方案详情
文
德国应用化学收录文章:在全PH值范围内的纳米结构化碳膜的超疏水性
方案详情

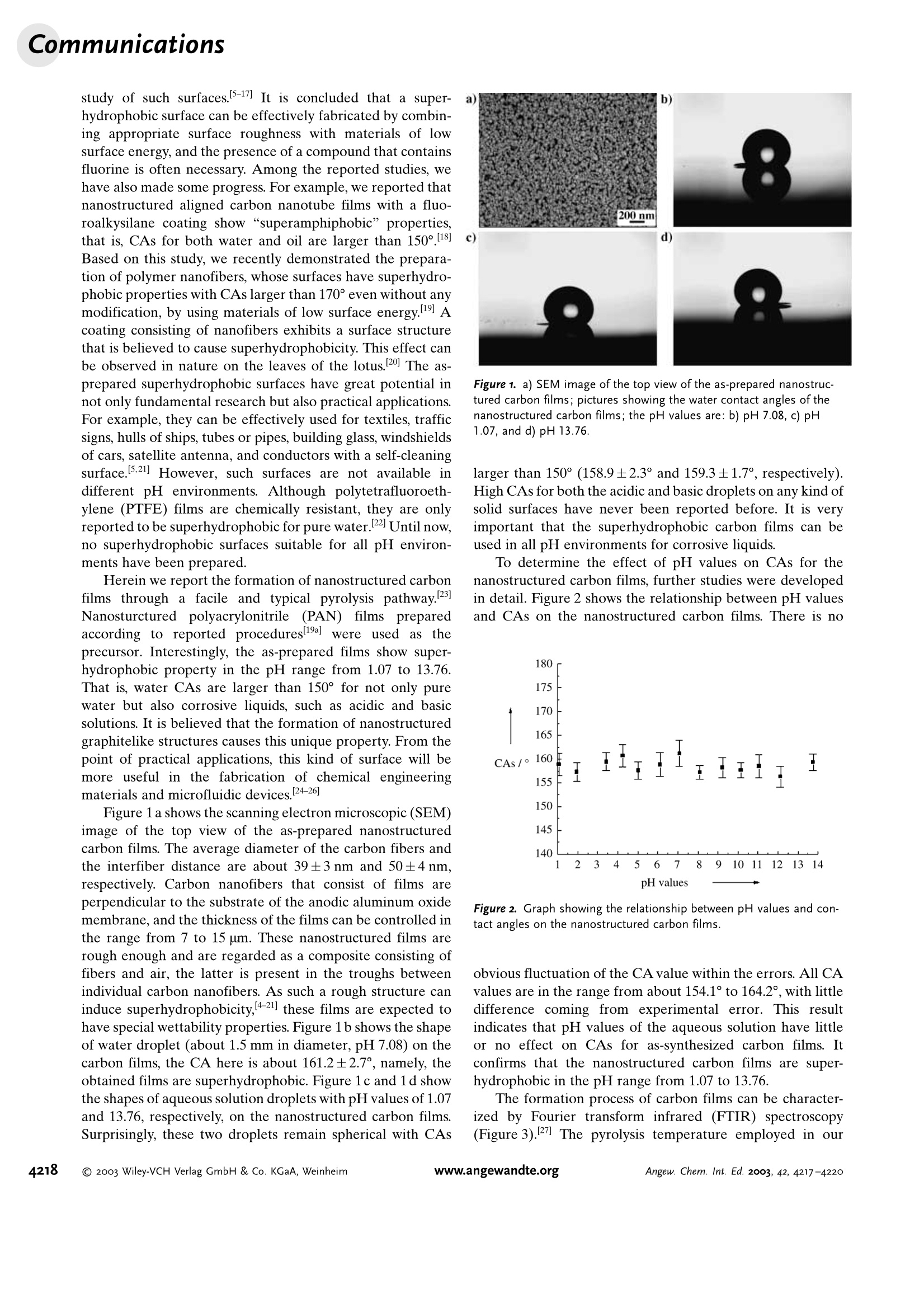
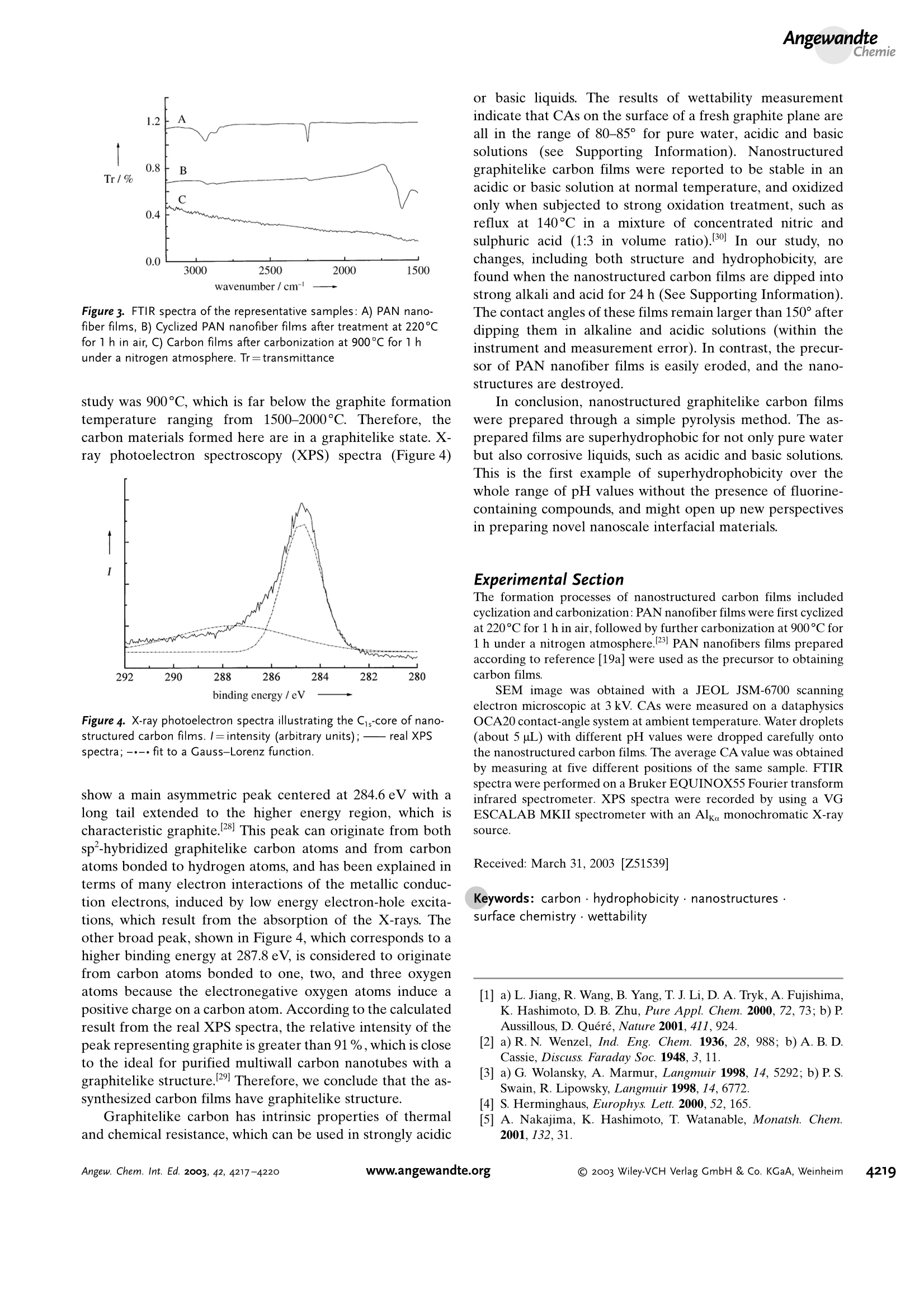
Angewandte Angewandte Chemie Superhydrophobic Carbon Films Superhydrophobicity of Nanostructured CarbonFilms in a Wide Range of pH Values** Lin Feng, Zhenglong Yang, Jin Zhai, Yanlin Song,Biqian Liu, Yongmei Ma, Zhenzhong Yang, * Lei Jiang,*and Daoben Zhu Superhydrophobic surfaces whose water contact angle (CA)is larger than 150° has aroused great interest. 1-4 Over the past60 years there have been numerous reports concerning the Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author. study of such surfaces. 5-17 It is concluded that a super-hydrophobic surface can be effectively fabricated by combin-ing appropriate surface roughness with materials of lowsurface energy, and the presence of a compound that containsfluorine is often necessary. Among the reported studies, wehave also made some progress. For example, we reported thatnanostructured aligned carbon nanotube films with a fluo-roalkysilane coating show “superamphiphobic”properties,that is, CAs for both water and oil are larger than 150°.18Based on this study, we recently demonstrated the prepara-tion of polymer nanofibers, whose surfaces have superhydro-phobic properties with CAs larger than 170° even without anymodification, by using materials of low surface energy.19 Acoating consisting of nanofibers exhibits a surface structurethat is believed to cause superhydrophobicity. This effect canbe observed in nature on the leaves of the lotus.[20] The as-prepared superhydrophobic surfaces have great potential innot only fundamental research but also practical applications.For example, they can be effectively used for textiles, trafficsigns, hulls of ships, tubes or pipes, building glass, windshieldsof cars, satellite antenna, and conductors with a self-cleaningsurface.[5,21] However, such surfaces are not available indifferent pH environments. Although polytetrafluoroeth-ylene (PTFE) films are chemically resistant, they are onlyreported to be superhydrophobic for pure water.