方案详情
文
模拟体液中(3-羟基丁酸酯)-(4-羟基丁酸酯)聚合物(PHA聚羟基脂肪酸酯)薄膜的降解Degradation of P(3HB-co-4HB) Films in Simulated Body Fluids
方案详情

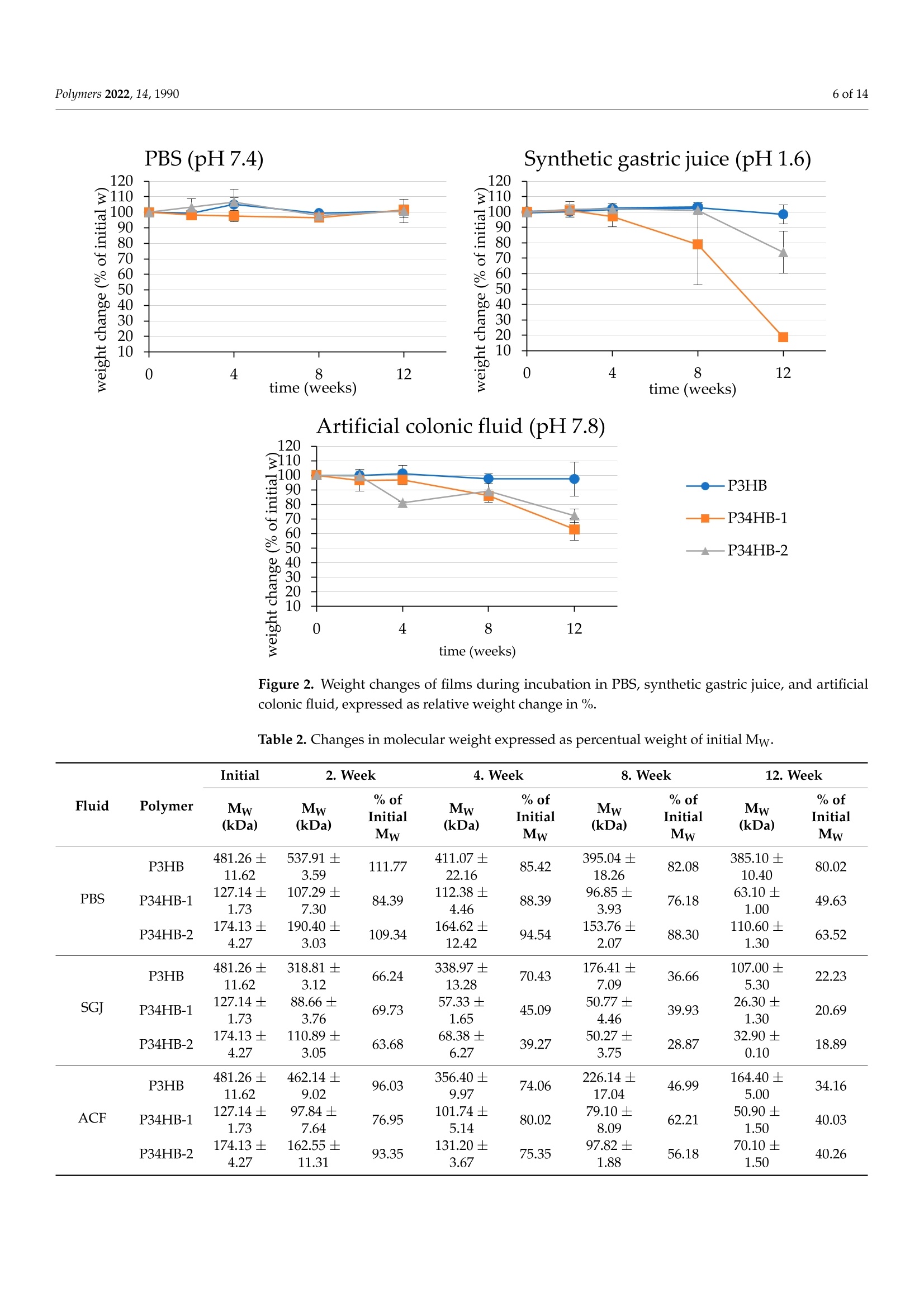
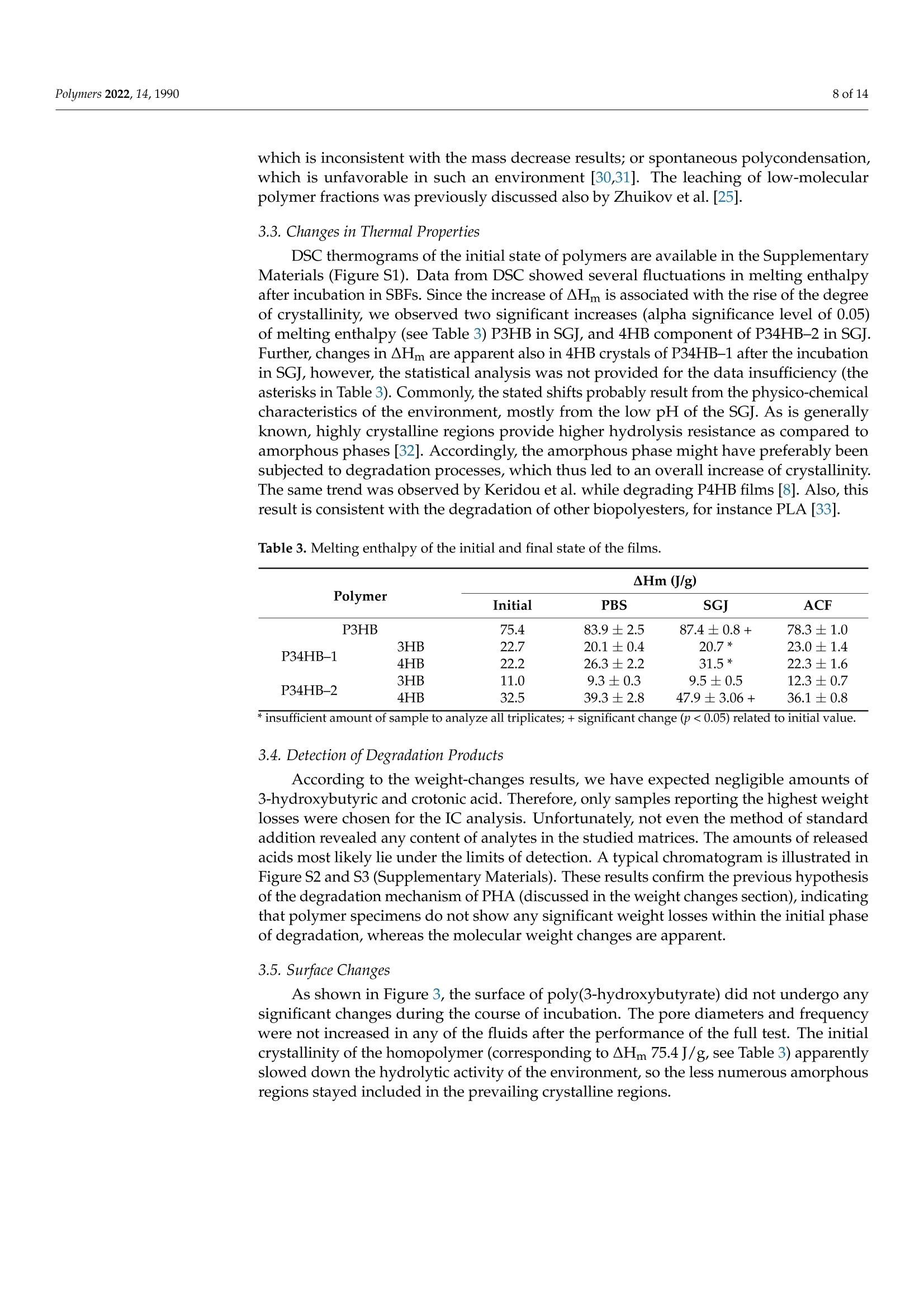
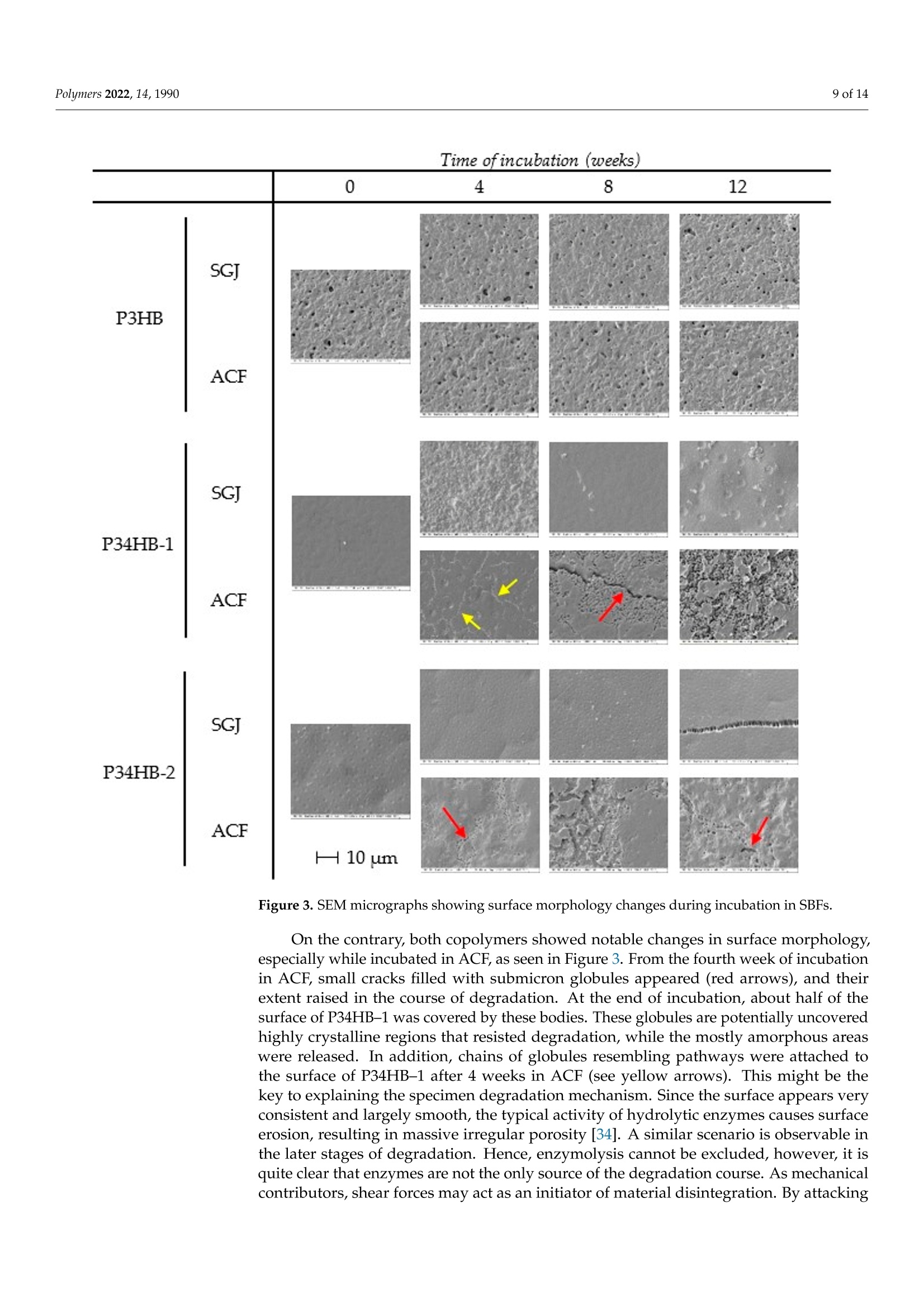
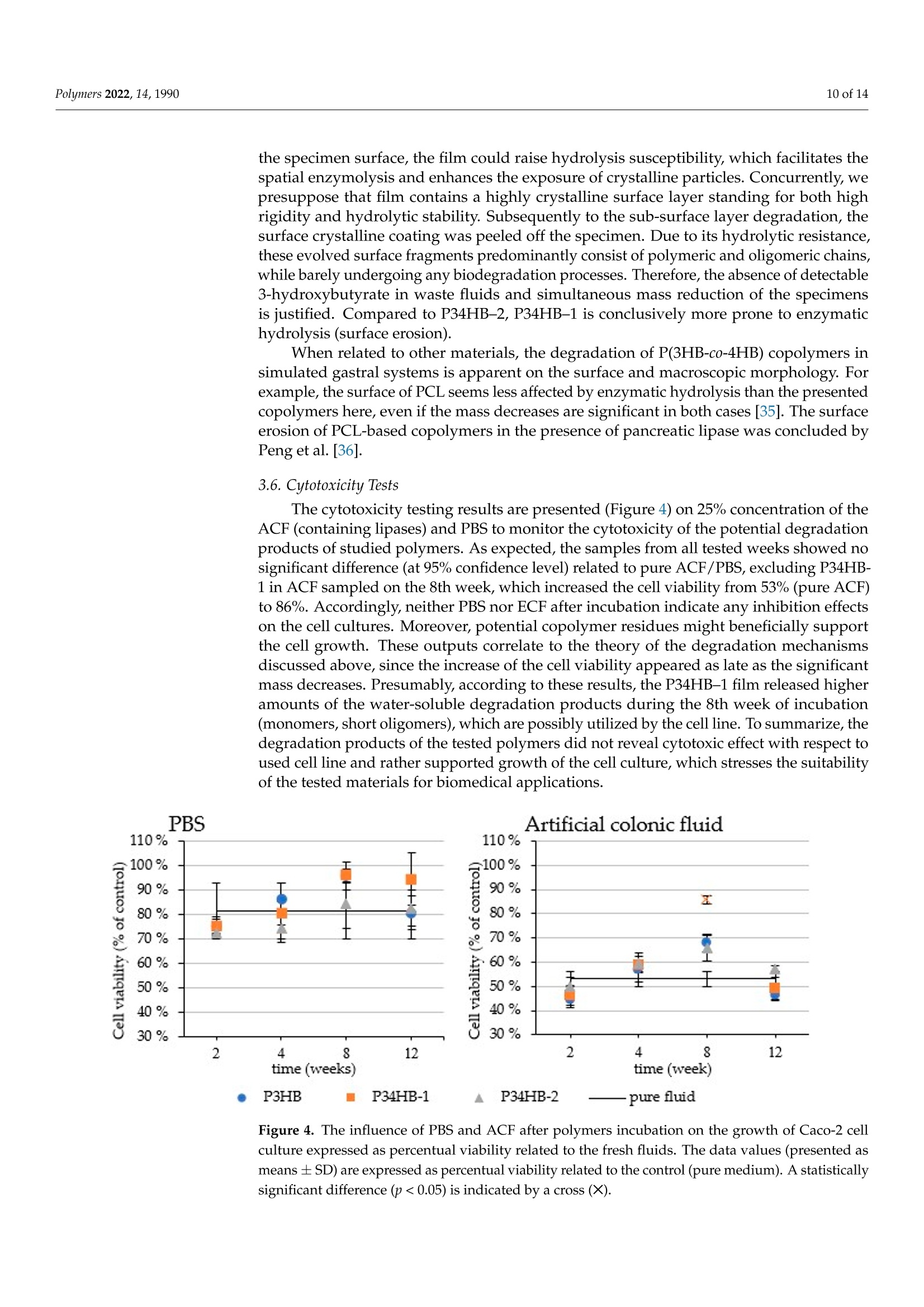
模拟体液中(3-羟基丁酸酯)-(4-羟基丁酸酯)聚合物(PHA聚羟基脂肪酸酯)薄膜的降解Degradation of P(3HB-co-4HB) Films in Simulated Body Fluidspolymers 2 of 14Polymers 2022,14,1990 Citation: Vodicka,J.; Wikarska, M.;Trudicova,M.;Juglova, Z.; Pospisilova, A.; Kalina, M.;Slaninova, E .;Obruca, S.;Sedlacek,P .Degradation of P(3HB-c0-4HB) Fi l ms in Simulated Body Fluids. Polymers 2022,14,1990. https://doi.org/ 10.3390/polym14101990 Academic Editors: Branka Musiě and Andrijana Sever Skapin Received : 6 Apr il 2022 Accepted: 10 May 2022 Published: 13 May 2022 Publisher's Note: MDPI stays neutral with regard to jurisdict i onal claims in published maps and institutiona l af fi l -iations. Copyright: @ 2022 by the authors.L i censee MDPI, Basel, Switzerland.This article is an open access article distr i buted under the terms and conditions of the Creative Commons Attribution (CC BY ) l icense (https://creativecommons.org/licenses/by/4.0/) Faculty of Chemistry, Brno University of Technology, Purkynova 118, 612 00 Brno, Czech Republic; xcvodickaj@vutbr.cz (J.V.); monika.wikarska@vut.cz (M.W.);xctrudicova@fch.vut.cz (M.T.); zuzana.juglova@vut .cz (Z.J .); xcpospisilovaan@fch.vut.cz (A.P.); kal i na-m@fch.vut.cz (M.K.); xcslaninovae@fch.vut.cz (E.S.); obruca@fch.vut.cz (S.O.) * Correspondence: sedlacek-p@fch.vut.cz; Tel .: +420-541-149-486 Abstract: A nove l model of biodegradable PHA copolymer films preparation was applied to evaluate the biodegradability of various PHA copolymers and to discuss its biomedical applicability. In this study, we i llustrate the potential biomaterial degradation rate affectability by manipulation of monomer composition via controlling the biosynthetic strategies. Within the experimental investi-gation, we have prepared two di f ferent copolymers of 3-hydroxybutyrate and 4-hydroxybutyrate一 P(3HB-co-36 mol .%4HB) and P(3HB-c0-66 mol.% 4HB), by cultivating the thermophilic bacterial strain Aneurinibacillus sp. H1 and further i nvestigated its degradability in simulated body f luids (SBFs). Both copolymers revealed faster weight reduction in synthetic gastric juice (SGJ) and arti-ficial colonic fluid (ACF) than simple homopolymer P3HB. In addition, degradation mechanisms differed across tested polymers, according to SEM micrographs. While incubated in SGJ, samples were fragmented due to fast hydrolysis sourcing from substantially low pH, which suggest abiotic degradation as the major degradation mechanism. On the contrary, ACF incubation indicated obvious enzymatic hydrolysis. Further, no cytotoxicity of the waste fluids was observed on CaCO-2 cell line.Based on these results in combination with high production f lexibi li ty, we suggest P(3HB-C0-4HB)copolymers produced by Aneurinibacillus sp. H1 as being very auspicious polymers for intestinal in vivo treatments. Keywords: polyhydroxyalkanoates (PHA); Aneurinibacillus sp. H1;PHA copolymers; biodegradation;simulated body f luids 1. Introduction With the reputation of being green polymers, polyhydroxyalkanoates (PHA) represent a very "hot" alternative to commercial polymers. PHA represents an extensive family of biopolymers, including homopolymers and copolymers, which are very diverse in monomer composition [1]. Unlike petrochemical polymers, PHA are entirely biodegrad-able and biocompatible. Their physico-mechanical properties evolve from the monomer composition and often balance the properties of conventional plastics. Amongst the PHA group, copolymers containing 4-hydroxybutyrate are recently being increasingly i nvesti-gated due to their suitability for various medical applications [2]. A wide range of bacterial strains are able to decompose PHA, simultaneously pro-duc i ng several types of highly speci f ic PHA-depolymerases [3,4]. However, enzymatic degradation of polyhydroxyalkanoates could also be ensured by the action of other es-terases, e.g., l ipases [5]. Due to the ubiquity of l ipases, a wide spectrum of organisms are expected to be able to decompose PHA. According to Mok et al., lipases from pan-creatic extracts from mice and chicken indicated similar PHA-depolymerizing activity on copolymer P(3HB-co-4HB) compared to several microbial lipases [6]. The decomposition of poly(4-hydroxybutyrate) f ilms by bacterial (Pseudomonas cepacia) and fungal (Rhizopus oryzae) l ipase in simulated physiological conditions was examined by Keridou et al.,and their data demonstrated notable differences between enzymatic and abiotic hydrolysis [7,8].In addition, the authors indicated different mechanisms of hydrolysis i n the presence of lipases compared to their absence. The rate of PHA-degradabi li ty depends on the crystal morphology-whilst highly crystalline regions are more resistant to hydrolysis, amorphous parts are not so stable and might undergo very quick hydrolysis [3,9,10]. Since the homopolymer of 3-hydroxybutyrate (3HB) occurs i n high-crystallinity form, various PHA copolymers appear more amorphous and thus much more prone to hydrolytic degradation. Moreover, higher amorphousness stands for enhancement of some of the material properties (higher elongation at break and lower melting and glass transition temperature), therefore ensuring higher flexibility of use [11,12]. One of the most promising copolymers with promoted mechanical properties is P(3HB-co-4HB). Combining in vivo biodegradability and biocompatibility, this copolymer is the auspicious candidate for in vivo applications in medicine [13]. There are only