方案详情
文
应用了Unisense氧气微电极穿刺老鼠肿瘤组织内1mm深处的氧气浓度。同时结合氧微电极测试了老鼠体内注射PNPs(全氟碳纳米颗粒)后并应用激光照射后测试老鼠肿瘤组织内的氧气浓度。
方案详情

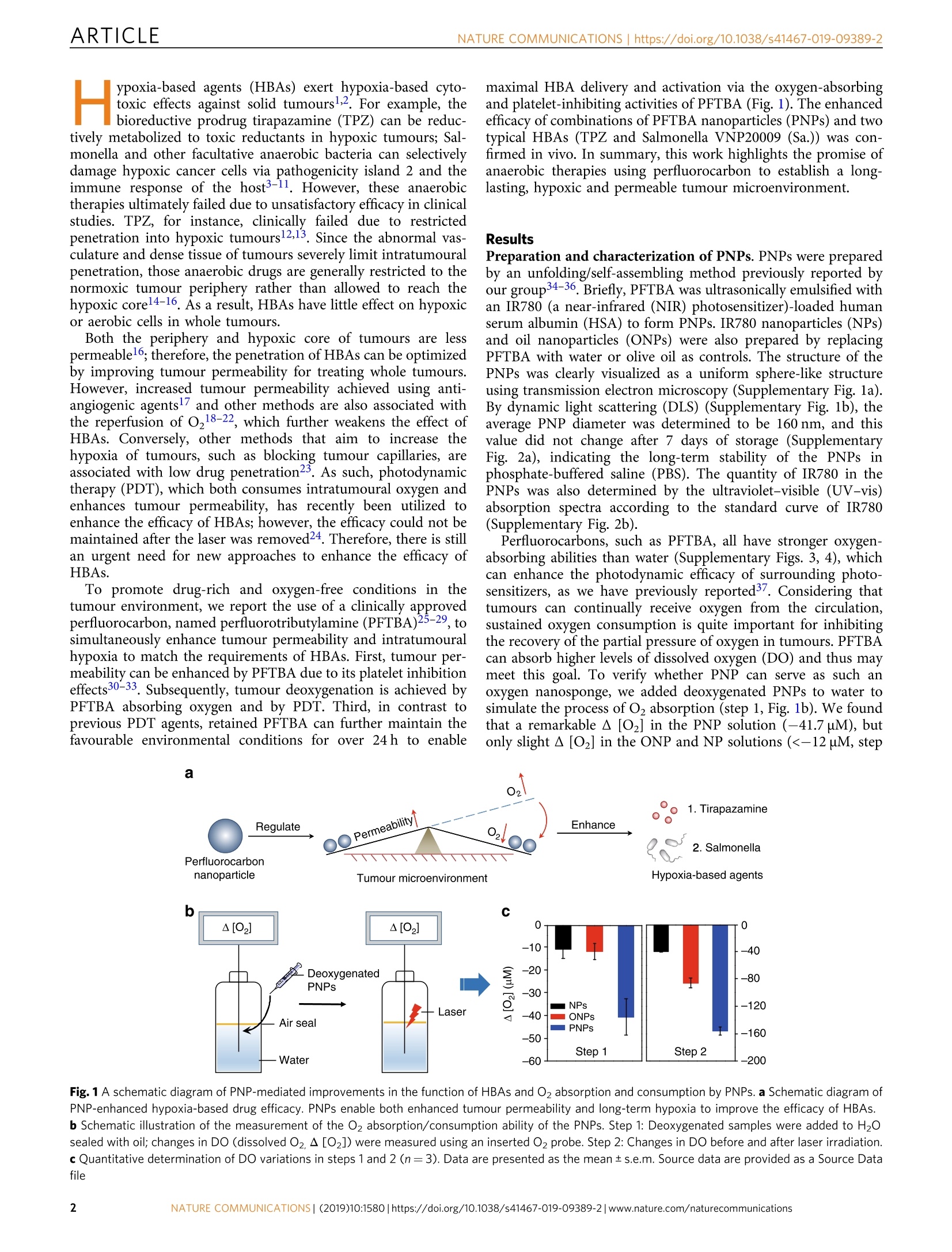
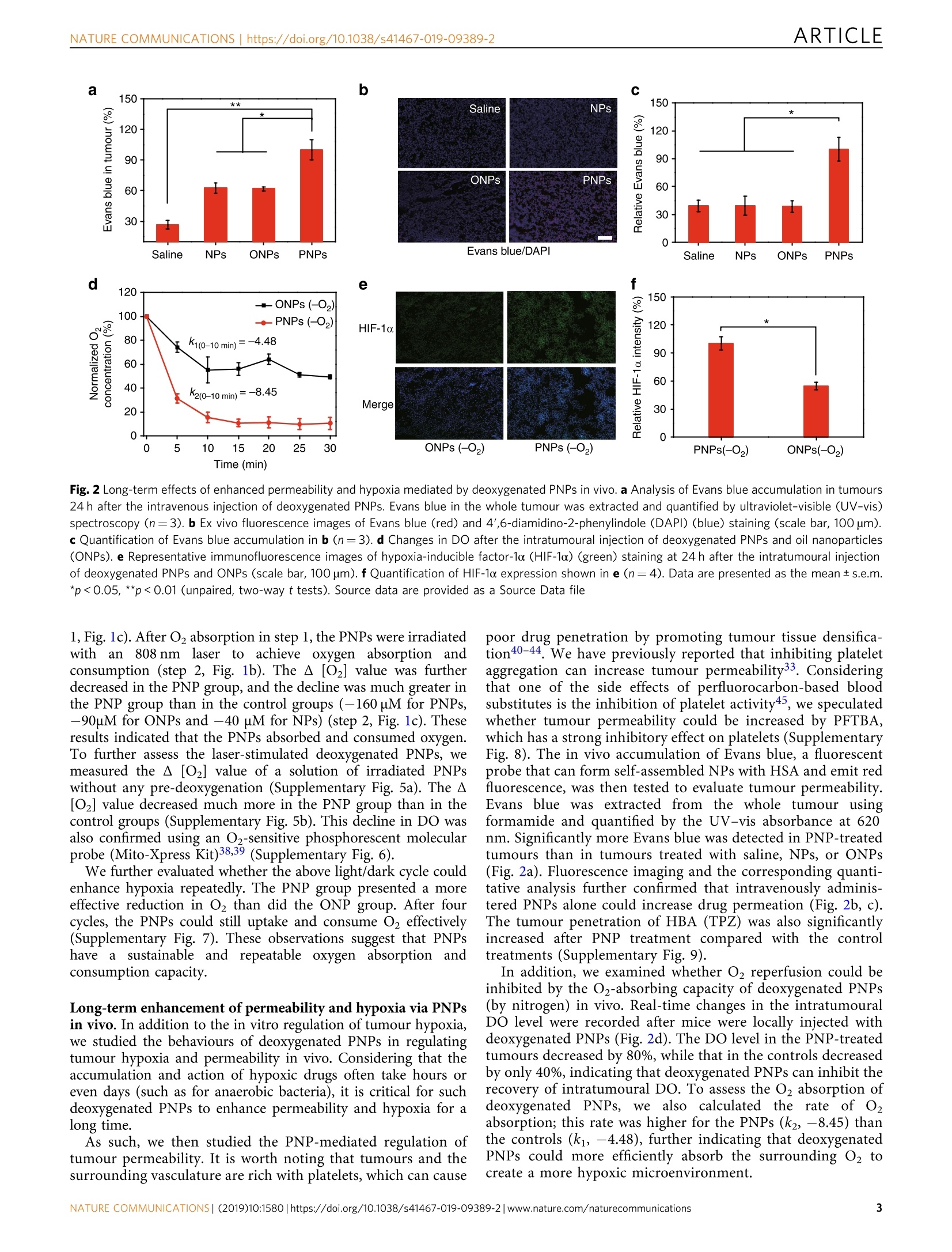
ARTICLEOPEN ARTICLENATURE COMMUNICATIONS |https://doi.org/10.1038/s41467-019-09389-2 https://doi.org/10.1038/s41467-019-09389-2 Wenguang Wang12, Yuhao Cheng12, Peng Yu12, Haoran Wang12,Yue Zhang12, Haiheng Xu12, Qingsong Ye12,Ahu Yuan12, Yiqiao Hu1,2,3 & Jinhui Wu12,3 Perfluorocarbon regulates the intratumouralenvironment to enhance hypoxia-based agentefficacv Hypoxia-based agents (HBAs), such as anaerobic bacteria and bioreductive prodrugs, requireboth a permeable and hypoxic intratumoural environment to be fully effective. To solve thisproblem, herein, we report that perfluorocarbon nanoparticles (PNPs) can be used to create along-lasting, penetrable and hypoxic tumour microenvironment for ensuring both the deliveryand activation of subsequently administered HBAs. In addition to the increased permeabilityand enhanced hypoxia caused by the PNPs, the PNPs can be retained to further achieve thelong-term inhibition of intratumoural O2 reperfusion while enhancing HBA accumulation forover 24 h. Therefore, perfluorocarbon materials may have great potential for reignitingclinical research on hypoxia-based drugs. ( I State K ey Laboratory of P h armaceutical Bi o technology, Medical Sch o ol of Nanjing Univ e rsity and Scho o l of Life Scie n ces, Nanj i ng University, 210093 Nanjing, China. 2Institute o f Drug R&D, Nanjing Uni v ersity, 210093 N anjing, China. 3Jiangsu Provincial Key Laboratory for Nano T echnology, N anjing U niversity, 210093 Nanjing, C hina. T hese authors contributed equally: Wenguang Wang, Yuha o Cheng, Peng Yu. Co r re s p ondence and requests for material s should b e addressed to A.Y. ( email: yua nn j u@n j u.e du . c n) o r t o Y.H. (email: h u y i q ia o @ n j u . edu . c n) or to J.W. (email : w u j @n j u . e du . cn ) ) ypoxia-based agents (HBAs) exert hypoxia-based cyto-Htoxic effects against solid tumours1.2. For example, thebioreductive prodrug tirapazamine (TPZ) can be reduc-tively metabolized to toxic reductants in hypoxic tumours; Sal-monella and other facultative anaerobic bacteria can selectivelydamage hypoxic cancer cells via pathogenicity island 2 and theimmune response of the host3-11. However, these anaerobictherapies ultimately failed due to unsatisfactory efficacy in clinicalstudies. TPZ, for instance, clinically failed due to restrictedpenetration into hypoxic tumours12,13. Since the abnormal vas-culature and dense tissue of tumours severely limit intratumouralpenetration, those anaerobic drugs are generally restricted to thenormoxic tumour periphery rather than allowed to reach thehypoxic corel4-16. As a result, HBAs have little effect on hypoxicor aerobic cells in whole tumours. Both the periphery and hypoxic core of tumours are lesspermeable16; therefore, the penetration of HBAs can be optimizedby improving tumour permeability for treating whole tumours.However, increased tumour permeability achieved using anti-angiogenic agentsl7 and other methods are also associated withthe reperfusion of O18-22, which further weakens the effect ofHBAs. Conversely, other methods that aim to increase thehypoxia of tumours, such as blocking tumour capillaries, areassociated with low drug penetration23. As such, photodynamictherapy (PDT), which both consumes intratumoural oxygen andenhances tumour permeability, has recently been utilized toenhance the efficacy of HBAs; however, the efficacy could not bemaintained after the laser was removed24. Therefore, there is stillan urgent need for new approaches to enhance the efficacy ofHBAs. To promote drug-rich and oxygen-free conditions in thetumour environment, we report the use of a clinically approvedperfluorocarbon, named perfluorotributylamine (PFTBA)25-29, tcsimultaneously enhance tumour permeability and intratumouralhypoxia to match the requirements of HBAs. First, tumour per-meability can be enhanced by PFTBA due to its platelet inhibitioneffects30-33. Subsequently, tumour deoxygenation is achieved byPFTBA absorbing oxygen and by PDT. Third, in contrast toprevious PDT agents, retained PFTBA can further maintain thefavourable environmental conditions for over 24h to enable maximal HBA delivery and activation via the oxygen-absorbingand platelet-inhibiting activities of PFTBA (Fig.1). The enhancedefficacy of combinations of PFTBA nanoparticles (PNPs) and twotypical HBAs (TPZ and Salmonella VNP20009 (Sa.)) was con-firmed in vivo. In summary, this work highlights the promise ofanaerobic therapies using perfluorocarbon to establish a long-lasting, hypoxic and permeable tumour microenvironment. Results Preparation and characterization of PNPs. PNPs were preparedby an unfolding/self-assembling method previously reported byour group34-36. Briefly, PFTBA was ultrasonically emulsified withan IR780 (a near-infrared (NIR) photosensitizer)-loaded humanserum albumin (HSA) to form PNPs. IR780 nanoparticles (NPs)and oil nanoparticles (ONPs) were also prepared by replacingPFTBA with water or olive oil as controls. The structure of thePNPs was clearly visualized as a uniform sphere-like structureusing transmission electron microscopy (Supplementary Fig. la).By dynamic light scattering (DLS) (Supplementary Fig. 1b), theaverage PNP diameter was determined to be 160 nm, and thisvalue did not change after 7 days of storage (SupplementaryFig. 2a), indicating the long-term stability of the PNPs inphosphate-buffered saline (PBS). The quantity of IR780 in thePNPs was also determined by the ultraviolet-visible (UV-vis)absorption spectra according to the standard curve of IR780(Supplementary Fig. 2b). Perfluorocarbons, such as PFTBA, all have stronger oxygen-absorbing abilities than water (Supplementary Figs. 3, 4), whichcan enhance the photodynamic efficacy ofsurrounding photo-sensitizers, as we have previously reported37. Considering thattumours can continually receive oxygen from the circulation,sustained oxygen consumption is quite important for inhibitingthe recovery of the partial pressure of oxygen in tumours. PFTBAcan absorb higher leveenBoals of dissolved oxygen (DO) and thus maymeet this goal. To verify whether PNP can serve as such anoxygen nanosponge, we added deoxygenated PNPs to water tosimulate the process of Oz absorption (step 1, Fig. 1b). We foundthat a remarkable A [O2] in the PNP solution (-41.7uM), butonly slight A[O2] in the ONP and NP solutions (<-12 uM, step Fig. 1 A schematic diagram of PNP-mediated improvements in the function of HBAs and O2 absorption and consumption by PNPs. a Schematic diagram ofPNP-enhanced hypoxia-based drug efficacy. PNPs enable both enhanced tumour permeability and long-term hypoxia to improve the efficacy of HBAs.b Schematic illustration of the measurement of the O2 absorption/consumption ability of the PNPs. Step 1: Deoxygenated samples were added to H2Osealed with oil; changes in DO (dissolvedO2,A[O2]) were measured using an inserted O2 probe. Step 2: Changes in DO before and after laser irradiation.c Quantitative determination of DO variations in steps 1 and 2 (n=3). Data are presented as the mean ±s.e.m. Source data are provided as a Source Datafile a b c 150 ** 150 Saline NPs c * 120 90 ONPs PNPs 60 一 120 c 30 90 60 30 Saline NPs ONPs PNPs Evans blue/DAPI Saline NPs ONPs PNPs Fig. 2 Long-term effects of enhanced permeability and hypoxia mediated by deoxygenated PNPs in vivo. a Analysis of Evans blue accumulation in tumours24 h after the intravenous injection of deoxygenated PNPs. Evans blue in the whole tumour was extracted and quantified by ultraviolet-visible (UV-vis)spectroscopy (n=3). b Ex vivo fluorescence images of Evans blue (red) and 4',6-diamidino-2-phenylindole (DAPI) (blue) staining (scale bar, 100 um).c Quantification of Evans blue accumulation in b (n=3). d Changes in DO after the intratumoural injection of deoxygenated PNPs and oil nanoparticles(ONPs). e Representative immunofluorescence images of hypoxia-inducible factor-1o (HIF-1o) (green) staining at 24 h after the intratumoural injectionof deoxygenated PNPs and ONPs (scale bar, 100 um). fQuantification of HIF-1o expression shown in e (n=4). Data are presented as the mean±s.e.m.