[22 Until now,no superhydrophobic surfaces suitable for all pH environ-ments have been prepared. Herein we report the formation of nanostructured carbonfilms through a facile and typical pyrolysis pathway.23Nanosturctured polyacrylonitrile (PAN) filmspreparedaccording to reported procedures[19a] were used as theprecursor. Interestingly, the as-prepared films show super-hydrophobic property in the pH range from 1.07 to 13.76.That is, water CAs are larger than 150° for not only purewater but also corrosive liquids, such as acidic and basicsolutions. It is believed that the formation of nanostructuredgraphitelike structures causes this unique property. From thepoint of practical applications, this kind of surface will bemore useful in the fabrication of chemical engineeringmaterials and microfluidic devices.[24-26] Figure 1 a shows the scanning electron microscopic (SEM)image of the top view of the as-prepared nanostructuredcarbon films. The average diameter of the carbon fibers andthe interfiber distance are about 39±3nm and 50±4 nm,respectively. Carbon nanofibers that consist of films areperpendicular to the substrate of the anodic aluminum oxidemembrane, and the thickness of the films can be controlled inthe range from 7 to 15 um. These nanostructured films arerough enough and are regarded as a composite consisting offibers and air, the latter is present in the troughs betweenindividual carbon nanofibers. As such a rough structure caninduce superhydrophobicity,4-21] these films are expected tohave special wettability properties. Figure 1 b shows the shapeof water droplet (about 1.5 mm in diameter, pH7.08) on thecarbon films, the CA here is about 161.2±2.7°, namely, theobtained films are superhydrophobic. Figure 1c and 1d showthe shapes of aqueous solution droplets with pH values of 1.07and 13.76, respectively, on the nanostructured carbon films.Surprisingly, these two droplets remain spherical with CAs a Figure 1. a) SEM image of the top view of the as-prepared nanostruc-tured carbon films; pictures showing the water contact angles of thenanostructured carbon films; the pH values are: b) pH 7.08, c) pH1.07, and d) pH 13.76. larger than 150°(158.9±2.3°and 159.3±1.7°, respectively).High CAs for both the acidic and basic droplets on any kind ofsolid surfaces have never been reported before. It is veryimportant that the superhydrophobic carbon films can beused in all pH environments for corrosive liquids. To determine the effect of pH values on CAs for thenanostructured carbon films, further studies were developedin detail. Figure 2 shows the relationship between pH valuesand CAs on the nanostructured