calibration should be verified with (at least) one separately prepared standard to ensurethe correctness of the calibration. 4.The TOC stock water blank must be subtracted proportionately from the obtained area value. The advantages of this method are obvious: You only need one standard solution of a higher concentration. Thus, the described difficultiesinvolved with standard solutions of very low TOC are eliminated. You need only one samplevial. A good sample metering pump integrated in the device operates with a higher precision andreliability.m The method offers enormous savings in labor and time. The examples in Figs. 1 and 2 show a comparison of two typical calibrations: one performedaccording to the principle of a standard dilution series and the other according to volume-dependent calibration with a single standard solution. Fig.1: Concentration-dependent, `classicalmulti-point calibration with KHP (V= 2000 pl) Alternative 2: Single-point calibration As the detector of the pharma TOC@ works linearly in the range between 15 and 500 pg/l,basically, single-point calibration by the measurement of a 500-ug/l solution would provide apossible method. A straight line is laid through the origin of the coordinate system, whichcorresponds to the assumption, that the (residual) device blank is negligible. These twoassumptions should however be verified experimentally first for every analytical system, beforetaking it into consideration as an alternative calibration method. How can this verification beperformed? The following method suggests itself: A volume-dependent calibration is performed and the blank of the TOC stock water subtractedproportionately each from the measuring points. The reduced residual axial section is thenconverted into the corresponding TOC concentration of the (residual) device blank. For theassessment of the calculated (residual) device blank, one can use, for instance, the smallest,experimentally determinable ultrapure-water concentration. If the (residual) device blank isdistinctly smaller (e.g. by a factor of 2) than the lowest measurable TOC concentration, can beconsidered negligible. The linearity of the detector system can also be verified by means ofvolume-dependent calibration. This method is based on the assumption that a high linearity of theoverall method requires a high linearity of the detector. The advantages of this calibration method are: No errors by negative axial sections Savings in time With volume-dependent calibration, typically the scatter of measured values for TOC standards ofless than 100 ug/l is distinctly lower than that obtained with the `classical'calibration by means of adilution series. As a rule, this is expressed both by the regression coefficient and by the size of the axial section ofthe calibration curve. A large (positive or negative) axial section causes great measuring errors inthe low-concentration range. Then, the axial section typically comes about by measured-valuevariations during calibration, and not by systematic effects. As a rule, the axial section of thecalibration curve of volume-dependent calibrations is near zero, i.e. in the range of low TOCconcentrations(<100 pg/l) the calibration-induced errorislower than thatcausedbyconcentration-dependent, classical calibration. Thus, it stands to reason to lay the axial sectiondeliberately through the origin of the coordinate system by single-point calibration and thuspreclude result distortion by too large axial section from the outset. This calibration method is themethod of choice for the pharma TOC", if exclusively TOC analyses of pure and ultrapure waterswith concentrations below 1000 pg/l are to be run. If, however, also higher TOC concentrations areto be determined regularly, which means that memory effects are to be expected, the axial sectioncannot be disregarded and the volume-dependent calibration method is to be preferred. 3. Which peculiarities result for the calibration if the TIC/TOC ratio is very unfavorable? In general, the TIC/TOC ratio is particularly unfavorable for the TOC analysis, if the TICconcentration of the sample is much higher than its TOC concentration. In this case, it is generalpractice, to expel the TIC of the sample through acidification and purging and determine the TOCdirectly (NPOC= Non-purgeable Organic Carbon). In this way, a too great mathematical error isprevented, which results from the subtraction of two result scatters. The problem with this methodresults from the fact, that sometimes it is impossible to accomplish complete purging of TIC withinan appropriate measuring time. In ultrapure water analysis, this `residual TIC’often cannot beneglected, as it would contribute to a distortion of the TOC data. In individual cases, it maytherefore be useful to determine the TOC of the samples in two steps,i.e. by a combination of theNPOC and the differential method: Step 1: NPOC-method: Acidification of the samples and purging of the major part of the produced carbon dioxideStep 2: Differential method: Determination of TOC content as difference from the measured values of the `residualTC'and the `residual TIC’. The TC/TIC values found in this way are pure arithmeticguantities. In the following, this method is referred to as `NPOC plus`method. Calibration of the NPOC plus method and calculation of the analytical results Basically, there are two calibration options: 1. Calibration and measurement analogous to the differential method: For the TIC and the TC channel of the analyzer, separate calibration curves are determinedwith appropriate standards. The calibration standards are neither acidified nor purged.Unlike the standard solutions, the samples are then acidified and purged (Step 1). The`residual TIC'and `residual TC'content arising after purging is determined by means of theseparate calibration curves. The TOC content results from the difference of these twoconcentrations (Step 2). 2.Alternatively to the calibration method described above, the TOC may also be calibrateddirectly by subtraction of the units of area (UA) of the `residual TIC’ from the `residualTOC: As standard solutions TOC solutions are used, which are acidified and purged in the sameway as the samples. Subsequently, the units of area of the TIC and TC are determined andthe calibration curve for the TOC content calculated on the basis of these values by thefollowing equation: The TIC and TC concentrations are not determined. The methods for the determination of the TOC of samples is performed as describedabove: After acidification and purging of the samples, the units of area of the TIC and theTC are determined. Subsequently, the found integrals are subtracted and the TOCcalculated from the thus found net integral on the basis of the calibration curve. The limited usability of Option 1 results from the fact that the calibration of the TIC in the lower pg/lrange causes great problems because of the increased risk of contamination. Hence, in this rangeespecially Option 2 would provide a solution, as here the TIC need not be calibrated. Theadvantages of this calibration method also show experimentally by the direct comparison of the twocalibration methods. The results shown in Fig. 3 represent the TOC concentrations of sucrosestandard solutions in a matrix of 40 mg/I TIC (sodium carbonate standard). The NPOC plus methodwas calibrated each according to the two options described above. Subsequently, the TOC contentof the standards was determined likewise. The obtained results agree better with the nominalconcentrations, if the TIC calibration is dispensed with (Option 2). This procedure, however,requires equal sensitivities and similar device blanks in the TIC and TC channels of the analyzer.On the pharma TOC, this is given in the lower pg/l range. Thus, the calibration method accordingto`Option 2'represents an appropriate option of the `NPOC-Plus’method, with which even verylow `TIC residual concentrations’ of the acidified and purged samples can reliably be taken intoaccount. Fig.3: NPOC plus method: Comparison of results, obtained with different calibration methods. Solutions:Sucrose in 40 mg/l sodium carbonate / hydrogen carbonate Printout and further use permitted with a reference to the source. C 2002 Analytik Jena AG Publisher:Analytik Jena AGKonrad-Zuse-StraBe 107745 Jena Telephone +49 (0)36 41/77-70 Fax +49 3641 77-92 79 Calibration methods for TOC analysis in the ultrapure water sectorKennziffer: Lit _SP_TOC_e Wb/ Str/
确定






还剩6页未读,是否继续阅读?
耶拿分析仪器(北京)有限公司为您提供《纯水中TOC分析的校正方法》,该方案主要用于饮用水中有机物综合指标检测,参考标准--,《纯水中TOC分析的校正方法》用到的仪器有德国耶拿 multi N/C 3100 TOC总有机碳/总氮分析仪、multi N/C UV HS新一代湿法总有机碳/总氮分析仪、multi N/CPharma HT制药专用干法总有机碳/总氮分析仪、multi N/C Pharma UV制药专用湿法总有机碳/总氮分析仪、德国耶拿multi N/C 2100 TOC总有机碳/总氮分析仪
相关方案
更多
该厂商其他方案
更多