a few described producers able to incorporate 4-hydroxybutyrate into t he polymer chain. As Pernicova et al. reported previously, Aneurinibacillus sp. H1 is a unique bacterial producer of P(3HB-co-4HB) able to produce t his copolymer containing various 4-hydroxybutyrate (4HB)portions according to the selected initial 1,4 butanediol:glycerol ratio [14]. Therefore, the properties of the final product are possibly adjustable by setting the cultivation conditions. Besides the material properties, the key characteristics with respect to medica l uses of biopolymers such as PHA also lie in its degradability/stability under physiological conditions. We consider this crucial behavior of these frequently investigated materials as insufficiently described in the term of biomedical applications. The future prospects set up requirements for the degradation course exploration in the body-simulating environment,fol l owing a major degradation rate and mechanisms. Therefore, in t his study, we prepared films of two various copolymers of P(3HB-co-4HB) differing in monomer ratios and com-pared their biodegradation i n synthetic human gastric juice (SGJ ) and arti f icial colonic fluid (ACF) to degradation of reference homopolymer (P3HB) in order to assess the benefits of application of PHA copolymers in biomedical technology. We have also performed unique attempts to evaluate the presence of degradation products and i ts potential health risks via cytotoxicity tests. 2. Materials and Methods 2.1. Preparation of Polymer Films Production of P(3HB-co-4HB) copolymers was performed by the cultivation of ther-mophilic bacterium Aneurinibacillus sp. H1, as reported previously [14]. The inoculum was generated by incubating the cells i n complex medium Nutrient Broth (10g/L beef extract,10 g/L peptone,5 g/L NaCl; HiMedia, Maharashtra, India) at 45 °℃, 180 rpm, for 24 h. The cells were subsequently inoculated to mineral salt medium (MSM) i n the ratio of 10%.MSM consisted of Na2HPO4·12H2O,9.0g/L;KH2PO4,1.5g/L;MgSO4·7H2O,0.2g/L;NH4NO3,1.0g/L;CaClz·2 H2O,0.02g/L;FENH4citrate, 0.0012 g/L; Tryptone,0.5g/L and microele-ment solution (MES, composition see Table S1 in Supplementary Materials), 1 mL/L (all chemicals from Lach-ner, Neratovice, Czech Republic). Two ratios of carbon sources were used: 4:6 g/L of 1,4-BD: glycerol (lower 4HB content), 4:2 g/L of 1,4-BD: glycerol (higher content of 4HB in t he copolymer). The production medium was incubated in shaking flasks (45°℃, 180 rpm) for 72 h. In order to produce a homopolymer of 3HB, Cupriavidus necator was employed. The inoculum was generated analogically to Aneurinibacillus, however, the incubation temperature was 30 °C . The MSM consisted of KH2PO4, 1.02g/L;(NH4)2SO4,1.0g/L;Na2HPO4·12 H2O, 11.1 g/L,MgSO47H2O,0.2 g/L; trace element solution (TES,composition see Table S2), 1 mL/L; as the source of carbon, fructose (20 g/L) was used (all from Lach-ner, Neratovice, Czech Republic). After the incubation, the bacterial cultures were centrifuged at 4800× g for 10 min, and the obtained biomass was dried at 70 °℃ in Petri dishes. The monomer composition and overall PHA content were determined by Gas Chromatography method according to Obruca et al. [15]. As an isolation step, chloroform extractions of the polymers f rom biomass were held by using SOXTHERM@ automatic extraction system (C. Gerhardt Analytical systems, Konigswinter, Germany). To generate polymer films, materials obtained were redissolved in chloroform (5%w/w) and solutions were filtered through 5 um-nylon filters. An automatic film applicator (TQC Sheen, Capelle aan der IJssel, The Netherlands) was used in order to prepare the films, where each film consisted of two layers applied in opposite directions. To determine film thickness, a mechanical profilometer (Bruker, Billerica, MA, USA) was employed. All chemicals used in t his study reached A.R. grade. 2.2. Biodegradation in Simulated Body Fluids Biodegradation of manufactured films was held i n two simulated body fluids (SBFs)一 simulated gastric juice (SGJ-pH 1.6) and artificial colonic fluid (ACF-pH 7.8); and phosphate buffer solution (PBS-0.01 M, pH 7.4) to monitor abiotic hydrolysis in a water environment. The SBFs were prepared according to Marques et al. (see ref. [16]); the compositions are available in Supplementary Materials (Tables S3-S5, Supplementary Materials). The films were cut i nto 2.5 ×2.5cm spec i mens, which were rinsed in ethanol to prevent medium contamination and then dried at room temperature. Specimens were incubated for 12 weeks at 37 °C and 160 rpm at an orbital incubator shaker. Fluids were refreshed weekly; samples of recycled f luids were held in the freezer for further analyses.After 2, 4, 8, and 12 weeks of incubation, triplicates of films were rinsed in ethanol, dried at room temperature, and weighed for the following analyses. 2.3. Molecular Weight Measurements Molecular weights of polymers were analyzed as previously [17]. In short, 1.5 mg of individual polymer samples were dissolved i n 1 mL of chloroform (HPLC-grade) and subsequently passed through nylon membrane filters (0.45 um). A total of 100 uL of each sample was injected to the size exclusion chromatography system (Infinity 1260 system with PLgel Mixed-C column, Agilent, Santa Clara, USA), for the detection, multi-angle l ight scattering (Dawn Heleos II, Wyatt Technology, Santa Barbara, CA, USA) and differential refractive index (Optilab T-rEX, Wyatt Technology, Santa Barbara, CA, USA) detectors were employed. As a mobile phase, HPLC-grade chloroform (filtered through 0.02 um membrane filters) at a flow rate 0.6 mL/min was used. To determine the weight-average molecular weight (Mw) and polydispersity index (PDI), ASTRA software (version 7.3.2,Wyatt Technology, Santa Barbara, CA, USA) was used employing Zimm equation. 