carbon films. There is no Figure 2. Graph showing the relationship between pH values and con-tact angles on the nanostructured carbon films. obvious fluctuation of the CA value within the errors. All CAvalues are in the range from about 154.1° to 164.2°, with littledifference coming from experimental error. This resultindicates that pH values of the aqueous solution have littleor no effect on CAs for as-synthesized carbon films. Itconfirms that the nanostructured carbon films are super-hydrophobic in the pH range from 1.07 to 13.76. The formation process of carbon films can be character-ized by Fourier transform infrared (FTIR) spectroscopy(Figure3).27 The pyrolysis temperature employed in our Figure 3. FTIR spectra of the representative samples: A) PAN nano-fiber films, B) Cyclized PAN nanofiber films after treatment at 220℃for 1 h in air, C) Carbon films after carbonization at 900°℃ for 1 hunder a nitrogen atmosphere. Tr=transmittance study was 900°C, which is far below the graphite formationtemperature ranging from 1500-2000°C. Therefore, thecarbon materials formed here are in a graphitelike state. X-ray photoelectron spectroscopy (XPS) spectra (Figure 4) Figure 4. X-ray photoelectron spectra illustrating the C1s-core of nano-structured carbon films. I=intensity (arbitrary units);- real XPSspectra;-·-. fit to a Gauss-Lorenz function. show a main asymmetric peak centered at 284.6 eV with along tail extended to the higher energy region, which ischaracteristic graphite.28 This peak can originate from bothsp-hybridized graphitelike carbon atoms and from carbonatoms bonded to hydrogen atoms, and has been explained interms of many electron interactions of the metallic conduc-tion electrons, induced by low energy electron-hole excita-tions, which result from the absorption of the X-rays. Theother broad peak, shown in Figure 4, which corresponds to ahigher binding energy at 287.8 eV, is considered to originatefrom carbon atoms bonded to one, two, and three oxygenatoms because the electronegative oxygen atoms induce apositive charge on a carbon atom. According to the calculatedresult from the real XPS spectra, the relative intensity of thepeak representing graphite is greater than 91%, which is closeto the ideal for purified multiwall carbon nanotubes with agraphitelike structure.29 Therefore, we conclude that the as-synthesized carbon