*p<0.05, **p<0.01 (unpaired, two-way t tests). Source data are provided as a Source Data file 1, Fig.1c). After O2 absorption in step 1, the PNPs were irradiatedwith aann 8808 nm laser to achieve oxygen absorption andconsumption (step 2, Fig. 1b). The A [O2] value was furtherdecreased in the PNP group,and the decline was much greater inthe PNP group than in the control groups (-160 uM for PNPs,-90uM for ONPs and -40 uM for NPs) (step 2, Fig. 1c). Theseresults indicated that the PNPs absorbed and consumed oxygen.To further assess the laser-stimulated deoxygenated PNPs, wemeasured the A [O2] value of a solution of irradiated PNPswithout any pre-deoxygenation (Supplementary Fig. 5a). The A[O2] value decreased much more in the PNP group than in thecontrol groups (Supplementary Fig. 5b). This decline in DO wasalso confirmed using an O2-sensitive phosphorescent molecularpr( e(Mito )ress Kit)38,39 entar We further evaluated whether the above light/dark cycle couldenhance hypoxia repeatedly. The PNP group presented a moreeffective reduction in O2 than did the ONP group. After fourcycles, the PNPs could still uptake and consume O2 effectively(Supplementary Fig. 7). These observations suggest that PNPshavea sustainable and repeatable oxygennabsorption andconsumption capacity. Long-term enhancement of permeability and hypoxia via PNPsin vivo. In addition to the in vitro regulation of tumour hypoxia,we studied the behaviours of deoxygenated PNPs in regulatingtumour hypoxia and permeability in vivo. Considering that theaccumulation and action of hypoxic drugs often take hours oreven days (such as for anaerobic bacteria), it is critical for suchdeoxygenated PNPs to enhance permeability and hypoxia for along time. As such, we then studied the PNP-mediated regulation oftumour permeability. It is worth noting that tumours and thesurrounding vasculature are rich with platelets, which can cause Ppoor drug penetration by promoting tumour tissue densifica-tion40-44. We have previously reported that inhibiting plateletaggregation can increase tumour permeability33. Consideringthat one of the side effects of perfluorocarbon-based bloodsubstitutes is the inhibition of platelet activity45, we speculatedwhether tumour permeability could be increased by PFTBA,which has a strong inhibitory effect on platelets (SupplementaryFig. 8). The in vivo accumulation of Evans blue, a fluorescentprobe that can form self-assembled NPs with HSA and emit redfluorescence, was then tested to evaluate tumour permeability.Evans blue was eextracted from the whole tumour usingformamide and quantified by the UV-vis absorbance at 620nm.Significantly more Evans blue was detected in PNP-treatedtumours than in tumours treated with saline, NPs, or ONPs(Fig. 2a). Fluorescence imaging and the corresponding quanti-tative analysis further confirmed that intravenously adminis-tered PNPs alone could increase drug permeation (Fig. 2b, c).The tumour penetration of HBA (TPZ) was also significantlyincreased after PNP treatment compared with the controltreatments (Supplementary Fig. 9). In addition, we examined whether O2 reperfusion could beinhibited by the O2-absorbing capacity of deoxygenated PNPs(by nitrogen) in vivo. Real-time changes in the intratumouralDO level were recorded after mice were locally injected withdeoxygenated PNPs (Fig.2d). The DO level in the PNP-treatedtumours decreased by 80%, while that in the controls decreasedby only 40%, indicating that deoxygenated PNPs can inhibit therecovery of intratumoural DO. To assess the O2 absorption ofdeoxygenated PNPs,S,we also calculated the rate of O,absorption; this rate was higher for the PNPs (k2, -8.45) thanthe controls (ki, -4.48), further indicating that deoxygenatedPNPs could more efficiently absorb the surrounding 02 tocreate a more hypoxic microenvironment. Pimonidazole/DAPI Fig. 3 Increased tumour permeability and hypoxia mediated by deoxygenated PNPs under laser irradiation. a The accumulation of Evans blue in tumourswas quantified 24 h after the intravenous injection of PNPs and 3 h after laser irradiation (5 min, 808 nm, 400mWcm-2),(n=3). b Ex vivo fluorescenceimages of Evans blue (red) in tumour sections from a. Nuclei were stained with 4',6-diamidino-2-phenylindole(DAPI) (blue), (scale bar, 100 um), (n=3).c Relative quantification of Evans blue accumulation in b(n=4). d Changes in dissolved oxygen (DO) during three cycles of laser irradiation (808 nm, 2 Wcm-2, 20 s) after intratumoural administration. e Changes in DO after laser irradiation (808 nm, 400 mW cm-2) for 5 min at 24 h post-intravenousinjection (n=3). f Western blot of intratumoural hypoxia-inducible factor-1o(HIF-1α) expression at 24 h after laser irradiation. g, h Tumour sections werestained with anti-pimonidazole antibody (green), anti-HIF-1o antibody (green) and DAPI (blue) at 24 h post irradiation (scale bars, 200 um).i Quantification of pimonidazole and HIF-1o staining (n3). Data are presented as the mean ±s.e.m. *p<0.05, **p<0.01 and ***p<0.001 (unpaired, two-way t tests). Source data are provided as a Source Data file Hypoxia-inducible factor-1a (HIF-1a) staining was performed24 h after injection. Significant green fluorescence was observed intumours treated with deoxygenated PNPs, whereas cells treatedwith1ONPsstillshowed negligible fluorescence (Fig.2e).Quantitative analysis of the HIF-1a expression in tumour tissuetreated with deoxygenated PNPs showed double the expressionobserved in tumour tissue treated with ONPs (Fig. 2f), suggestingthat PNPs could actively extract surrounding O2, resulting inlong-lasting tumour hypoxia. Irradiation of deoxygenated PNPs to regulate permeability andhypoxia. To evaluate the effect of laser irradiation on theobserved hypoxia, the effects of deoxygenated PNPs on hypoxiaand permeability after laser stimulation were evaluated in vitro.Enhanced cellular hypoxia was first confirmed using a hypoxiastress detection kit. The results showed that the hypoxia signals incells treated with laser-stimulated PNPswere significantlyincreased compared to those in cells treated with NPs and ONPs(Supplementary Fig. 10a). Cellular hypoxia was also confirmedusing anti-HIF-1a antibody (Supplementary Fig. 10b), suggesting that longer-lasting hypoxic environments could be created by thestimulated PNPs with a laser. The in vitro generation of singletoxygen (1O2) under different O2 environments was confirmed,and the cellular 102 level was determined (SupplementaryFig. 