2.4. DSC Measurements DSC measurements were provided according to previous method [17]. Briefly, change in crystallinity after 12-weeks incubation in SBFs was investigated by DSC measurements using a calorimeter (DSC 2500, TA Instruments, New Castle, DE,USA). Samples were analyzed in hermetically sealed TzeroTM aluminum pans (TA Instruments, New Castle,DE, USA) under a dynamic nitrogen atmosphere. Single-heating program (ramp 10°C/min to 195 °C) and cooling (ramp 10 °C/min to 30 °C) was applied. As a calibrant, indium was used. 2.5. Detec t ion of Degradation Products Samples of f luids were investigated for the residual 3-hydroxybutyric acid and cro-tonic acid content as the putative products of polymer decomposition. The ion-exchange chromatography (IC) system (Metrohm, Herisau, Switzerland) coupled with Metrosep Or-ganic Acids-250/7.8 column (Metrohm, Herisau, Switzerland) and conductivity detector (850 Professional IC Anion, Metrohm, Herisau , Switzerland) were employed for this pur-pose. For the isocratic elution, 0.5 mM HClO4 with 10 mM LiCl was used. The samples of SGJ were pre-neutralized by 0.01 M NaOH. All samples were pre-filtered through 0.45 um syringe f ilters (nylon membrane). 2.6. Surface I maging The smal l specimens from each sample were gold-coated in a sputtering coater (Po-laron) and investigated using a ZEISS EVO LS 10 scanning electron microscope (Carl Zeiss NTS, Munich, Germany). Observations were realized in secondary electron (SE) mode,andthe accelerating voltage was 5 kV (avoiding sample charging). The method corresponds to a previously used SEM procedure by Sedlacek et al. [17]. 2.7. Cytotoxicity Tests Cell viability assay study for determination of cytotoxicity was performed using Human Colon Adenocarcinoma cel l line, permanent cel l l i ne CaCO-2 (Cell Lines Ser-vice, Eppelheim, Germany, SRN). The cells were grown in Eagle's Minimum Essential Medium-MEM supplemented with 10% fetal bovine serum, 1% Antibiotic-Antimycotic blend containing 100 mg/mL of streptomycin, 100 units/mL of penicillin and 0.25 ug/mL of Gibco Amphotericin B in humidified,5% (V/V) CO2 atmosphere at 37°C . Every second day, the medium was refreshed, and the cells were eventually subcultured at 80-90% con-fluency by treatment with 0.25% trypsin-EDTA. For the MTT assay, the cells were seeded at a density of 1800 cells per well (100 pL) and cultivated overnight . The samples obtained by polymers foil digestion from weeks 2, 4,8,and 12 were filtered through a 200 nm PET syringe f ilter. The growth medium was replaced after 24 h of seeding and the samples were added in the concentration range 25%, 12%, 6.5%, and 3.25%, using ethanol as a negative control and growth media as a control culture. After 24 h of cultivation, 20 uL of MTT dissolved in PBS (2.5 mg/mL) was added to each well and the plate was placed for 3 h in cultivator (BioTek, Winooski, VT, USA). Then, 100 uL of 10% SDS in PBS was added and the plate was stored in laboratory temperature until the next day. The evaluation was performed the next day by ELISA Reader (Synergy HTX Multimode reader, BioTek,Winooski, VT, USA) at 562 nm. The test design was inspired by Li et al . [18]. 2.8. Statistical Analysis All results are presented as mean values from triplicates. Statistical comparisons were provided by one-way analysis of variance (ANOVA), and differences were considered significant at p <0.05. Al l statistical analyses were provided in Statistica@ 13.3 software (TIBCO, Palo Alto, CA, USA). 3. Results and Discussion 3.1. Weight Changes Parameters of manufactured films are presented in Table 1. The specimens for degra-dation test and its macroscopic morphology at the end of incubation are shown in Figure 1.Within the first four weeks of the test, no significant mass changes were observed, exclud-ing P34HB-2 in colonic f luid (Figure 2). On the contrary, molecular weight decreases are noticeable at this point, especially in the case of gastric juice (Table 2). In the later stage of the test, f ilms of both copolymers became more brittle and appeared fragmented. A substantial part of the material disappeared. Moreover, some smaller fragments were lost due to the manipulation difficulties during the fluid refreshment, which most l ikely stands for markedly higher deviations. Additionally, only l arge-scale cracks appear on the SEM micrographs (see Figure 3), and no surface erosion is observed. Based on these results, it is obvious that the low pH of t he gastric juice might i nduce abiotic hydrolysis, resulting in polymer cracking. Table 1. Parameters of manufactured films. Polymer 4HB (mol.%) Film Thickness (um) Mw (kDa) Mn (kDa) PDI(-) P3HB 0 11.0±1.1 481.26±11.62 380.1±23.47 1.27±0.07 P34HB-1 36 11.1±1.4 127.14±1.73 72.83±7.18 1.76±0.20 P34HB-2 66 11.1±0.6 174.13±4.27 134.0±5.38 1.3±0.03 P34HB-2 initial P34HB-2 in SGI P34HB-2 in ACF Figure 1. Changes in the morphology of the f ilms at the end of incubation in SBFs. The weight of the samples i s generally expected to decrease during degradation.Nevertheless, it was reported that the change in weight is not a reliable indicator for the degree of PHA degradation evaluation. Based on available studies, degradation of PHA specimens shows lag-phase in weight loss or very mild development of the mass decrease in different environments [19-23]. The process is possibly initiated by the scission of macromolecules to shorter chains and oligomers, while the mass of a specimen undergoes only slight f luctuations. The shorter polymer chains are still too hydrophobic to dissolve in an aqueous environment, so they remain adhered