films have graphitelike structure. Graphitelike carbon has intrinsic properties of thermaland chemical resistance, which can be used in strongly acidic or basic liquids. The results of wettability measurementindicate that CAs on the surface of a fresh graphite plane areall in the range of 80-85°for pure water, acidic and basicsolutions (see Supporting Information). Nanostructuredgraphitelike carbon films were reported to be stable in anacidic or basic solution at normal temperature, and oxidizedonly when subjected to strong oxidation treatment, such asreflux at 140°C in a mixture of concentrated nitric andsulphuric acid (1:3 in volume ratio).30] In our study, nochanges, including both structure and hydrophobicity, arefound when the nanostructured carbon films are dipped intostrong alkali and acid for 24 h (See Supporting Information).The contact angles of these films remain larger than 150° afterdipping them in alkaline and acidic solutions (within theinstrument and measurement error). In contrast, the precur-sor of PAN nanofiber films is easily eroded, and the nano-structures are destroyed. In conclusion, nanostructured graphitelike carbon filmswere prepared through a simple pyrolysis method. The as-prepared films are superhydrophobic for not only pure waterbut also corrosive liquids, such as acidic and basic solutions.This is the first example of superhydrophobicity over thewhole range of pH values without the presence of fluorine-containing compounds, and might open up new perspectivesin preparing novel nanoscale interfacial materials. Experimental Section The formation processes of nanostructured carbon films includedcyclization and carbonization:PAN nanofiber films were first cyclizedat 220°C for 1 h in air, followed by further carbonization at 900℃ for1 h under a nitrogen atmosphere.23] PAN nanofibers films preparedaccording to reference [19a] were used as the precursor to obtainingcarbon films. SEM image was obtained with a JEOL JSM-6700 scanningelectron microscopic at 3 kV. CAs were measured on a dataphysicsOCA20 contact-angle system at ambient temperature. Water droplets(about 5 pL) with different pH values were dropped carefully ontothe nanostructured carbon films. The average CA value was obtainedby measuring at five different positions of the same sample. FTIRspectra were performed on a Bruker EQUINOX55 Fourier