10c, 11). The laser stimulation of PNPs also resulted in thebest cytotoxicity among all treatments (Supplementary Fig. 12);this approach might enhance tumour penetration by severelydamaging the dense tumour tissue. To explore the distribution of the PNPs in vivo, intrave-nously dosed mice were examined by NIR imaging at 12, 24 and36hhpost administration(Supplementary Fig.13). PNPsemitted the strongest fluorescence in tumours 24 h postinjection, and this time point was chosen for performing laserirradiation if needed. Laser-stimulated 102 generation in vivowasthen detected using 2', 7'-dichlorodihydrofluoresceindiacetate (HDCFDA) (Supplementary Fig. 14). In addition,the effect of photothermal treatment was evaluated in vivo. Noobvious temperature increase sufficient to effectively ablatetumours was observed in the PNP or NP groups (Supplemen-tary Fig. 15). ONPs+TPZ PNPs+TPZ 6 8 9 C2500 -TPZ +Saline 2000 -PNPs -ONPs 1500 1000 500 0 ) 10 11 12 Time (days) f Saline PNPs+TPZ 吖工 Fig. 4 PNPs enhanced TPZ chemotherapy. a Fluorescence images of CT26 cells cultured with TPZ and oil nanoparticles (ONPs) or PNPs under laserirradiation (808 nm, 400 mWcm-2). Viable cells were stained with Calcein-AM (green), and dead/late apoptotic cells were stained with propidium iodide(Pl) (red), (scale bar, 100um). b Viability of treated cells (n=3). c Tumour growth curves of treated mice (n≥6 per group). Both PNPs and TPZ wereinjected intravenously into mice on days 1, 3 and 5. Tumours in groups 3, 4, 5 and 6 were irradiated three times (808 nm, 400mW cm-2) for 5 min at 24 hafter the administration of the PNPs. d Normalized averages of the tumour weight on day 12. e Changes in body weight. f Haematoxylin and eosin (H&E)(scale bars, 100 um) and terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) (green) (scale bars, 100 um) staining of CT26 tumoursections.Samples were collected from different groups on day 8 post administration. Data are presented as the mean± s.e.m. *p<0.05, **p<0.01, *** p<0.001 (unpaired, two-way t tests). Source data are provided as a Source Data file To evaluate the effects of irradiated PNPs on HBA permeationin vivo, Evans blue was employed as a fluorescent probe to reflecttumour permeability. In addition to the platelet inhibition ofPNPs, in vivo 102 production was expected to cause tumourtissue damage, thus further improving drug accumulation andpermeation through vascular infiltration, as well as tumourapoptosis and necrosis. Evans blue was extracted from wholetumour and analysed 3 h post irradiation (Fig. 3a). Twice as muchEvans blue was present in tumours treated with PNPs than intumours treated with saline, NPs or ONPs. Tumour sections(Fig. 3b) and the corresponding quantified data (Fig. 3c) alsosuggested that PNP-mediated PDT significantly increased thepenetration of Evans blue. Therefore, tumour permeability couldbe further improved with the application of PDT. To evaluate the effects of laser-irradiated PNPs on the tumourhypoxia status, in vivo real-time oxygen monitoring was employedto reflect the change in DO post irradiation. Tumour hypoxiacaused by O2/02 conversion was explored in situ using a real-timeO2 microelectrode inserted into the tumour. We first evaluated whether PNP-based oxygen consumption occurred in a laser-responsive manner. Exposure to a 2W cm-2 808 nm laser for 20 swas used to study the triggered hypoxia, and changes in intracellularO2 were monitored immediately after PNPs were injected into miceintratumourally. DO in the tumours was significantly decreasedafter laser exposure. Additionally, the DO level would recoverimmediately after cessation of the short-term laser irradiation,mainly due to the incomplete activation of PNPs (Fig. 3d). Considering the potential for skin damage with high-intensitylaser irradiation, we then applied 400 mW cm-2 laser irradiationfor 5 min to create a hypoxic environment within the tumour.Similarly, PNPs could significantly decrease the tumour O2 level,resulting in more hypoxia than observed in the other groups(Fig. 3e). Interestingly, we found that the DO level continued todecline even after the laser had been removed and did not recoverfor a long period. This may be due to the transient occlusion ofthe oxygen supply from the circulation by the damage of tumourvessels (Supplementary Fig. 16) and the oxygen absorption ofdeoxygenated PFTBA (Fig. 2d-f). Fig. 5 PNPs enabled hypoxia-based bacterial cancer therapy. a The hypoxia intensity, Salmonella VNP20009(Sa.) accumulation and nuclei weredetermined by staining with anti-glut-1 antibody (red), anti-Sa. antibody (green) and 4',6-diamidino-2-phenylindole (DAPI) (blue), respectively (scale bar,100 um). Sa. (5×106CFU) was intravenously administered to mice 1 h before irradiation. The mice were exposed to the laser (808 nm, 400 mW cm-2) for5 min at 24 h after they were intravenously injected with PNPs, oil nanoparticles (ONPs) or saline. Immunofluorescence images of tumour sections wereanalysed on day 3 post irradiation (n≥3). b Semiquantitative analysis of the relative hypoxia intensity (the black y-axis) and Sa. distribution (the red y-axis)in a. c The distribution of Sa. in tumours was also measured by colony-forming assay on day 3 (n=3). d Tumour growth curves of mice subjected todifferent treatments (n=4-9). e Normalized tumour weight at day 10 post treatment. f Haematologic indexes and blood biochemistry of mice that wereintravenously injected with VNP20009. The experiment was carried out on days 1 and 7 after the injection of VNP20009 (5×106 CFU mouse-1, n=3)and saline. Values are the mean ±s.e.m. *p<0.05, **p<0.01, ***p<0.001 (unpaired,two-way t tests). Source data are provided as a Source Data file Next, we investigated whether laser-stimulated PNPs could beused to maintain tumour hypoxia for longer periods, so weperformed pimonidazole and HIF-1a staining at24h postirradiation. Pimonidazole can form protein adducts only underhypoxic conditions, and the hypoxia intensity is then indicated bygreen fluorescence. Significantly enhanced green fluorescence wasobserved in the PNP group compared with the control groups(Fig. 3g). Similarly, the HIF-1a fluorescence was significantlystronger in the PNP-treated tumours than in those treated with NPs(Fig. 3h). The quantitative results showed that the hypoxia intensity(pimonidazole and HIF-1a) in PNP-treated tumour sections wastwo times higher than that in ONP-treated tumour sections(Fig. 3i), indicating the superior ability of PNPs to create andmaintain tumour hypoxia after laser irradiation. Western blotting was also used to investigate tumour hypoxiaat 24 h after the treatment. The results showed that, after laser irradiation, the HIF-1a expression in the PNP-treated tumourswas much higher than that in the control groups (Fig. 3f). Thequantitative results showed that the expression of HIF-1a in thePNP group was almost 2 times as much as that in the ONPgroup (Supplementary Fig. 17), indicating that PNPs couldcreate and maintain long-term tumour hypoxia for at least 24 hin vivo. PNPs enhanced TPZ chemotherapy. To explore whether thePNPs could synergistically improve the efficacy of HBAs intreating tumours, we chose TPZ, a widely studied bioreductiveprodrug, as a typical HBA. We applied laser-stimulated PNPs tobuild a permeable and hypoxic environment that could bemaintained long enough for the in vivo study and was easy toachieve. Calcein-AM/propidium iodide (PI) apoptosis detectionwas first performed to measure the apoptosis-inducing capability of the HBA plus the PNPs in vitro. All cells showed greenfluorescence before laser irradiation, indicating that both thePNPs and TPZ were non-toxic (Supplementary Fig. 18). Afterirradiation, cells treated with PNPs plus TPZ displayed strongerred fluorescence, whereas those treated with ONPs plus TPZshowed relatively weak red fluorescence (Fig. 4a), indicating theoptimal cytotoxicity of TPZ plus PNPs with laser exposure. Staining with Alamar Blue was then performed to confirm thecytotoxicity in vitro. Few cells incubated with TPZ were damagedafter