to t he matrix until the l ength of oligomers is reduced enough for t he mass release into environment. For t his reason, changes in molecular weight are much more evident than weight changes. This phenomenon has already been reported by several authors. Han et al. investigated biodegradation of copolymer P(3HB-co-3HV) films in a phosphate buffer solution with porcine pancreas lipase; after 180 days of incubation, the authors observed only slight weight deflections (remaining more t han 95% of initial weight), while the molecular weight decrease reached 34-80%[24]. Zhuikov et al. reported similar results as various PHA copolymers incubated in the f luid containing pancreatic lipase, where the mass decreased by 8-9% and the molecular weight reduction reached up to 80%[25]. Artificial colonic fluid (pH 7.8) Figure 2. Weight changes of films during incubation in PBS, synthetic gastric j uice, and arti f icial colonic f luid, expressed as relative weight change in %. T able 2. Changes in molecular weight expressed as percentual weight of i nit i al Mw Initial 2. Week 4. Week 8. Week 12. Week % of % of % of % of Fluid Polymer Mw (kDa) Mw (kDa) Initial Mw Mw (kDa) Initial Mw Mw (kDa) Initial Mw Mw (kDa) Initial Mw P3HB 481.26土 11.62 537.91士 3.59 111.77 411.07± 22.16 85.42 395.04士 18.26 82.08 385.10± 10.40 80.02 PBS P34HB-1 127.14土 1.73 107.29土 7.30 84.39 112.38士 4.46 88.39 96.85土 3.93 76.18 63.10± 1.00 49.63 P34HB-2 174.13± 4.27 190.40土 3.03 109.34 164.62± 12.42 94.54 153.76土 2.07 88.30 110.60± 1.30 63.52 P3HB 481.26土 11.62 318.81土 3.12 66.24 338.97土 13.28 70.43 176.41土 7.09 36.66 107.00土 5.30 22.23 SGJ P34HB-1 127.14土 1.73 88.66土 3.76 69.73 57.33土 1.65 45.09 50.77土 4.46 39.93 26.30± 1.30 20.69 P34HB-2 174.13土 4.27 110.89 土 3.05 63.68 68.38土 6.27 39.27 50.27土 3.75 28.87 32.90土 0.10 18.89 P3HB 481.26土 11.62 462.14土 9.02 96.03 356.40土 9.97 74.06 226.14土 17.04 46.99 164.40土 5.00 34.16 ACF P34HB-1 127.14土 1.73 97.84土 7.64 76.95 101.74土 5.14 80.02 79.10土 8.09 62.21 50.90土 1.50 40.03 P34HB-2 174.13土 162.55土 93.35 131.20土 75.35 97.82土 56.18 70.10± 40.26 4.27 11.31 3.67 1.88 1.50 Compared to the copolymers, P3HB did not exhibit any significant weight changes during the whole incubation in any f luid (Figure 2). These results correlate to outputs presented by Kovalcik et al., who observed a weight reduc t ion of 3.6% of PHB scaffolds in synthetic gastric juice and lipase-supplemented PBS after 81 days of incubation [26]. We report an average mass decrease of 1.5% and 2.5% in gastric j uice and colonic f luid after 12 weeks of incubation, respectively. The 3HB-homopolymer is obviously more resistant to degradation processes, so i t is predisposed to quite different biomedical applications compared to copolymers, where i ts prolonged disintegration is beneficial. Similar results were obtained by Chuah et al. who observed the biodegradation of various PHA in PBS with porcine pancreas lipase [27]. The author reported complete degradation of P(98 mol%4HB-co-3HB) f ilms, while no degradation of P3HB films was detected [27]. Additionally, our results show l arge deviations due to coagulation of the f luid compo-nents (proteins and bile salts) on the films, especially in the colonic fluid. Even though these components were rinsed off the specimens until visua l absence, some particles persisted on the surface and i nside the pores of the homopolymer. 3.2. Molecular Weight Changes As stated before, changes in molecular weight offer a more reliable view on the biodegradation process than simple weight change monitoring. The course of the molecular weight changes is shown in Table 2. In general , the largest Mw losses were detected in simulated gastric juice, where all samples reached 80% Mw reduction. Since this medium did not contain any esterases, we do not suppose enzymatic hydrolysis as a reason of this Mw reduction. Pepsinolysis also seems improbable as far as pepsin is able to digest peptide bonds nearby large-side chain amino acids (no substrate analogy). Hence, acid-catalyzed hydrolysis appears as the most probable source of chain scission. Simple hydrolysis of the samples is also noticeable in PBS medium. While the Mw losses are quite mild in a comparison with films i ncubated in SGJ, the influence of low pH in SGJ is more observable.As SGJ i s a substantially aggressive environment for polyester incubation, it represents a medium suitable for rapid decomposition of P(3HB-co-4HB). On the contrary, incubation in ACF showed slightly consistent molecular weight decreases, so this medium suggests a different degradation mechanism. Nevertheless, the presence of enzymes (pancreatin)obviously accelerates hydrolysis. This is also supported by Freier et al. [28]. Interestingly, P3HB reached nearly the same final degree of chain scission (approxi-mately 20% of initial Mw) as the copolymers in SGJ. Commonly, in ACF, the homopolymer almost reached the highest Mw loss amongst the tested PHAs. The very analogic courses in both SBFs of decomposition suggest overall aggressivity of SGJ and ACF environments,possibly deleting the crystal-morphological handicap of homopolymer. Similar results were also obtained by Kovalcik et al . whi l e degrading PHB and P(3HB-co-3HV) i n SGJ and PBS with microbial lipase [26]. The authors reported an even higher rate of Mw decreases in PHB than in copolymers and suggest an explanation, stating that the decrease-rate differences are disproportionally lower than Mw differences between polymers. This hypothesis is based on differences in the probability of hydrolysis, in which a larger set of hydrolysis targets (ester bonds) provides a higher probabil i ty of hydrolysis, which might explain the phenomena of our tests. On the