transforminfrared spectrometer. XPS spectra were recorded by using a VGESCALAB MKII spectrometer with an Alka monochromatic X-raysource. Received: March 31,2003 [Z51539] Keywords: carbon· hydrophobicity· nanostructures·surface chemistry· wettability ( [1] a) L . Jiang, R. Wang, B . Yang, T. J. L i, D. A. Tryk, A. Fujishima,K. H ashimoto, D. B. Z hu, Pure Appl. Chem. 2000,72 , 73; b) P.Aussillous, D. Quere, Nature 2001, 411,924. ) [2] a) R. N. Wenzel, Ind. Eng. Chem. 1936, 28, 988; b) A. B. D.Cassie, Discuss. Faraday Soc. 1948,3, 11. [3] a) G. Wolansky, A. Marmur, Langmuir 1998, 14, 5292; b) P. S.Swain, R. Lipowsky, Langmuir 1998, 14,6772. ( [4] S. Herminghaus, E u rophys. Le t t. 2000, 52, 165. ) ( [5] A. Nakajima, K. Hashimoto, T. Watanable, Monatsh. C hem.2001,132,31. ) ( [6] S. H. Li, H. J. Li, X. B. Wang, Y. L. Song, Y. Q. Liu,L. Jiang, D. B . Zhu, J. Phys. Chem. B 2002, 1 06,9274. ) ( [7] J. Genzer, K. Efimenko, Science 2000,290,21 3 0. ) ( [8] a ) T . O n da, S. Shibuichi, N. Satoh, K. Tsujii, Langmuir 1996 , 12,2125; b) S. Shibuichi,T.Onda,N. Satoh, K. Tsujii,J. Phys. Chem.1996,100, 1 9512. ) ( [9] H. Y. E rbil, A . L . D emirel, Y. A v ci, O. Mert, Sc i ence 2003,299, 1377. ) ( [10] D. Oner, T.J. McCarthy, Langmuir 2000, 16,7777. ) ( [11] a) Y . Wu, H. S ugimura, Y. Inoue, O . T a kai, C h em. Vap. Deposition 2 002, 8 , 47; b) A. H ozumi, O . Ta k ai, Th i n So l idFilms 1997,303,222. ) ( [12] a) K. Tsujii, T. Yamamoto, T. Onda, S. Shibuichi, Angew. Chem. 1997,109,1042;Angew. Chem. Int. E d . Engl. 1997,36,1 0 11;b) S.Shibuichi, T. Yamamoto, T . Onda,K. Tsujii, J. C olloid I nterface Sci. 1998,208,287. ) ( [13] a) A. Nakajima, A . Fujishima,K. Hashimoto, T. Watanabe, Adv.Mater. 1999, 1 1,1365;b) A. Nakajima, K. Abe, K. Hashimoto, T . Watanable, Thin Solid Films 2000, 376, 140. ) ( [14] a) K. Tadanaga,N. Katata, T. Minami, J. Am. Ceram. Soc. 1997,80, 1040; b)K. Tadanaga, N . K a tata, T . Minami, J. Am. Ceram. Soc. 1997,80,3213. ) ( [15] J. B ico, C . Marzolin, D. Q uere, Europhys. L e tt. 1 999, 47, 220. [16] H. Tada, H. Nagayama, Langmuir 1995, 1 1 , 136. ) ( [17] Z. Z. Gu, H. Uetsuka,K . Takahashi, R . Nakajima, H. Onishi, A .F ujishima, O. Sato , Angew. Chem. 2003 , 115,922; Angew. Chem.Int. Ed.2003,42,894. ) ( [18] H. J. Li, X. B. Wang, Y. L. S ong, Y.Q. Liu, Q. S. Li, L. Jiang, D.B. Zhu, Angew. Chem. 2 0 01, 1 1 3, 1 7 93; Angew. C h em. In t . E d . 