laser irradiation (Fig. 4b). Cells treated with PNPs plus TPZshowed significant cytotoxicity (46.6 and 25.2%), whereasrelatively moderate cytotoxicity (63.6 and 36.2%) was observedin tumours treated with PNPs alone when the concentration ofIR780 was 1.3 and 7.5 ug mL-1, respectively. These results furtherindicated that PNP-mediated PDT could effectively increase thecytotoxicity of TPZ due to the hypoxic environment caused byPNPs. TPZ has no cytotoxic effects under normoxic conditionsand only shows toxicity under hypoxic conditions (Supplemen-tary Fig. 19). As PNPs continuously absorb and consume thesurrounding O2, TPZ is converted into toxic radicalsls ianddamages tumour cells more effectively. We then evaluated the enhanced efficacy of the combinedtherapy by pretreating CT26 tumour-bearing mice with PNPsfollowed by intratumoural injections of TPZ and monitoring thetumour volume. Tumours treated with PNPs plus TPZ weresignificantly inhibited compared with tumours in the othergroups, which suggested the successful destruction of tumourcells by PNPs combined with TPZ (Supplementary Figs. 20a, b,21). The results of haematoxylin and eosin (H&E) and terminaldeoxynucleotidyl transferase dUTP nick-end labelling (TUNEL)staining also indicated that pretreatment with PNPs significantlyenhanced the efficacy of TPZ (Supplementary Fig.20d). Further-more, no obvious changes in body weight or tissue damage innormal organs were observed, suggesting that the combinedtherapy has few side effects (Supplementary Figs. 20c,22). The antitumour efficacy of the PNP-enhanced chemotherapywith laser irradiation was then assessed in vivo after theintravenous administration of both PNPs and TPZ. CT26tumour-bearing mice were randomly divided into six groups:the control (saline), PNP, ONP, TPZ, ONP plus TPZ and PNPplus TPZ group. At 24 h post injection, all tumours were exposedto an 808 nm laser for 5 min at a density of 400 mW cm-2. Thefastest tumour growth was detected in the saline group, whilemoderately restricted tumour growth was observed in the PNPand ONP plus TPZ groups. In the TPZ plus PNP group, completetumour inhibition was achieved (Fig. 4c, Supplementary Fig. 23,24). The mice were sacrificed on day 13, and the excised tumourswere weighed. These results also showed the strongest tumourinhibition in the PNP plus TPZ group (Fig.4d). The body weightof the mice did not significantly change with any treatment(Fig. 4e), indicating that PNP-based PDT enhanced the antic-ancer efficacy of TPZ without causing additional toxicity. H&E and TUNEL staining was also performed to investigatethe level of tumour apoptosis ex vivo. H&E staining showedprominent tumour cell necrosis after treatment with PNPs plusTPZ, whereas moderate damage was achieved by PNPs and TPZalone (Fig. 4f). The TUNEL assay also showed the highest level ofapoptosis in tumours treated with PNPs plus TPZ, furtherconfirming the excellent antitumour performance of the combi-nation of TPZ and PNPs. PNPs enhanced hypoxia-based bacterial cancer therapy. Aftersuccessfully demonstrating the effect of TPZ combined withPNPs, Sa. was chosen as another example for combination withPNP pretreatment. A phase I clinical study of VNP20009 failed because of the lack of therapeutic efficacy7. Some studies haveshown that Sa. only distributes in the necrotic areas of hypoxictumours, which are far from tumour vessels5. In this case, sus-tained whole-tumour hypoxia is quite important for ensuring thatinjected bacteria accumulate as much as possible throughout theentire tumour, extensively distributing and proliferating in timeand space. To confirm whether pretreatment with PNPs could enhancethe efficacy of the bacteria, we first investigated the intratu-moural distribution of VNP200099by performing ex vivoimmunofluorescence staining withi glut-1 protein antibody(red) and Salmonella antibody (green). At day 3, sections ofPNP-treated tumours showed obviously increased hypoxialevels and VNP20009 distributions compared with the controlgroups, and VNP20009 was exactly distributed in the hypoxicareas exhibiting greater glut-1 protein expression (Fig. 5a).Semiquantitative analysis of the glut-1 protein and VNP20009levels revealed that the percentage of the VNP20009 distribu-tion was consistent with the level of glut-1 protein expression(Fig. 5b), demonstrating that PNPs can expand and reinforcethe hypoxic zone for to support the infiltration and prolifera-tion of VNP20009. Next, we evaluated the intratumoural VNP20009 level bycolony-forming assay (CFU). Approximately 12.7 times as muchVNP20009 accumulated in tumours treated with PNPs plusVNP20009 as in tumours treated with VNP20009 alone (Fig. 5c).In addition, this level was almost 1000 times greater than that inother organs, including the spleen, liver, kidneys, heart and lungs(Supplementary Fig. 25). These results confirmed that PNPscould sguide VNP20009 to penetrate and proliferate withintumours more effectively. Next, we studied the in vivo efficacy of PNP-enhancedbacterial cancer therapy in CT26 tumour-bearing mice. MaleBALB/c mice bearing CT26 tumours ~60-80 mm’ in size wererandomly divided into the following six groups: the saline, PNP,ONP, Sa., ONP plus Sa., and PNP plus Sa. groups. At 24 h postinjection, Sa. was intravenously injected if needed; then, allmice were irradiated with an 808 nm laser (400 mW cm-2) for5 min. PNPs plus Sa. achieved the most effective tumourinhibition (Fig. 5d). The mice were sacrificed on day 10, and thetumours were excised, photographed and weighed (Fig. 5e,Supplementary Fig. 26). These experiments also confirmed thatPNPs plus Sa. inhibited tumour growth the most. The micewere also weighed (Supplementary Fig. 27). Collectively, theseresults show that the hypoxic and permeable environmentcreated by PNPs could also improve the effect of hypoxia-basedbacterial cancer therapies. While the safety of such intravenous bacterial treatments hasbeen previously tested46-49, we analysed the blood biochemicaland haematologic indexes of the mice to assess the safety of ourtherapy. The blood biochemistry of mice that were injected withVNP20009 intravenously varied significantly onday 1,especially the AST and ALT levels, which were ~2.94 and2.54 times those in mice treated with saline; however, no long-term side effects of VNP20009 were observed on day 7 postinjection (Fig. 5f). These results indicate that the intravenousinjection of VNP20009 at a dose of 5×106 CFU per mouse isfeasible and safe Discussion HBAs (i.e., TPZ, TH-302,AQ4N and VNP20009) alone usuallycannot achieve satisfactory antitumour effects50-55. To treattumours effectively, HBAs face at least three obstacles. First, thedense perivascular tissues are not only insensitive to HBAs butalso prevent HBAs from penetrating hypoxic regions. Second, after drug penetration is improved (e.g. via increasing vascularpermeability using tumour necrosis factor-a, promoting thenormalization of tumour vessels using losartan, or degradingthe extracellular matrix (ECM) using matrix metalloproteinasesand relaxin), the accompanied reperfusion of blood oxygen willease the hypoxia and limit drug efficacy. Lastly, the intratu-moural distribution of hypoxic drugs often takes hours or evendays (such as for Sa.); even if the hypoxic environment anddrug penetration are simultaneously increased (i.e. by photo-dynamic O2 to cytotoxic 102 conversion), additional strategiesare still needed to maintain these environmental conditions forlong periods of time. In this study, we construct a perfluorocarbon-based nano-therapeutic that can enhance anaerobic therapies