other hand, PBS showed a significant difference between incubated specimens, where P3HB showed a very weak tendency to lose molecular weight (approximately 20% loss) compared to copolymers. Likewise, Doi et al. observed different rates of Mw decrease as incubating P(3HB-co-4HB) in PBS, depending on monomer com-position [29]. Indeed, simple hydrolysis was in progress, however, the environment did not offer more efficient catalytic sources, so it was insufficient for the decomposition of highly crystalline regions of the homopolymer. The authors further reported that random hydrolytic scission acted through the whole polymer matrix [29]. Surprisingly, an increase of P3HB molecular weight was observed in PBS after 2 weeks of incubation. The exact reason of this phenomenon is not obvious within the scope of this work. Two possible explanations are offered: t he leaching of t he short polymer chains, which is inconsistent with the mass decrease results; or spontaneous polycondensation,which is unfavorable in such an environment [30,31]. The leaching of low-molecular polymer fractions was previously discussed also by Zhuikov et al. [25]. 3.3. Changes in Thermal Properties DSC thermograms of the initia l state of polymers are avai l able i n the Supplementary Materials (Figure S1). Data from DSC showed several f luctuations in melting enthalpy after incubation in SBFs. Since the increase of AHm is associated with the rise of the degree of crystallinity, we observed two significant increases (alpha significance level of 0.05)of melting enthalpy (see Table 3) P3HB in SGJ, and 4HB component of P34HB-2 in SGJ.Further, changes in AHm are apparent also in 4HB crystals of P34HB-1 after the incubation in SGJ, however, the statistical analysis was not provided for the data insufficiency (the asterisks in Table 3). Commonly, the stated shifts probably result from the physico-chemical characteristics of the environment, mostly from the low pH of the SGJ. As is generally known, highly crystalline regions provide higher hydrolysis resistance as compared to amorphous phases [32]. Accordingly, the amorphous phase might have preferably been subjec t ed to degradation processes, which thus led to an overall increase of crystallinity.The same trend was observed by Keridou et al. while degrading P4HB films [8]. Also, this result is consistent with the degradation of other biopolyesters, for instance PLA [33]. Table 3. Melting enthalpy of the i nitial and f i nal state of the films . Polymer AHm (J/g) Initial PBS SGJ ACF P3HB 75.4 83.9±2.5 87.4±0.8+ 78.3±1.0 P34HB-1 3HB 22.7 20.1±0.4 20.7* 23.0±1.4 4HB 22.2 26.3±2.2 31.5* 22.3±1.6 P34HB-2 3HB 11.0 9.3±0.3 9.5±0.5 12.3±0.7 4HB 32.5 39.3±2.8 47.9±3.06+ 36.1±0.8 * insufficient amount of sample to analyze all triplicates; + significant change (p<0.05) related to init i a l value. 3.4. Detection of Degradation Products According to the weight-changes results, we have expected negligible amounts of 3-hydroxybutyric and crotonic acid. Therefore, only samples reporting the highest weight losses were chosen for the IC analysis. Unfortunately, not even the method of standard addition revealed any content of analytes in the studied matrices. The amounts of released acids most likely l ie under the limits of detection. A typical chromatogram is illustrated in Figure S2 and S3 (Supplementary Materials). These results confirm the previous hypothesis of the degradation mechanism of PHA (discussed in the weight changes section), indicating that polymer specimens do not show any significant weight losses within the initial phase of degradation, whereas the molecular weight changes are apparent. 3.5. Surface Changes As shown in Figure 3, the surface of poly(3-hydroxybutyrate) did not undergo any significant changes during the course of incubation. The pore diameters and frequency were not increased in any of the fluids after the performance of the full test. The initial crystallinity of the homopolymer (corresponding to AHm 75.4 J/g, see Table 3) apparently slowed down the hydrolytic ac t ivity of t he environment, so the less numerous amorphous regions stayed included in the prevailing crystal li ne regions. Time ofincubation (weeks ) Figure 3. SEM micrographs showing surface morphology changes during incubation i n SBFs On the contrary, both copolymers showed notable changes in surface morphology,especially while incubated in ACF, as seen in Figure 3. From the fourth week of i ncubation in ACF, small cracks f illed with submicron globules appeared (red arrows), and their extent raised in the course of degradation. At t he end of incubation, about half of the surface of P34HB-1 was covered by these bodies. These globules are potentially uncovered highly crystal l ine regions that resisted degradation, while the mostly amorphous areas were released. In addi t ion, chains of globules resembling pathways were attached to the surface of P34HB-1 after 4 weeks in ACF (see yellow arrows). This might be the key to explaining t he specimen degradation mechanism. Since t he surface appears very consistent and l argely smooth, the typical activity of hydrolytic enzymes causes surface erosion, resulting in massive irregular porosity [34]. A similar scenario is observable in the later stages of degradation. Hence, enzymolysis cannot be excluded, however, it is quite clear t hat enzymes are not the only source of the degradation course. As mechanical contributors, shear forces may act as an initiator of material disintegration. By attacking the specimen surface, the film could raise hydrolysis susceptibility, which facilitates the spatial enzymolysis and enhances the exposure of crystal l ine partic l es. Concurrently, we presuppose