2001,40, 1 743. ) ( [19] a ) L . F eng, S. H. L i , H . J. Li, J. Zhai, Y. L. Song, L. Jiang, D. B.Zhu,Angew. Chem. 2002,114,1269;Angew. Chem. Int. Ed.2002,41,1221;b) L. Feng, Y. L. Song, J. Zhai, J. X u , L. Jiang, D. B.Zhu, Angew. Chem. 2003,115,82 4 ; Angew. Ch e m. Int. Ed. 2003,42,800. ) ( [20] a ) L . F eng, S. H. L i , Y.S. Li, H. J. L i , L . J. Zhang, J. Zhai, Y. L. Song, B. Q. L iu, L . Jiang, D. B. Zhu, Adv. Mater. 2002, 14, 1 857;b) W . Barthlott, C. N einhuis, P l anta 1997, 202,1;c) C. Neinhuis,W. B arthlott, Ann. B ot. 1 997, 79, 667. ) ( [21] D . C yranoski, Nature 2 0 01, 414, 240. ) ( [22] | a) B. D. Washo,Org. C o at. Appl. Polym. Sc i . Proc.1982,47, 69;b)J. P. Youngblood, T.J. McCarthy, Macromolecules 1 999, 32,6800; c)J. D. M iller, S. Veeramasuneni, J. Drelich, M . R.Yaamanchili, G. Y amauchi, Polym. En g . Sci. 1 9 96, 36,1849. ) ( [23] H. Bu, J. Rong, Z. Yang, Macromol. Rapid Commun. 20 0 2, 23,460. ) ( [24] C. M. Henry, Chem. E ng. N ews 2001,79(10),35. ) ( [25] H. Kind, J.M. B onard, C . Emmenegger, L. O. Ni l sson, K . H ermadi,S. E.Ma i llard, L. Schlapbach, L. Forro, K. Kern, Adv.Mater. 1999,11, 1 285. ) ( [26] H. Gau, S . Herminghaus, P. L enz, R . Lipowsky, Science 1999, 283,46. ) ( [27] Two s tretching vibrationbbands from 1 CH2 st retching (~2931 c m-) a n d C = N (~ 2 243 cm-) are observed in sa m ple A. B oth b ands are s trong, revealing the presence of PAN. The corresponding absorptions are weak in t h e s p ectrum of sample B, and a new band appear at ~1594 cm- o riginating from theC-N bond after cy c lization. I n t h e s p ectrum of sample C, allcharacteristic bands disappear, thus i n dicating the carbonizationfrom the cyclized s tructure. ) ( [28] P .M. Th. M. van Attekum, G.K. Wetheim, Phys.Rev. Lett. 1979,43,1896. ) ( [29] H. A go, T. Kugler, F . Cacialli, W. R. S alaneck, M. S. P. Shaffer,A. H. Windle, R. H. Friend, J. Phys. Chem. B 1999, 103, 8116. ) ( [30] K. Esumi, M. Ishigami, A. Nakajima, K. Sawada, H. Honda,Carbon 1996, 34,279. ) Angew. Chem. Int. Ed. DOI: anie.o Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Wiley-VCH Verlag GmbH & Co. KGaA, Weinheimwww.angewandte.orgAngew. Chem. Int. Ed.
确定


还剩2页未读,是否继续阅读?
北京东方德菲仪器有限公司为您提供《德国应用化学收录文章:在全PH值范围内的纳米结构化碳膜的超疏水性》,该方案主要用于碳材料中--检测,参考标准--,《德国应用化学收录文章:在全PH值范围内的纳米结构化碳膜的超疏水性》用到的仪器有
相关方案
更多