in a two-stepmanner. First, drug permeation and tumour hypoxia wereimproved via PDT. Synergism between the co-encapsulatedphotosensitizer and PFTBA enable PNPs to exert a superiorphotodynamic effect compared to ordinary photosensitizers.Based on the unique affinities between O/O2 and PFTBA, withirradiation, the PNPs can efficiently convert tumour O2 intocytotoxic 102, resulting in oxygen consumption, vascular per-foration, tissue necrosis and apoptosis for improved HBApenetration. Second, the PNPs maintained the increased intratumoural drugpermeability and hypoxia for over 24 h after laser irradiation,which is long enough to allow both the delivery and activation ofHBAs. This function is achieved by the PFTBA rather than thephotodynamic treatment. The oxygen capacity of PFTBA is 20times higher than that of water, which enables the PNPs to serveas buffers to restrict tumour oxygen recovery. Pimonidazole andHIF-1a staining showed that PNP-treated tumours were morehypoxic than tumours in the control groups even at 24 h afterirradiation. In addition, PFTBA has an inhibitory effect on pla-telets30-33, which plays a crucial role in limiting the penetrationof drugs into tumours40-44. Other assays revealed that intrave-nously injected Evans blue, TPZ and VNP20009 accumulatedmoreein PNP-treateddtumours, even withouti aany laserstimulation. A possible problem with using PNPs to regulate the tumourenvironment is the potential pro-progression effects. Enhancedhypoxia may promote tumour aggression and immune suppres-sion and further promote tumour spreading56. additionally,increased tumour penetration may provide more opportunitiesfor tumour spreading. However, at the same time, recent studieshave reported that the pro-progression effects of pre-hypoxiatreatments could be reversed by HBAs58,59. HBAs may suppresshypoxia-stimulated tumour metastasis and down-regulate theexpression of Snail transcription factors, which are responsible forcancer invasion and metastasis60. In addition, increased tumourpenetration may enhance the infiltration and response of immunecells61. In conclusion, the advantages and disadvantages of usingPNPs to regulate the tumour microenvironment need to be fur-ther verified. Additionally, it should be emphasized that particlediameter is a critical aspect of extravascular penetration.Although we achieved therapeutic efficacy against CT26 solidtumours with 160 nm PNPs, it is possible to achieve better resultsby adjusting the particle size to adapt to tumours of differenttypes and stages. Another issue worth discussing is the potential challenge oflight source in treating non-superficial diseases2. Currently,many technical solutions have been reported to solve thisproblem, and some good progress has been made. For example,using invasive fibre optics for internal irradiation63, usingupconversion nanoparticles64 and triplet fusion upconver-sion65 and so on to convert penetrating NIR light into pho-todynamic visible light in situ. It is worth expecting that new PDT with additional device will be used for non-superficialdiseases soon. In summary, we successfully constructed a biocompatiblenanotherapeutic that can specifically accumulate in tumours andsimultaneously increase the tumour permeability and hypoxia toactivate hypoxia-based therapies such as bioreductive and bac-terial cancer therapies. Considering that the PNPs were formedby combining clinically approved blood substitutes and photo-sensitizers, these PNPs have significant potential for clinicaltranslation in combination with HBAs for the treatment of solidtumourS. Methods Materials and reagents. HSA solution was purchased from CSL Behring AG.Olive oil was obtained from Aladdin Industrial Corporation. PFTBA was suppliedby Meryer Chemical Technology Co., Ltd. (Shanghai, China). HIF-1a antibody wasobtained from Thermal Fisher (MA5-16009), Pimonidazole (Hypoxyprobe-1 PlusKit, Hypoxyprobe Inc.,USA) and its antibody (clone 4.3.11.3; FITC-MAb1), IR780,calcein-AM and propidium iodide (PI) were provided by Sigma-Aldrich ChemicalCorporation. Alamar Blue cell viability reagent was supplied by Thermo Fisher.Singlet Oxygen Sensor Green and carbon-H2DCFDA were obtained from Mole-cular Probes. The O2 Consumption Rate Assay Kit was supplied by CaymanChemical Company, and the Hypoxia/Oxidative Stress Detection Kit was pur-chased from Enzo. Cell Counting Kit 8 was supplied by Dojindo Laboratories(Japan). For western blot detection of HIF-1a, anti-HIF-1a antibody was obtainedfrom Abcam (ab51608); anti-B-actin antibody was supplied by Seville (GB13001-1).For ex vivo immunofluorescence staining, anti-Salmonella antibody (FITC) waspurchased from Abcam (ab69253), Glut-1polyclonal antibody was obtained fromProteintech (21829-1-AP) and anti-CD31 antibody was provided by BioLegend(102508). The mouse CT26 colorectal cancer cells and 4T1 breast cancer cells werepurchased from China Type Culture Collection obtained from the American TypeCulture Collectio. BALB/c mice were purchased from the Yangzhou UniversityMedical Centre (Yangzhou, China); the mice weighed ~20-25 g. Synthesis of NPs, ONPs and PNPs. NPs were formed according to protocolsdescribed below. Briefly, 120 mg of HSA was added to 60 mL of deionized waterand stirred for 10 min. Then, IR780, which was dissolved in ethanol, was slowlyadded to the HSA solution to form NPs. To remove free IR780, the NPs werehyper-filtrated by a 30kDa Millipore filter. Centrifugation (1438×g,5 min) wasused to remove large aggregates of IR780 post ultrafiltration. To prepare ONPs and PNPs, an unfolding/self-assembling method wasadopted36. Briefly, 100 mg of HSA was mixed with 3 mL of deionized water andstirred for 5 min. Specified quantities of IR780, PFTBA or oil were added to theHSA solution. ONPs and PNPs were formed under sonication at 300 W in an icebath for 10 min. Free IR780 was removed by ultrafiltration in centrifuge tubes(Millipore) for 10 min. Characterization of NPs, ONPs and PNPs. DLS (90Plus, Brookhaven Instru-ments Corp.) was employed to measure the particles size. The structure of thedifferent samples was characterized by TEM (JEM-2100, Japan). To measure theamount of IR780, the NPs were dissolved in DMSO and then detected by UV-visabsorption spectra (UV-2450, Shimadzu, Japan). In vitro O2 absorption and consumption. An O2 microelectrode (Unisense) wasused to explore the hypoxic conditions mediated by PNPs in vitro. Briefly, 1 mL ofwater was added to a vial and covered with mineral oil for to separate it from air.Then,0.06 mL of PNPs was mixed into the water, with a final IR780 concentrationof 2 ug mL- (1% PFTBA, v/v) and 4 ug mL-1 (2% v/v PFTBA). DO was mon-itored using the microelectrode before and after 10 min of laser irradiation(808 nm, 400 mW cm-2). To evaluate O2 absorption, the sample (8ug mL-1 IR780, 4% v/v PFTBA) wasdeoxygenated (N2 flow rate=0.01 Lmin-1 for 30 s,step 1) and then (step 2)irradiated at the same intensity as above with O2 microelectrode detection.Repeated O2 consumption profiles were measured using the same method, exceptthe samples were alternately exposed to irradiation or kept in the dark every 10min. The irradiated PNPs were then combined with air-saturated water as an O,supply before the next exposure. Hypoxia assessment using an O2 microelectrode in vivo. To detect the PNP-mediated O2 absorption in vivo, 50 uL of deoxygenated PNPs (IR780, 1.4 mg kg;20% v/v PFTBA) were injected into the tumour. DissolvedO2 was monitored usingthe microelectrode before and after the injection. To measure laser-triggered O2alterations, the tumour was subjected to laser irradiation (808 nm, 2 W cm-2) for20 s immediately after the intratumoural injection of 50 uL of PNPs (1.4 mg kgIR780, 20%v/v PFTBA). These mice were exposed to the laser three times. Then, low-intensity laser irradiation (808 nm, 400 mW cm-2) was used tcincrease the tumour hypoxia in vivo. At 24 h after the injection of 200 uL ofPNPs (1.4mgkg-IR780, 20% v/v PFC), the mice were irradiated (808nm, 400mW cm-2) for 5 min. The O2 microelectrode was placed ~1 mm into the tumourto monitor the changes in O2. Ex vivo immunofluorescence staining. Ex vivo immunofluorescence staining ofthe hypoxyprobe (pimonidazole hydrochloride) and HIF-1a were employed toevaluate the hypoxic conditions in the tumours. Briefly, 200 uL of the samples (1.4mgkg-IR780;20% v/v PFTBA) was intravenously injected into the CT26tumour-bearing mice; the mice were then irradiated (808 nm, 400 mWcm-2) for5 min at 24 h post injection. The mice then received an injection of pimonidazolehydrochloride intravenously at a dose of60 mg kg. Tumour sections weresequentially stained for HSA, treated with Triton X-100 and stained with anti-pimonidazole antibody (1:200, clone 4.3.11.3; FITC-MAb1). Finally, the sectionswere imaged using a digital microscope (Nikon, Japan). To measure the expressionof HIF-1a, treatments similar to pimonidazole staining were performed, and themice were sacrificed 24 h later. The tumours were removed and treated accordingto the instructions of the HIF-1a Immunocytochemistry Kit (anti-HIF-1a antibodywas diluted with 1:200). Deoxygenated PNPs (N flow rate=0.01 L min-l for 1min) were intratumourally injected into mice for this evaluation of HIF-1aexpression. Images of the HIF-1a expression in tumour sections were captured bymicroscopy. The hypoxia status, as indicated by the staining intensity of eachsection, was statistically analysed using the ImageJ software. Assessment of PNP-induced Evans blue penetration in vivo. Evans blue wasemployed to evaluate changes in tumour penetration mediated by PNPs underlaser irradiation. Briefly, 200 uL of the samples (PNPs [1.4 mgkg-IR780;20% v/vPFTBA], ONPs, NPs or saline) were injected intravenously into the mice when theCT26 tumour diameter reached 6-8 mm. Then, the tumours were irradiated (808nm, 400 mW cm-2) for 5 min at 24 h post administration. Evans blue dye(20 mgkg-in 0.1 mL of PBS) was also injected intravenously 1 h before laserirradiation; 3 h later, the mice were sacrificed, and the tumours were removed andweighed. Evans blue dye in the tumour was extracted with formamide andquantified using a UV-vis spectrometer (optical density=620 nm). Ex vivo imagesof tumour sections stained with Evan blue were also obtained after the sametreatments. To explore PNP-mediated changes in tumour permeability withoutlaser irradiation, similar treatments were performed without laser irradiation. Western blot analysis of HIF-1o protein in tumour. Tumour-bearing miceinjected with different samples intravenously were exposed laser (808 nm, 400 mWcm-2) for 5 min, and then those tumours were washed with ice-cold PBS andground in 4 mL extract buffer on ice for 30 min. Using a micro-centrifuge,theextract was spun down at a rate of 10,000× g for 5 min at 4℃, and the lysate wasacquired for further analysis of corresponding proteins. Then, proteins wereresolved using sodium dodecyl sulphate-polyacrylamide gel electrophoresis andtransferred onto a PVDF membrane. The membrane was then incubated with adilute solution (1:1000) of anti-HIF-1a and anti-actin antibody, which was used todetect the actin proteins as a loading control for whole-cell lysate after blockingwith 0.05% Tween-20 and 5% skimmed milk in PBS. All the antibodies werepurchased from Wuhan Servicebio Technology Co., Ltd. The results of western blotwere performed to visualize using the chemiluminescence technology. Unprocessedscans of the most important blots were supplied in the Source Data file. In addition,corresponding quantifications were measured by the Alpha software. Animal model. Five-week-old BALB/c male mice weighing ~20-22 g were pur-chased from the Yangzhou University Medical Centre (Yangzhou, China), and theanimals were cared for and used in accordance with the regulations of the Insti-tutional Animal Care and Use Committee (IACUC) of Nanjing University. Duringthe study, these animals had free access to food and water. Before treatment, thehair on the flanks of the mice was removed; then, anaesthesia was induced with anintraperitoneal injection of 1% pentobarbital sodium (100 uL) anaesthesia. Toestablish the subcutaneous tumour models, 1×107 CT26 cells suspended in 100 uLof serum-free Dulbecco's modified Eagle's medium were injected into the lowerflanks of the mice subcutaneously. When the tumour volume reached ~150 mm’,the tumour mass was cut into smaller pieces with a volume of 2-5 mm’, whichwere then subcutaneously implanted into other mice. During the study, animals were observed every day for any clinically relevantabnormalities. If any mouse was moribund due to treatment-induced toxicity, theappearance of severe ulceration around the ectopic tumour or the loss of 20% of bodyweight compared with the start of study before scheduled killing, it was euthanized.Mice were treated in accordance with NJU-IACUC-approved animal use guidelines. ( PNPs enhanced TPZ chemotherapy by the intravenous injection of TPZ.Mice ( 20-25g) b e aring CT26 tumours (60-80 mm’) were rand o mly divided into s ix groups (n≥6). T h e tr e atment scheme was as follows: 1. sal i ne; 2. TP Z ; 3. ONPs; 4. PNP s ; 5. ONPs plus T PZ; and 6 . PNPs p lus TPZ. Both PNPs (1.4mgkg- I R 7 80; 20% v/vPFTBA) and TPZ (20 mg kg) were injected intravenously into mice on days 1, 3 and 5. T u mours in groups 3, 4, 5 and 6 were irradiated three times (8 0 8 nm, 400mW ) cm-2) for 5 min at 24 h after the administration of the PNPs. Then, the tumourlength (L) and width (W) were recorded every day using a digital calliper, and thevolume (V) was calculated according to the following formula:V=L×W2/2. Mice ineach group were sacrificed on day 13, and the tumours were isolated and weighed.The therapeutic efficacy of the different treatments was also evaluated by H&E andTUNEL staining on day 8. Haematologic indexes and blood biochemistry. CT26 tumour-bearing mice(20-25 g, n=3) were used in this study. When the tumour size reached 60-100mm3,VNP20009 (5×107 CFU mL-1, 100 pL) or PBS was intravenously injectedinto the mice. At different time points (1 and 7 days), blood and serum sampleswere obtained. Haematologic indexes were measured using an autohaematologyanalyser (Sysmex XE2100), and blood biochemical parameters were evaluatedusing corresponding kits according to their instructions. VNP20009 distribution in CT26 tumour-bearing mice. The VNP20009 strainwas transferred to a fresh LB medium. After the OD value reached 0.8, theVNP20009-containing medium was centrifuged at 3500×g at 4°C. Then,VNP20009 was collected and washed twice with sterile PBS and quantified byUV-vis spectroscopy (OD=600 nm). A total of nine mice bearing CT26 tumourswere divided into three groups. The mice received 200 uL of PNPs (1.4mgkgIR780; 20% v/v PFTBA), ONPs or saline intravenously. VNP20009 (5×10 CFUmouse-l) was intravenously injected into the mice 1 h before laser irradiation. Alltumours were irradiated (808 nm, 400 mW cm-2) for 5 min at 24 h after theinjection of VNP20009. The mice were sacrificed 3 days after irradiation, and theheart, liver, spleen, lungs, kidneys and tumour were removed under sterile con-ditions. After being weighed, these tissues were homogenized in pre-cooled