that f ilm contains a highly crystalline surface layer standing for both high rigidity and hydrolytic stability. Subsequently to the sub-surface layer degradation, the surface crystalline coating was peeled off the specimen. Due to its hydrolytic resistance,t hese evolved surface fragments predominantly consist of polymeric and oligomeric chains,while barely undergoing any biodegradation processes. Therefore, the absence of detectable 3-hydroxybutyrate in waste f luids and simultaneous mass reduction of the specimens is j ustified. Compared to P34HB-2, P34HB-1 is conclusively more prone to enzymatic hydrolysis (surface erosion). When related to other materials, the degradation of P(3HB-co-4HB) copolymers in simulated gastral systems i s apparent on the surface and macroscopic morphology. For example, the surface of PCL seems less affected by enzymatic hydrolysis than the presented copolymers here, even if the mass decreases are significant in both cases [35]. The surface erosion of PCL-based copolymers in the presence of pancreatic lipase was concluded by Peng et al. [36]. 3.6. Cytotoxicity Tests The cytotoxicity testing results are presented (Figure 4) on 25% concentration of the ACF (containing l ipases) and PBS to monitor the cytotoxicity of the potential degradation products of studied polymers. As expected, the samples f rom all tested weeks showed no significant difference (at 95% confidence level) related to pure ACF/PBS, excluding P34HB-1 in ACF sampled on the 8th week, which increased the cell viability from 53% (pure ACF)to 86%. Accordingly, neither PBS nor ECF after incubation indicate any inhibition effects on the cell cultures. Moreover, potential copolymer residues might beneficially support the cell growth. These outputs correlate to the theory of the degradation mechanisms discussed above, since the increase of the cell viability appeared as late as the significant mass decreases. Presumably, according to these results, the P34HB-1 film released higher amounts of t he water-soluble degradation products during the 8th week of incubation (monomers, short oligomers), which are possibly utilized by the cell line. To summarize, the degradation products of the tested polymers did not revea l cytotoxic effect with respect to used cell l ine and rather supported growth of the ce ll culture, which stresses the suitability of the tested materials for biomedical applications. P3HB ■ P34HB-1 P34HB -2 —pure f luid Figure 4. The influence of PBS and ACF after polymers incubation on the growth of Caco-2 cell culture expressed as percentual viability related to the fresh fluids. The data values (presented as means ± SD) are expressed as percentual viability related to the control (pure medium). A statistically significant di f ference (p <0.05) is indicated by a cross (X). 4. Conclusions In this study, we have tested the biodegradability/stability of PHB and P(3HB-co-4HB)copolymers in various simulated body fluids. All the tested materials exhibited evident degradability in simulated body fluids. According to the presumption, copolymers showed markedly higher promptness to biodegradation compared to a homopolymer, as observed on surface morphology. On the other hand, results of further analyses also demonstrated the degradation processes i n P3HB f ilms (molecular weight changes, changes in melting enthalpy). No toxic products of the material degradation in waste SBFs were observed on cel l viability. Additionally,3-hydroxybutyrate as a free monomer was not detected by ion-exchange chromatography. It is likely that degradation products consist predominantly of water-soluble oligomers, which were not detected by the chromatographic analysis. Within the f luids used for incubation, synthetic gastric juice appeared as the most aggressive environment due to its low pH, which resulted in the highest weight reductions of both copolymers. Most likely, this weight loss cannot be attributed to enzymatic hydrolysis, and we suggest solely an abiotic consequence of degradation. Artificial colonic fluid appears as a very promising environment for the P(3HB-co-4HB) copolymers biodegradation, apparently causing surface erosion of this material. Possible mechanisms of decomposition are subject to further investigation. There are still many economic challenges and l imitations of t he PHA biotechnological production, which are mainly affec t ed by relatively high production costs and the difficulty of the extraction steps when compared to other biopolymers. Nevertheless, many research groups currently investigate novel production approaches and cultivation strategies in order to increase the competitiveness of these highly promising biopolymers [39-42]. The study presented here is consistent with this concept as far as the thermophilic (bacterial)PHA producent has been employed, which suggests the potential reduction of sterility requirements. The i ntroduced aspects should i mprove the competitiveness of PHA copoly-mers and extend the applicability spec t rum. Furthermore, in-depth exploration of structural and func t ional properties of the presented materials is suggested for further production and development . Supplem ne e n nt t a ary Materials: The following suppor t ing information can be downloaded at: https://www.m dp i.com/ar t icle/10.3390/p olym14101990/s1, Figure S1: DSC thermograms of initial-state polymer films. Figure S2: IC chromatogram of pure ACF with 3HB and crotonic acid standards.Figure S3: IC chromatograms of waste ACF. Table S1: MES composition. Table S2: TES composition.T able S3: composition of phosphate buffer solution (PBS) for films incubation. Table S4: composition of simulated gastric j uice (SGJ). Table S5: composition of artificial colonic fluid (ACF). Author Contributions: Conceptualization and methodology, S.O. and P.S.; investigation, J.V., M.W.,M.T .,Z.J., A.P ., M.K. and E.S.; wri t ing-original draft preparation, J.V. and M.W.; writing-review and editing, S.O., P.S., A.P. and M.K.; visualization, S.O., J.V., Z.J. and M.W.; supervision, S.O. and P.S.; project administration and funding acquisition, J.V. All authors have read and agreed to the publ i shed version of the manuscript. Funding: This work was funded by the grant FCH-K-21-7008 investigated under the project Quality Internal Grants of BUT (KInG BUT ), Reg. No. CZ.02.2.69/ 0.0/0.0/19_073/0016948, which is subsidized from t he OP RDE, Ministry of Education, Youth and Sports, Czech Republic. Informed Consent Statement: Not applicable. The study involved no experiments on humans or animals. The paper properly credits t he contributions of co-authors and co-researchers. All sources used are properly cited. Data Availability Statement: The data that support the findings of t his study are available from t he corresponding author, P.S., upon reasonable request. Acknowledgments: The authors express their acknowledgments to Andrea Nemcova for assisting with photographing and completing the f inal pictures, and Veronika Melcova for assisting with the DSC measurements. Conflicts of Interest: The authors declare no conf li ct of interest. The funders had no role i n t he design of the study; in the collection, analyses, or interpretation of data ; i n the writing of the manuscript, or in the decision to publish the results. References 1. Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022,9,74. [CrossRef ] [PubMed ] 3. Jendrossek, D.; Handrick, R. Microbia l Degradation of Polyhydroxyalkanoates. Annu. Rev. Microbiol. 2002,56,403-432. [CrossRef ]P ubMed l 4. Koller, M.;Mukherjee, A. Polyhydroxyalkanoates—Linking Properties, Applications and End-of-life Options. Chem. Biochem.Eng. Q. 2020,34, 115-129. [CrossRef ] 5. Jaeger, E.K.; Steinbuchel, A.; Jendrossek, D. Substrate specificities of bacterial polyhydroxyalkanoate depolymerases and l ipases:Bacterial l ipases hydrolyze poly(omega-hydroxyalkanoates). Appl . Environ. Microbiol . 1995, 61,3113-3118. [CrossRef ] 6. Mok, P.-S.; Ch'Ng,D.H.-E.;Ong, S.-P.; Numata, K.; Sudesh, K. Characterization of the depolymerizing activity of commercial lipases and detection of lipase-like act i vities in animal organ extracts using poly(3-hydroxybutyrate-co-4-hydroxybutyrate) t hin film. AMB Express 2016, 6, 97. [CrossRef ] 7. Keridou, I .; Franco, L.; Del Valle, L.J.; Martinez, J.C.; Funk, L .; Turon, P .; Puiggali, J. Microstructural Changes during Degradation of Biobased Poly(4-Hydroxybutyrate) Sutures. Polymers 2020, 12, 2024.[CrossRef] [PubMed ] 8. Keridou, I .; Franco, L.; del Valle, L.J;Martinez, J.C.; Funk, L.; Turon, P.; Puiggali , J . Hydrolytic and enzymatic degradation of biobased poly(4-hydroxybutyrate) fi lms . Selective etching of spherulites. Polym. Degrad. Stab. 2020, 183,109451. [CrossRef ] 9. Boyandin, A.N.; Prudnikova, S.V.; Fi li penko, M.L .; Khrapov, E.A.; Vasil'Ev, A.D.; Volova, T.G. Biodegradation of polyhydroxyalka- noates by soil microbial communities of different structures and detection of PHA degrading microorganisms. Appl. Biochem.Microbiol. 2011,48, 28-36. [CrossRef ] 10. Prudnikova, S.V.; Vinogradova,O.N.; Trusova, M.Y. Specific character of bacterial biodegradation of polyhydroxyalkanoates with different chemical structure in soi l . Dokl. Biochem. Biophys. 2017,473,94-97.[CrossRef ] 11. Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as t issue engineering materials. Biomaterials 2005, 26, 6565-6578.[CrossRef ] [PubMed ] 12. Luo, Z.; Wu, Y.; Li, Z.; Loh, X.J. Recent Progress in Polyhydroxyalkanoates-Based Copolymers for Biomedical Applications.Biotechnol. J . 2019,14,1900283.[CrossRef] [PubMed ] 13. Rodriguez-Contreras, A. Recent Advances in the Use of Polyhydroyalkanoates in Biomedicine. Bioengineering 2019, 6,82.[CrossRef ] [PubMed ] 14. Pernicova, I.; Novackova, I.; Sedlacek, P.; Kourilova, X.; Kalina, M.; Kovalcik, A.; Koller, M.; Nebesarova, J.; Krzyzanek, V.;Hrubanova, K.; et al . Introducing the Newl y Isolated Bacterium Aneurinibacillus sp. H1 as an Auspicious Thermophilic Producer of Various Polyhydroxyalkanoates (PHA) Copolymers-1. Isolation and Characterization of the Bacterium. Polymers 2020,12,1235.[CrossRef l 15. C Obruca, S.; Benesova,P; Oborna, J.; Marova, I . Application of protease-hydrolyzed whey as a complex nitrogen source to increase poly(3-hydroxybutyrate) production from oils by Cupriavidus necator. Biotechnol. Lett. 2013,36,775-781. [CrossRef] 16. Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Fluids. Dissolution Technol. 2011,18,15-28.[CrossRef ] 17. Sedlacek, P.; Pernicova , I.; Novackova, I.; Kourilova, X.; Kalina, M.; Kovalcik, A.; Koller, M.; Nebesarova, J .; Krzyzanek, V.;Hrubanova, K.; et al . Introducing the Newly Isolated Bacterium Aneurinibacillus sp. H1 as an Auspicious Thermophilic Producer of Various Polyhydroxyalkanoates (PHA) Copolymers -2. Material Study on the Produced Copolymers. Polymers 2020, 12,1298.[CrossRef l 18. Li, X.; Turánek, J.; Knotigova, P.; Kudlackova, H.; Masek, J .; Parkin, S.; Rankin, S.; Knutson, B.L .; Lehmler, H.-J. Hydrophobic tai l length, degree of fluorination and headgroup stereochemistry are determinants of the biocompatibility of (fluorinated)carbohydrate surfactants. Colloids Surf. B Biointerfaces 2009,73,65-74.[CrossRef ] 19. Volova, T .G.; Gladyshev, M.I.; Trusova, M.Y.; Zhila, N.O. Degradation of polyhydroxyalkanoates and t he composition of microbial destructors under natural conditions. Microbiology 2006,75,593-598.