PBS,diluted and coated onto LB plates. The number of VNP20009 colonies was cal-culated after 12-16h of culture. After similar tumour treatments, ex vivo immu-nofluorescence analysis was also performed for glut-1 and Sa. Anti-glut-1 and anti-Sa. antibodies were diluted with 1:200. PNPs enhanced bacterial cancer therapy. To explore the enhancement of bac-terial cancer therapy mediated by the PNPs, male BALB/c mice bearing CT26tumours 60-80 mm’ in size were randomly divided into six groups. The treatmentscheme was as follows: 1. saline; 2. PNPs; 3. ONPs; 4. Sa.;5. ONPs plus Sa.; and 6.PNPs plus Sa. The mice were treated and sacrificed on day 10; then, the tumours ineach group were weighed. Statistical analysis. Statistical analysis was performed using two-tailed Student’s ttest for two groups and one-way analysis of variance for multiple groups. P values:*p<0.05, **p<0.01, ***p<0.001 (unpaired, two-way t tests). P values <0.05 wereconsidered statistically significant. Reporting summary. Further information on experimental design is available inthe Nature Research Reporting Summary linked to this article. Data availability The authors declare that the main data that support the findings of this study areavailable from the corresponding author upon reasonable request. A reporting summaryfor this study is available as a Supplementary Information file. The source dataunderlying Figs. 1c, 2a, 2c-d, 2f, 3a, 3c-e, 3i, 4b-f and 5b-f and Supplementary Fig. 1,2a-b, 4b-c, 5-7,9,11-14,16-17,18a,18c,20, 21a-c, 26 and 28 are provided in a sourcedata file. Received: 19 January 2018 Accepted: 6 March 2019 Published online: 05 April 2019 References 1. Wilson, W. R. & Hay, M. P. Targeting hypoxia in cancer therapy. Nat. Rev.Cancer 11,393-410(2011). 2. Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment.Nat. Rev. Cancer 4, 437 (2004). 3. Workman, P. & Stratford, I. J. The experimental development of bioreductivedrugs and their role in cancer therapy.Cancer Metastas. Rev. 12, 73-82(1993). 4 Brown, J. M. SR 4233 (tirapazamine): a new anticancer drug exploitinghypoxia in solid tumours. Br. J. Cancer 67, 1163-1170 (1993). 5. Ganai, S., Arenas, R. B., Sauer, J. P., Bentley, B. & Forbes, N. S. In tumorsSalmonella migrate away from vasculature toward the transition zone andinduce apoptosis. Cancer Gene. Ther. 18, 457 (2011). 6. Liu, F., Zhang, L., Hoffman, R. M. & Zhao, M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumorvascularity. Cell Cycle 9, 4518-4524(2010). 7. Toso, J. F. et al. Phase I study of the intravenous administration of attenuatedSalmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol.20,142-152(2002). ( 8. Zhang Y . e t al. Toxicology and e f ficacy of tumor-targeting Salmonella typhimurium A1-R compared to VNP 2 0 009 in a syngeneic mouse tumor model i n immunocompetent mice. Oncotarget 8, 54616-54628 (2017). ) ( . 9. Zheng, J. H. & Min, J.-J. Targeted cancer therapy using engineered Salmonella t yphimurium. Chonnam Med. J. 52,173-184(2016). ) ( 1 0. P awelek, J. M . et al. Salmonella pathogenicity island-2 and anticancer activity in mice. Cancer Gene. Ther. 9,813-818 (2002). ) ( 11.Le e , C. H., H sieh, J. L., Wu, C. L., Hsu, P. Y. & Shiau, A. L. T cell augments theantitumor activity o f tumor-targeting S a lmonella. Appl. Microbiol. Biotechnol.90,1381-1388 (2011). ) ( 12. B ] rown, J. M. Exploiting t h e hypoxic cancer cell: mechanisms and the r apeutic strategies. Mol. Med. Today 6, 1 57-162 ( 2 000). ) ( 1 3. R eddy, S. B. & Williamson, S. K . T i rapazamine: a n o v el ag e nt targeting h ypoxic tumor cells. E x pert. O p in. Investig. Drugs 18 , 77 - 87 (20 0 9). ) ( 14 . Chauhan, V. P. & J ain, R. K. St r ategies for a d vancing ca n cer nanomedicine. Nat. M ater. 1 2, 958-962 (2013). ) 15.Brown,J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment.Nat. Rev. Cancer 4, 437-447 (2004). 16.Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. & Peer, D. Progress and challengestowards targeted delivery of cancer therapeutics. Nat. Commun. 9, 1410(2018). ( 17. J J ain, R . K . Normalization o f tumor vasculature: an emerging concept inantiangiogenic therapy. Science 3 0 7, 58-62 (2005). ) ( 18. Stylianopoulos, T ., Munn, L. L . & Ja i n, R. K. Reengineering the tum o rvasculature: improving drug delivery and efficacy . Trends Cancer 4, 258-259 (2018). ) 19.C(hauhan, V. P. et al. Angiotensin inhibition enhances drug delivery andpotentiates chemotherapy by decompressing tumour blood vessels. Nat.Commun. 4, 2516 (2013). 20.Ojha, T. et al. Pharmacological and physical vessel modulation strategies toimprove EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 119,44-60(2017). ( 21. P ] ark, J. S. et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumormicroenvironment. Cancer Cell. 31, 157-158(2017). ) ( 22. S eki, T. et al. Tumour necrosis fa c tor-alpha increases extravasation of virus particles into tumour tissue by activating the Rho A/Rho kinase pathway. J.Control Rel. 156, 381-389 ( 2011). ) ( 23Z . hang,C. et al. M a gnesium silicide nanoparticles as a deoxygenation agent forcancer s tarvation therapy. Nat. Nanotechnol. 12, 378-386 (2017). ) ( 24. L iu, Y. et al. Hypoxia induced by u pconversion-based photodynamic therapy:towards highly effective synergistic bioreductive therapy in tumors. Angew.Chem. Int. Ed. Engl. 54,8105-8109(2015). ) ( 25 . Jiang, X . et al. Enhancement of radiotherapy efficacy by pleiotropic liposomes encapsulated paclitaxel and p erfluorotributylamine. Drug Deliv. 2 4, 1419-1428 (2017). ) ( 26 . Song, X., Feng, L., Liang, C., Yang, K . & Li u , Z. Ultrasound tri g gered t umor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated re s istance in cancer therapies. Na n o Lett. 16, 614 5 - 6 153 (2016). ) ( 27. Castro, C. I. & Briceno,J. C. Perfluorocarbon-based oxygen carriers: review of products and trials. A rtif. Organs 34, 622-6 3 4 (2 0 10). ) ( 28. v on der H a rdt, K. et a l . C omparison of aerosol therapy wi t h di f ferent perfluorocarbons in s u rfactant-depleted an i mals. Crit. Ca r e Med. 32, 1200-1206 (2004). ) ( 29 Y . oung, L . H., Jaffe, C. C., Revkin , J . H., McNulty, P . H. & Cleman, M. Metabolic a n d functional effects of p e rfluorocarbon dis t al pe r fusion during c oronary angioplasty. Am. J. Cardiol. 6 5, 9 86-990 (1990). ) ( 30. . Lowe, K. C. Second-generation perfluorocarbon emulsion blood substitutes. A rtif. Cells Blood Substit. Immobil. B iotechnol. 2 8, 25-38 (2000). ) ( 31. Smith, D . J . & L a ne, T. A. E f fect of high concentration perflubron emulsion onplatelet function. Biomater. Artif. Cell s Immobil. Biotechnol. 21, 173-181 ( 1 9 93). ) 32.Spence, R. K. Perfluorocarbons in the twenty-first century: clinical ( applications a s t ransfusion alternatives. Artif. Cells Blood Substit . Immobil. B iotechnol. 23, 367-380 (1995). ) ( 33. Z 2 hou Z. et a l . P erfluorocarbon nanoparticles m ediated p l atelet blocking d isrupt vascular barriers to improve the efficacy of oxygen-sensitive antitumor drugs. S mall 14, e1801694 (2018). ) ( 34. Ding, D. et a l. Novel self-assembly endows human serum al b uminnanoparticles with an enhanced antitumor efficacy. AAPS PharmSciTech. 1 5 , 213-222(2014). ) ( 35. Gong, G. et al. Fabrication of a nanocarrier system t h rough self-assembly of plasma protein a nd its tumor t argeting. Nanotechnology 22, 295603 ( 2011). ) 36.Ren H. et al. Relighting photosensitizers by synergistic integration of albuminand perfluorocarbon for enhanced photodynamic therapy. ACS Appl. Mater.Interfaces 9, 3463-3473 (2017). 37. Cheng, Y. et al. Perfluorocarbon nanoparticles enhance reactive oxygen levelsand tumour growth inhibition in photodynamic therapy. Nat. Commun. 6,8785 (2015). ( 38.Fercher, A., Borisov, S. M., Zhdanov, A . V ., Klimant, I. & P apkovsky, D. B . Intracellular O2 sensing probe based on c e ll-penetrating phosphorescent nanoparticles. ACS Nano 5,5499-5508 (2011). ) ( 39.Will, Y., Hynes, J., Ogurtsov, V. I. & Papkovsky, D . B . Analysis ofmitochondrial f unction u sing phosphorescent oxygen-sensitive p robes. Nat. Protoc. 1,2563-2572 (2006). ) ( 40. . H o-Tin-Noe, B . , D emers, M. & W a gner, D. D. How platelets s a feguard vascular integrity. J. Thromb. Haemost. 9, Suppl 1.56-65 (201 1 ). ) ( 41. H o-Tin-Noe, B . , Goerge, T . & Wagner, D . D. P l atelets: guardians of tumorvasculature. Cancer Res. 69, 5623-5626 ( 2009). ) ( 42 e . . K D isucka, J . et a l. Platelets and p latelet a dhesion support angiogenesis while preventing e xcessive hemorrhage.Pr o c. Natl. Acad. Sc i . U S A 103, 855-860 (2006). ) ( 43. . Boulaftali, Y . et al. P latelet I TAM sig n aling is crit i cal for vascular integrity in i nflammation. J. Clin. Invest. 123,908-916 (2013). ) ( 44. H o-Tin-Noe, B . , Goerge, T., Cifuni, S. M., Duerschmied,D . & Wagner, D. D P latelet granule secretio n continuously prevent s intratumor hemorrhage.Cancer Res. 68,6851-6858 (2008). ) ( 45. Winslow, R . M . B l ood substitutes. A dv. Drug D e liv. Rev.40, 1 3 1-142 (2000). ) ( 46 Pawelek, J . M., Low, K . B. & B ermudes, D . Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 57, 4 537-4544(1 9 97). ) ( 47. .1 Morrissey, D., O’Sullivan, G. C. & Tangney , M. Tumour targeting with s ystemically administered bacteria. Curr. Gene Ther. 10, 3-14 (2010). ) 48. Low, K. B. et al. Lipid A mutant Salmonella with suppressed virulence andTNFalpha induction retain tumor-targeting in vivo. Nat. Biotechnol. 17, 37-41(1999) 49.Toso, J. F. et al. Phase I study of the intravenous administration of attenuatedSalmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol.20, 142-152(2002). 50.Denny, W. A. The role of hypoxia-activated prodrugs in cancer therapy.Lancet Oncol. 1, 25-29(2000). 51.G(anjoo, K. N. et al. A phase I study of the safety and pharmacokineticsof the hypoxia-activated prodrug TH-302 in combination with doxorubicinin patients with advanced soft tissue sarcoma. Oncology 80, 50-56(2011). 52.K1im, Y. et al. Probing nanoparticle translocation across the permeableendothelium in experimental atherosclerosis. Proc. Natl. Acad. Sci. USA 111,1078-1083 (2014). ( 53. 5 . W eiss, G. J.e t al. P hase 1 s t udy of the safety, tolerability, andpharmacokinetics of TH-302, a h y poxia-activated prodrug, in p atients with advanced solid malignancies. Clin. Cancer Res. 17, 2997-3004 (2011). ) 54. Williams, K. J. et al. In vivo activation of the hypoxia-targeted cytotoxin AQ4N in human tumor xenografts. Mol. Cancer Ther. 8, 3266-3275 (2009). 55.Feng, L. et al. Theranostic liposomes with hypoxia-activated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy. ACS Nano 11,927-937 (2017). ( 56. N oman, M. Z. e t a l. PD - L1 is a n o v e l direct target of H IF - 1alpha, and its b lockade under hypoxia enhanced M D SC-mediated T cell activation. J. Exp. Med. 211,781- 7 90 (2014). ) 57.Q(uail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19,1423-1437 (2013). ( 58. Wang, Y. et al. Tumor-penetrating nanoparticles fo r enhanced ant i canceractivity of combined p hotodynamic and hypoxia-activated therapy. ACS nano 11,2227-2238 (2017). ) ( 59. L I iu, Y. et al. Radiation-/hypoxia-induced solid tumor metastasis and regrowthinhibited b y h ypoxia-specific upconversion nanoradiosensitizer. Biomaterials 49,1-8(2015). 60. . J J in, H . e t a l. Snail is critical for tumor growth and metastasis of ovarian ) carcinoma. Int.J. Cancer 126, 2102-2111(2010). 61..Shrimali, R. K. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy ofcancer. Cancer Res. 70,6171-6180(2010). ( 62. P I alumbo, G. Photodynamic therapy and c ance r : a brief sightseeing tour. E xpert O pin. D rug Deliv. 4 , 131-148 (2007). ) ( .63. N 1 agaya T. e t al. Near infrared photoimmunotherapy using a fibe r optic diffuser for t reating peritoneal gastric cancer dissemination. Gastric Cancer h ttps: / / d oi.or g/ 10 .1 007 / s10120-018-0871- 5 ( 2 018). ) ( 64. z. I dris, N. M. et al. In vivo photodynamic therapy using upconversionnanoparticles as remote-controlled n anotransducers. Nat. M ed. 1 8 , 1580- 1 585(2012). ) 65.Ravetz, B. D. et al. Photoredox catalysis using infrared light via triplet fusionupconversion. Nature 565, 343-346 (2019). Acknowledgements This paper was supported by National Key R&D Program of China (2017YFA0205400);National Natural Science Foundation of China (No. 31872755,81872811); this project wasalso supported by the Central Fundamental Research Funds for the Central Universities. Author contributions W. W. and J. W. conceived and designed the experiments. W.W., P. Y., H. W., Y. Z.,H. X. and Q. Y. performed the experiments. Y. C., A. Y., Y. H. and J. W. assisted in thedata analysis. J. W., W.W. and Y. C. prepared the manuscript. J. W. and Y. H. supervisedthe project. Additional information Supplementary Information accompanies this paper at https://doi.org/10.1038/s41467-019-09389-2. Competing interests: The authors declare no competing interests. Reprints and permission information is available online at http://npg.nature.com/reprintsandpermissions/ Journal peer review information: Nature Communications thanks the anonymous reviewersfor their contribution to the peer review of this work. Peer reviewer reports are available. Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims inpublished maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribu-@ tion 4.0 International License, which permits use, sharing, adaptation,distribution and reproduction in any medium or format, as long as you give appropriatecredit to the original author(s) and the source, provide a link to the Creative Commonslicense, and indicate if changes were made. The images or other third party material in thisarticle are included in the article’s Creative Commons license, unless indicated otherwise in acredit line to the material. If material is not included in the article’s Creative Commonslicense and your intended use is not permitted by statutory regulation or exceeds thepermitted use, you will need to obtain permission directly from the copyright holder. Toview a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. ATURE COMMUNICATIONS|(https://doi.org/swww.nature.com/naturecommunications 大多数肿瘤在生长过程中会出现不同程度的乏氧,而乏氧是导致肿瘤转移和耐受的重要原因之一。因此在肿瘤治疗中清除乏氧肿瘤细胞对预防肿瘤复发和转移尤为重要。乏氧依赖性药物则是在乏氧条件下起效的一类物质,主要包括乏氧性细菌、乏氧性前药(如:沙门氏菌、替拉扎明)等。这些物质可与其他抗肿瘤手段联合,清除乏氧肿瘤细胞,防止肿瘤复发和转移。然而临床上联合乏氧依赖性药物对肿瘤的疗效并不理想,这主要是由于肿瘤的乏氧部位渗透性差,药物浓度过低所导致的。尽管已经有一些手段可增加乏氧药物的渗透性,然而渗透性增加必然伴随着氧气浓度增加,反而降低了乏氧药物的疗效。研究人员报道了一种全氟碳纳米颗粒(PNPs)可以用来创建一个持久穿透性和低氧肿瘤微环境,探索这种材料全氟化碳在临床肿瘤治疗应用的一个新方向。 图1、PNP介导的HBA和O2吸收及消耗功能的改善示意图 临床上联合乏氧依赖性药物对肿瘤的疗效并不理想,这主要是由于肿瘤的乏氧部位渗透性差,药物浓度过低所导致。为了在增加药物渗透的同时,降低氧气的渗透,研究人员发明了一种全氟化碳纳米粒。 该纳米粒可通过光动力效应破坏血管,增加肿瘤部位的药物渗透,同时还作为一种“吸氧海绵”,将渗透进入肿瘤组织的氧气,富集并消耗完毕,为乏氧依赖性药物创造了能够起效的乏氧微环境。本论文研究了发明了一种全氟化碳纳米粒子, 该纳米粒可通过光动力效应破坏血管,增加肿瘤部位的药物渗透,同时还作为一种“吸氧海绵”,将渗透进入肿瘤组织的氧气,富集并消耗完毕,为乏氧依赖性药物创造了能够起效的乏氧微环境。具体方法将全氟化碳纳米粒子PNPs注入到老鼠体内,全氟化碳纳米粒子能够与药物共同作用降低肿瘤中的氧气浓度,创造乏氧环境,全氟化碳纳米粒可显著增加乏氧药物的疗效,实验中使用了unisense氧微电极用于原位的测试老鼠肿瘤组织中的氧气浓度实现了对于老鼠体内的氧气浓度的在线监测,从而提出了基于全氟化碳纳米粒子缺氧剂能够增加乏氧药物的疗效的相关机理,研究采用的全氟化碳纳米粒对机体的组织器官没有毒性,安全可靠,极有可能成为全氟化碳在临床应用的一个新方向。这也看出unisense氧微电极在医学缺氧肿瘤临床应用方面存在着很好的应用前景。
确定






还剩9页未读,是否继续阅读?
上海谓载科技有限公司为您提供《老鼠组织,血管中氧浓度检测方案(溶解氧测定仪)》,该方案主要用于其他中氧浓度检测,参考标准--,《老鼠组织,血管中氧浓度检测方案(溶解氧测定仪)》用到的仪器有丹麦Unisense溶氧仪、丹麦Unisense氢气测量仪
相关方案
更多