[CrossRef ] 20. Qu, X.-H.; Wu, Q.; Zhang, K.-Y.; Chen, G. In vivo studies of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) based polymers:Biodegradation and tissue reactions. Biomaterials 2006, 27, 3540-3548. [CrossRef ] 21. Boskhomdzhiev, A.P.; Bonartsev, A.P.; Makhina, T.K.; Myshkina, V.L.;Ivanov, E.A.; Bagrov, D.;Filatova, E.V.;Iordanskii, A.L.;Bonartseva, G. Biodegradation kinetics of poly(3-hydroxybutyrate)-based biopolymer systems. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem. 2010,4,177-183. [CrossRef ] 22. Gutierrez-Wing, M.T.; Stevens, B.E.; Theegala, C.S.; Negulescu, I.I.; Rusch, K.A. Aerobic Biodegradation of Polyhydroxybutyrate in Compost. Environ. Eng. Sci. 2011,28,477-488.[CrossRef] 23. Deroine, M.; Cesar, G.; Le Duigou, A.; Davies,P.; Bruzaud, S. Natura l Degradation and Biodegradation of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) in Liquid and Solid Marine Environments. J. Polym. Environ. 2015, 23, 493-505. [CrossRe f ] 24. Han, J; Wu, L.; Liu, X.-B.; Hou, J.; Zhao, L .-L.; Chen, J .-Y.; Zhao, D.-H.; Xiang, H. Biodegradation and biocompatibility of haloarchaea-produced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymers. Biomaterials 2017,139, 172-186. [CrossRef ][PubMed ] 25. Zhuikov, V.A.; Zhuikova, Y.V.; Makhina, T.K.; Myshkina, V.L.; Rusakov, A.; Useinov, A.; Voinova, V.V.; Bonartseva, G.A.; Berlin,A.A.; Bonartsev, A.P.; et al. Comparative Structure-Property Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)s Films under Hydrolytic and Enzymatic Degradation: Finding a Transition Point in 3-Hydroxyvalerate Content. Polymers 2020, 12,728. [CrossRef ] 26. Kovalcik, A.;Obruca, S.; Kalina, M.; Machovsky, M.; Enev, V.;Jakesova,M.; Sobkova, M.;Marova, I . Enzymatic Hydrolysis of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Scaffolds. Materials 2020, 13, 2992.[CrossRef ] 27..Chuah,J.-A.; Yamada, M.; Taguchi,S.; Sudesh, K.;Doi, Y.;Numata, K. Biosynthesis and characterization of polyhydroxyalkanoate containing 5-hydroxyvalerate units: Effects of 5HV units on biodegradability, cytotoxicity, mechanical and thermal properties.Polym. Degrad. Stab. 2012, 98,331-338. [CrossRef ] 28. Freier, T.; Kunze, C.; Nischan, C.; Kramer, S.; Sternberg,K.;SaB, M.; Hopt, U.T .; Schmitz, K.-P. In vitro and in vivo degradation studies for development of a biodegradable patch based on poly(3-hydroxybutyrate). Biomaterials 2002, 23, 2649-2657. [CrossRef ] 29. YDoi, Y.; Kanesawa, Y.; Kunioka, M.; Saito, T. Biodegradation of microbial copolyesters: poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1990, 23, 26-31.[CrossRef ] 30. Bonartsev, A.; Boskhomodgiev, A.P.; Iordanskii, A.L.; Bonartseva, G.; Rebrov , A.V.;Makhina, T.K.; Myshkina, V.L.; Yakovlev, S.A.;Fi l atova, E.A.; Ivanov, E.A.; et al . Hydrolytic Degradation of Poly(3-hydroxybutyrate), Polylactide and their Derivatives: Kinetics,Crystallinity, and Surface Morphology. Mol. Cryst. Liq. Cryst. 2012,556,288-300.[CrossRef 31. C Olkhov, A.; Tyubaeva, P.; Vetcher, A.; Karpova, S.; Kurnosov, A.; Rogovina, S.; Iordanskii, A.; Berlin, A. Aggressive Impacts Affecting the Biodegradable Ultrathin Fibers Based on Poly(3-Hydroxybutyrate), Polylactide and Their Blends: Water Sorption,Hydrolysis and Ozonolysis. Polymers 2021, 13, 941. [CrossRef ] [PubMed ] Numata, K.; Abe, H.; Iwata, T. Biodegradability of Poly(hydroxyalkanoate) Materials. Materials 2009, 2, 1104-1126. [CrossRef ]33Chang, H.-M.; Huang, C .-C.; Tsai, H.-C.; Imae, T .; Hong, P.-D. Characterization and morphology analysis of degradable poly(l-lactide) film in in-vitro gastric juice incubation. Appl. Surf. Sci. 2012,262,89-94.[CrossRef ] 34. Tarazona, N.A.;Machatschek, R.; Lendlein, A. Inf l uence of Depolymerases and Lipases on the Degradation of Polyhydroxyalka-noates Determined i n Langmuir Degradation Studies. Adv. Mater. Interfaces 2020,7,2000872. [CrossRef ] 35. Chang, H.-M.; Prasannan, A.; Tsai , H.-C.; Jhu , J.-J. Ex vivo evaluation of biodegradable poly(e-caprolactone) f ilms in digestive fluids. Appl. Surf. Sci . 2014,313,828-833. [CrossRef ] 36. Peng, H.; Ling, J;Liu, J.; Zhu, N.; Ni, X.; Shen, Z. Controlled enzymatic degradation of poly(e-caprolactone)-based copolymers in the presence of porcine pancreatic lipase. Polym. Degrad. Stab. 2010,95,643-650. [CrossRef ] 37. Ladd, M.R.; Costello, C.M.; Gosztyla, C.; Werts, A.D.; Johnson, B.; Fulton, W.B.; Martin, L .Y.; Redfield, E.J .; Crawford, B.;Panaparambil , R.; et al . Development of Intestinal Scaffolds that Mimic Native Mammalian Intestinal Tissue. Tissue Eng. Part A 2019,25,1225-1241.[CrossRef ] 38. Wu, L.; Li, X.; Li, P.; Pan, L.; Ji, Z.; Feng, Y.; Shi, C. Bioabsorbable flexible elastomer of PTMC-b-PEG-b-PTMC copolymer as intestinal anastomosis scaffold. Polym. Adv. Technol. 2021,32,3633-3645. [CrossRef ] 39. Pernicova,I.; Kucera, D.; Nebesarova,J .; Kalina, M.;Novackova, I .;Koller, M.; Obruca, S. Production of polyhydroxyalkanoates on waste f rying oi l employing selected Halomonas strains. Bioresour. Technol . 2019,292,122028.[CrossRef ] 40. Kourilova, X.; Pernicova, I.; Sedlar, K.; Musilova,J.; Sedlacek, P.; Kalina, M.; Koller, M.; Obruca, S. Production of polyhydrox-yalkanoates (PHA) by a thermophilic strain of Schlegelella thermodepolymerans from xylose rich substrates. Bioresour. Technol .2020,315,12388.[CrossRe f 41. Sabbagh, F;Muhamad, I.I. Production of poly-hydroxyalkanoate as secondary metabolite with main focus on sustainable energy.Renew. Sustain. Energy Rev. 2017, 72,95-104. [CrossRef ]
确定










还剩12页未读,是否继续阅读?
产品配置单
中国格哈特为您提供《模拟体液中(3-羟基丁酸酯)-(4-羟基丁酸酯)聚合物(PHA聚羟基脂肪酸酯)薄膜样品的提取》,该方案主要用于可降解材料中含量检测,参考标准--,《模拟体液中(3-羟基丁酸酯)-(4-羟基丁酸酯)聚合物(PHA聚羟基脂肪酸酯)薄膜样品的提取》用到的仪器有格哈特全自动快速溶剂萃取仪Sox416、德国加液器MM、滤纸筒
相关方案
更多
该厂商其他方案
更多












